Addition of Carbonaceous Material to Aquatic Sediments for Sorption of Lindane and p,p’-Dichlorodiphenyldichloroethylene
Abstract
:1. Introduction
2. Materials and Methods
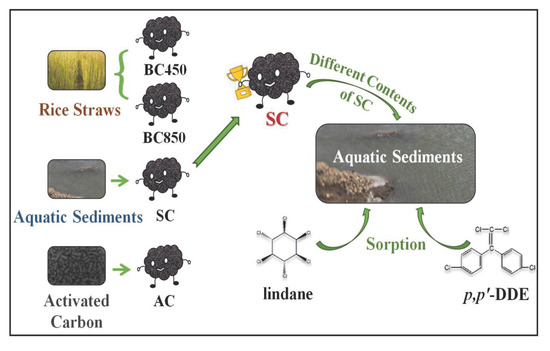
2.1. Materials
2.2. Batch Sorption Experiments of Lindane and p,p’-DDE by the Sediments
2.3. Effects of CMs on the Sorption of Lindane and p,p’-DDE
2.4. Determination of Lindane and p,p’-DDE
2.5. Data Analysis
3. Results
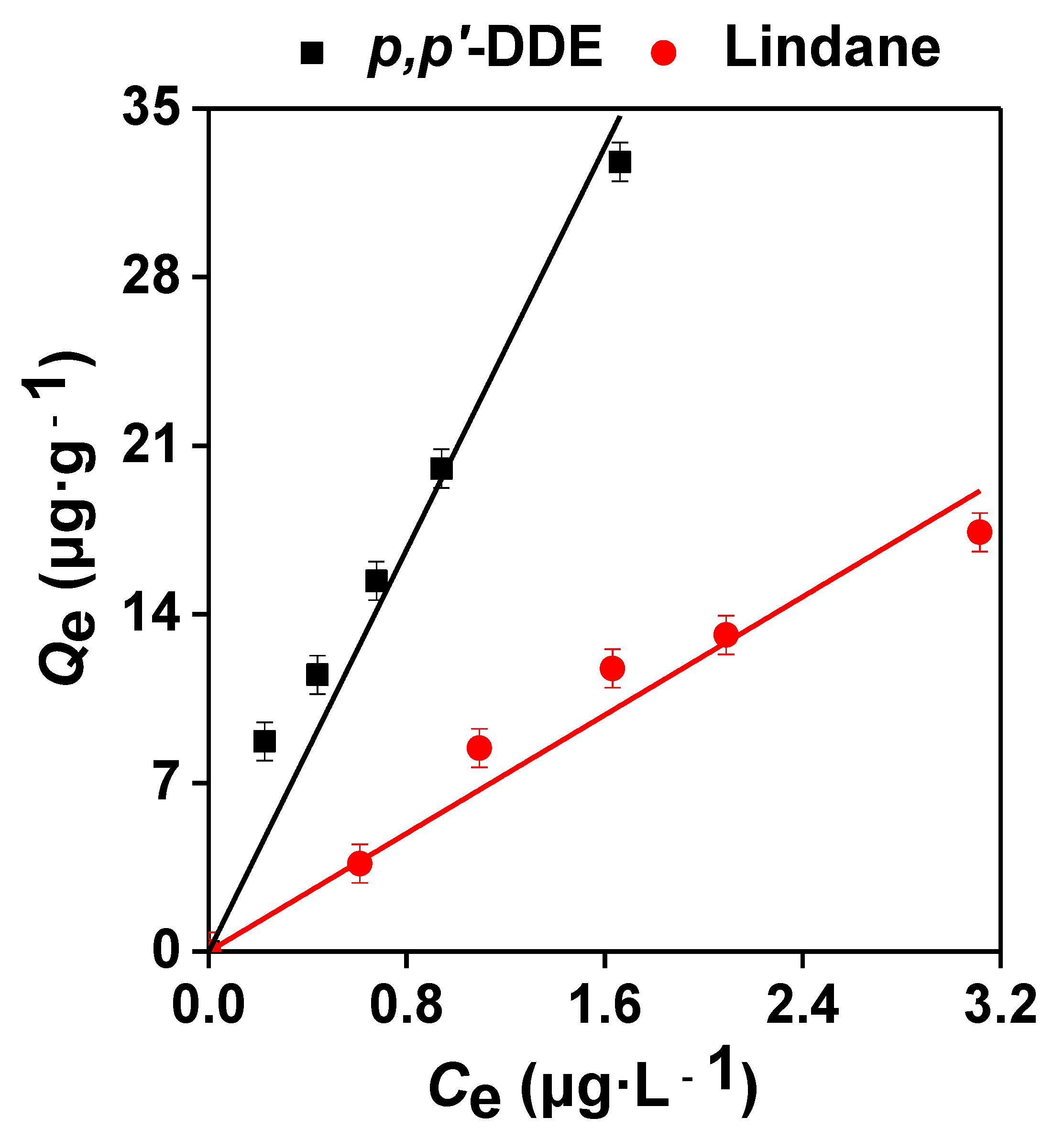
3.1. Sorption Isotherms of Lindane and p,p’-DDE on the Sediments
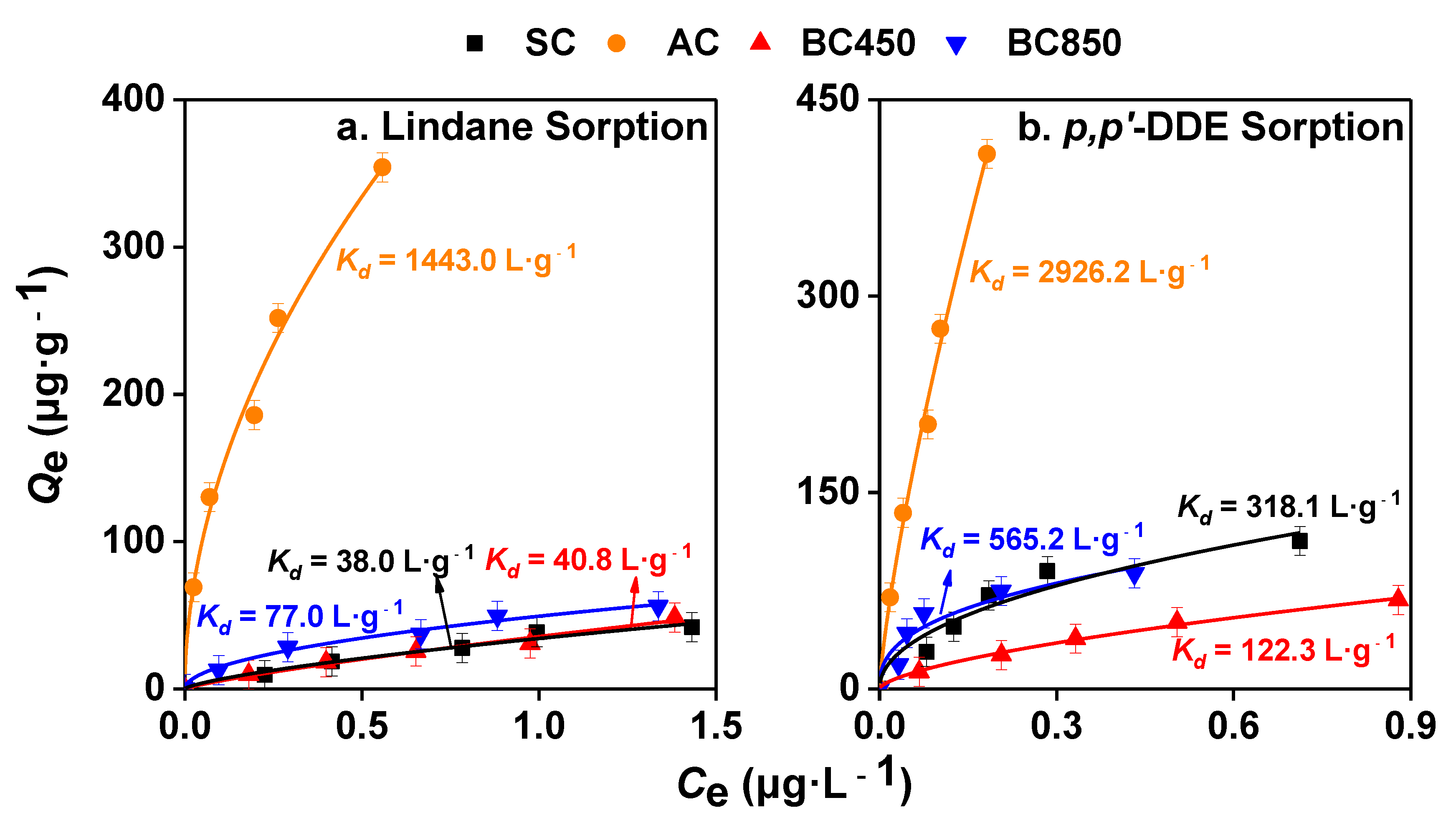
3.2. Sorption Isotherms of Lindane and p,p’-DDE on CMs
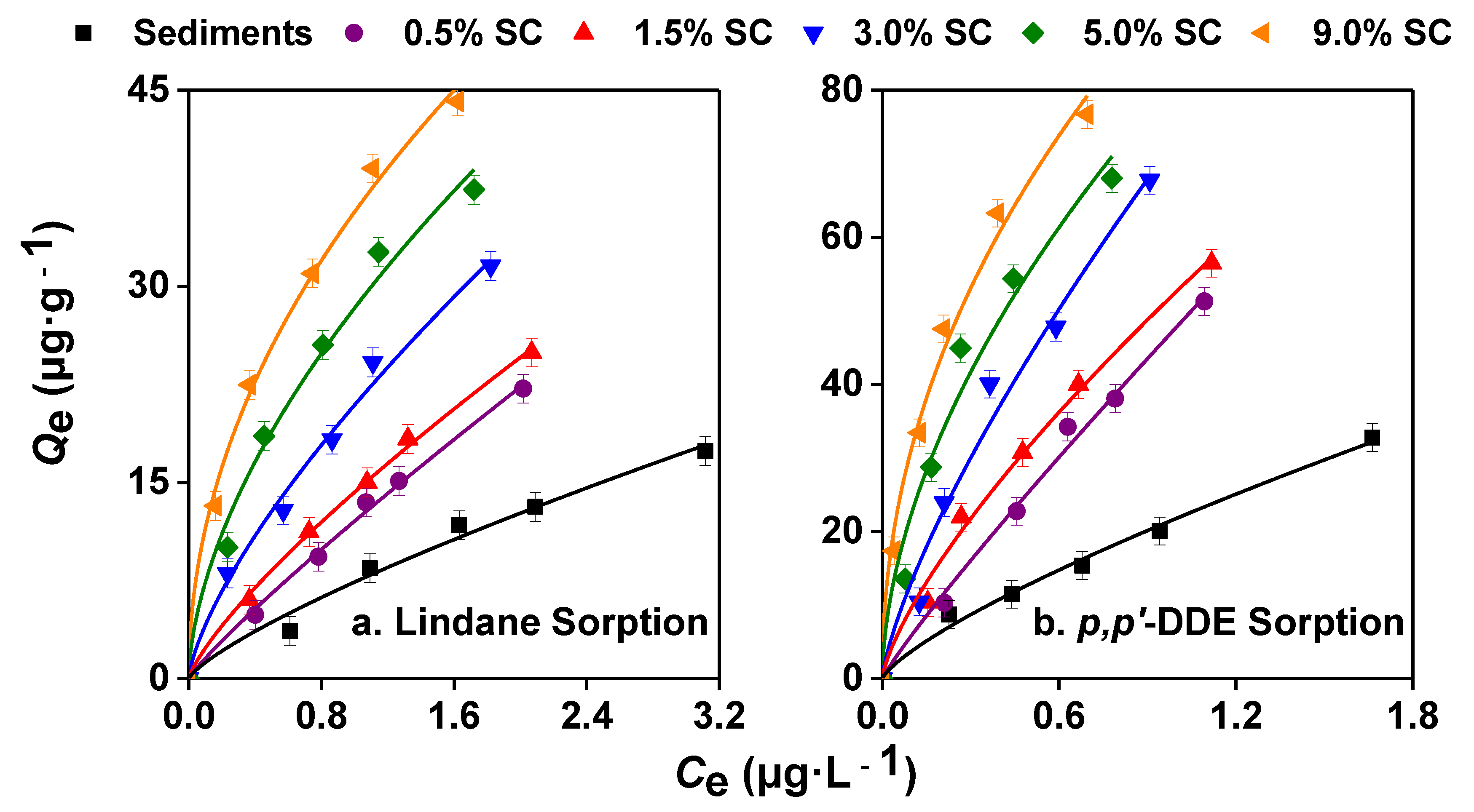
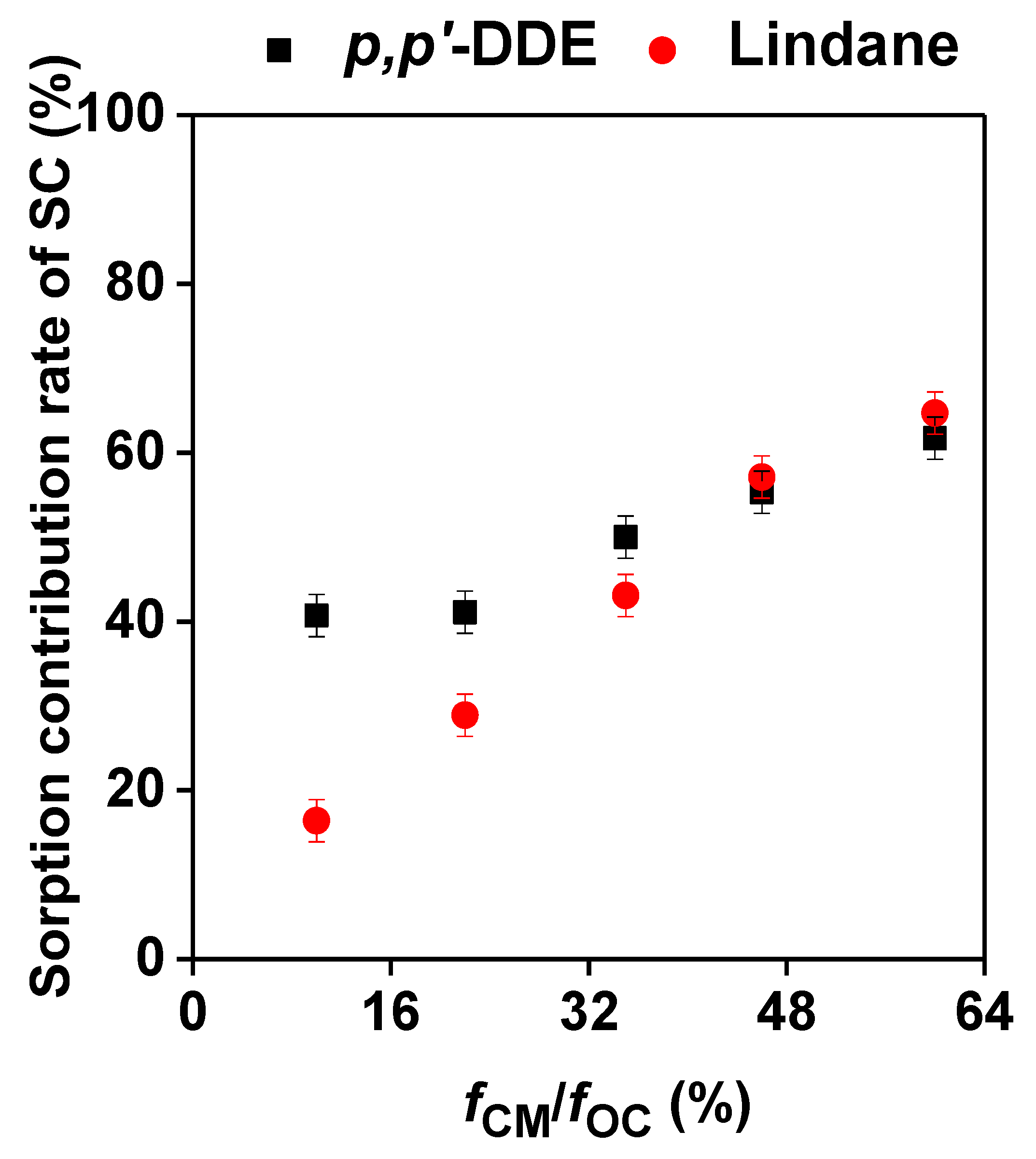
3.3. Effects of SC with Different Contents on the Sorption of Lindane and p,p’-DDE by the Sediments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parween, M.; Ramanathan, A.L.; Khillare, P.S.; Raju, N.J. Persistence, variance and toxic levels of organochlorine pesticides in fluvial sediments and the role of black carbon in their retention. Environ. Sci. Pollut. Res. 2014, 21, 6525–6546. [Google Scholar] [CrossRef]
- Meng, J.; Hong, S.J.; Wang, T.Y.; Li, Q.F.; Yoon, S.J.; Lu, Y.L.; GiesyJohn, J.P.; Khim, J.S. Traditional and new POPs in environments along the Bohai and Yellow Seas: An overview of China and South Korea. Chemosphere 2017, 169, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.C.; Jamil, S.; Singh, V.; Singh, A.; Singh, N.; Srivastava, S.C. Occurrence and distribution of hexachlorocyclohexane isomers in vegetation samples from a contaminated area. Chemosphere. 2008, 72, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Macleod, M.; Borga, K.; Cabrerizo, A.; Dachs, J.; Di Guardo, A.; Ghirardello, D.; Hansen, K.M.; Jarvis, A.; Lindroth, A.; et al. Past, present, and future controls on levels of persistent organic pollutants in the global environment. Environ. Sci. Technol. 2010, 44, 6526–6531. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Nizzetto, L.; Guo, Z.G.; Li, Y.Y.; Li, J.; Zhang, G. DDTs and HCHs in sediment cores from the coastal East China Sea. Sci. Total Environ. 2016, 539, 388–394. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Oturan, N.; Romero, A.; Santos, A.; Oturan, M.A. Lindane degradation by electrooxidation process: Effect of electrode materials on oxidation and mineralization kinetics. Water Res. 2018, 135, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; He, X.X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical-and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]
- Tavares, R.S.; Amaral, S.; Paiva, C.; Baptista, M.; Ramalho-Santos, J. In vitro exposure to the organochlorine p,p’-DDE affects functional human sperm parameters. Chemosphere 2015, 120, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, M.D.; Crosse, J.; Hamilton, P.B.; Johnson, A.C.; Jones, K.C. The long shadow of our chemical past – High DDT concentrations in fish near a former agrochemicals factory in England. Chemosphere 2016, 162, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasinghe, S.; Lind, L.; Salihovic, S.; Larsson, A.; Lind, P.M. High serum levels of p, p’-DDE are associated with an accelerated decline in GFR during 10years follow-up. Sci. Total Environ. 2018, 644, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.J.; Dsikowitzky, L.; Kucher, S.; Ricking, M.; Schwarzbauer, J. Formation and fate of point-source nonextractable DDT-related compounds on their environmental aquatic-terrestrial pathway. Environ. Sci. Technol. 2019, 53, 1305–1314. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Dong, D.M.; Hua, X.Y.; Zhang, L.W.; Zhu, S.J.; Lan, X.H.; Liang, D.P. Cr and As decrease lindane sorption on river solids. Environ. Chem. Lett. 2015, 13, 111–116. [Google Scholar] [CrossRef]
- Kohusova, K.; Havel, L.; Vlasak, P.; Tonika, J. A long-term survey of heavy metals and specific organic compounds in biofilms, sediments, and surface water in a heavily affected river in the Czech Republic. Environ. Monit. Assess. 2011, 174, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Hua, X.Y.; Lan, X.H.; Sun, Y.Y.; Dong, D.M. Evidence for a mutual effect of biofilms, suspended particles and sediments on DDT sorption. Environ. Chem. Lett. 2012, 10, 407–411. [Google Scholar] [CrossRef]
- Oliveira, A.H.B.; Cavalcante, R.M.; Duavi, W.C.; Fernandes, G.M.; Nascimento, R.F.; Queiroz, M.E.L.R.; Mendonça, K.V. The legacy of organochlorine pesticide usage in a tropical semi-arid region (Jaguaribe River, Ceará, Brazil): Implications of the influence of sediment parameters on occurrence, distribution and fate. Sci. Total Environ. 2016, 542, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ogbeide, O.; Chukwuka, A.; Tongo, I.; Ezemonye, L. Relationship between geosorbent properties and field-based partition coefficients for pesticides in surface water and sediments of selected agrarian catchments: Implications for risk assessment. J. Environ. Manage. 2018, 217, 23–27. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhang, Q.; Sun, K.; Liu, X.T.; Zheng, W.J.; Zhao, Y. Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environ. Pollut. 2011, 159, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Lim, B.; Cachier, H. Determination of black carbon by chemical oxidation and thermal treatment in recent marine and lake sediment and Cretaceous-Tertiary clays. Chem. Geol. 1996, 131, 143–154. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Bugna, G.C. Analysis of black carbon in sediments and soils using multi-element scanning thermal analysis (MESTA). Org. Geochem. 2008, 39, 1562–1571. [Google Scholar] [CrossRef]
- Dong, D.M.; Li, L.F.; Zhang, L.W.; Hua, X.Y.; Guo, Z.Y. Effects of lead, cadmium, chromium, and arsenic on the sorption of lindane and norfloxacin by river biofilms, particles, and sediments. Environl. Sci. Pollut. Res. 2018, 25, 4632–4642. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2017, 532, 112–126. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B.S. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Zhang, L.W.; Dong, D.M.; Xie, Y.J.; Guo, Z.Y.; Hua, X.Y. Coexisting sediments and suspended particles change the sorption of lindane and ciprofloxacin in waters. Environ. Chem. Lett. 2018, 16, 1043–1048. [Google Scholar] [CrossRef]
- Cornelissen, G.; Gustafsso, O.; Bucheli, T.D.; Jonker, M.T.O.; Koelmans, A.A.; Van Noort, P.C.M. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005, 39, 6881–6895. [Google Scholar] [CrossRef]
- Braida, W.J.; Pignatello, J.J.; Lu, Y.F.; Ravikovitch, P.I.; Neimark, A.V.; Xing, B.S. Sorption hysteresis of benzene in charcoal particles. Environ. Sci. Technol. 2003, 37, 409–417. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Kwon, S.; Lu, Y.F. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Attenuation of surface activity by humic and fulvic acids. Environ. Sci. Technol. 2006, 40, 7757–7763. [Google Scholar] [CrossRef]
- Chen, Z.M.; Chen, B.L.; Zhou, D.D.; Chen, W.Y. Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ. Sci. Technol. 2012, 46, 12476–12483. [Google Scholar] [CrossRef]
- Lian, F.; Chang, C.; Du, Y.; Zhu, L.; Xing, B.; Liu, C. Adsorptive removal of hydrophobic organic compounds by carbonaceous adsorbents: A comparative study of waste-polymer-based, coal-based activated carbon, and carbon nanotubes. J. Environ. Sci. 2012, 24, 1549–1558. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G.; Diez, M.A. Kinetics and equilibrium study of phenol adsorption on nitrogen-enriched activated carbons. Fuel. 2013, 114, 235–243. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Sorption and desorption behaviors of diuron in soils amended with charcoal. J. Agric. Food Chem. 2006, 54, 8545–8550. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.D.; Masek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kupryianchyk, D.; Hale, S.; Zimmerman, A.R.; Harvey, O.; Rutherford, D.; Abiven, S.; Knicker, H.; Schmidt, H.P.; Rumpel, C.; Cornelissen, G. Sorption of hydrophobic organic compounds to a diverse suite of carbonaceous materials with emphasis on biochar. Chemosphere. 2016, 144, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.H.; Dai, Z.N.; Li, Y.R. The role of black carbon in the sorption and desorption of phenanthrene on river sediments. Environ. Earth Sci. 2011, 64, 2287–2294. [Google Scholar] [CrossRef]
- Ali, U.; Bajwa, A.; Chaudhry, M.J.I.; Mahmood, A.; Syed, J.H.; Li, J.; Zhang, G.; Jones, K.C.; Malik, R.N. Significance of black carbon in the sediment–water partitioning of organochlorine pesticides (OCPs) in the Indus River, Pakistan. Ecotox. Environ. Safe. 2016, 126, 177–185. [Google Scholar] [CrossRef]
- Bajwa, A.; Ali, U.; Mahmood, A.; Chaudhry, M.J.I.; Syed, J.H.; Li, J.; Zhang, G.; Jones, K.C.; Malik, R.N. Organochlorine pesticides (OCPs) in the Indus River catchment area, Pakistan: Status, soil-air exchange and black carbon mediated distribution. Chemosphere 2016, 152, 292–300. [Google Scholar] [CrossRef]
- Jin, L.X.; He, M.C.; Zhang, J.H.; Xia, X.H. Norfloxacin sorption to different fractions in sediments from typical water systems in China. Soil Sediment Contam. 2011, 20, 564–580. [Google Scholar] [CrossRef]
- Zhang, L.; Dickhut, R.; DeMaster, D.; Pohl, K.; Lohmann, R. Organochlorine pollutants in Western Antarctic Peninsula sediments and benthic deposit feeders. Environ. Sci. Technol. 2013, 47, 5643–5651. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigm. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhang, Y.B.; Chen, H.; Zhao, Y.Z.; Quan, X. Contribution of black carbon to nonlinearity of sorption and desorption of acetochlor on sediment. Front. Environ. Sci. Eng. China. 2009, 3, 69–74. [Google Scholar] [CrossRef]
| Organochlorine Pesticide | Structural Formula | Molecular Formula | Melting Point (°C) | Solubility (mg·L−1) | Vapor Pressure (mmHg) | Octanol-Water Partition Coefficient (LogKow) | Bioconcentration Factor |
|---|---|---|---|---|---|---|---|
| Lindane |  | C6H6Cl6 | 112.5 | 7.30 | 4.2 × 10−5 (20 °C) | 3.72 | 1.4 × 104 |
| p,p’-DDE | 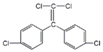 | C14H8Cl4 | 89.0 | 0.12 | 6.0 × 10−6 (25 °C) | 6.51 | 9.8 × 105 |
| SD | SC | AC | BC450 | BC850 | |
|---|---|---|---|---|---|
| fCM (%) | 0.17 a | 14.49 | 56.93 | 12.47 | 37.29 |
| SSA (m2·g−1) | - | 21.4 | 525.1 | 26.5 | 79.6 |
| Sediments | 0.5% SC | 1.5% SC | 3.0% SC | 5.0% SC | 9.0% SC | |
|---|---|---|---|---|---|---|
| fCM (%) | 0.17 | 0.67 | 1.67 | 3.17 | 5.17 | 9.17 |
| fOC (%) | 6.16 | 6.66 | 7.66 | 9.16 | 11.16 | 15.16 |
| fCM/fOC | 0.03 | 0.10 | 0.22 | 0.35 | 0.46 | 0.60 |
| KOC-lindane [(µg·g−1)·(μg·L−1)−n] | 119.82 | 180.81 | 186.17 | 228.41 | 253.94 | 235.44 |
| KOC-p,p’-DDE [(µg·g−1)·(μg·L−1)−n] | 346.23 | 718.90 | 688.12 | 801.81 | 731.11 | 621.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Chen, T.; Wang, X.; Zhang, L.; Wang, L.; Dong, D.; Hua, X. Addition of Carbonaceous Material to Aquatic Sediments for Sorption of Lindane and p,p’-Dichlorodiphenyldichloroethylene. Appl. Sci. 2019, 9, 1722. https://doi.org/10.3390/app9091722
Guo Z, Chen T, Wang X, Zhang L, Wang L, Dong D, Hua X. Addition of Carbonaceous Material to Aquatic Sediments for Sorption of Lindane and p,p’-Dichlorodiphenyldichloroethylene. Applied Sciences. 2019; 9(9):1722. https://doi.org/10.3390/app9091722
Chicago/Turabian StyleGuo, Zhiyong, Tianyi Chen, Xinzhou Wang, Liwen Zhang, Liting Wang, Deming Dong, and Xiuyi Hua. 2019. "Addition of Carbonaceous Material to Aquatic Sediments for Sorption of Lindane and p,p’-Dichlorodiphenyldichloroethylene" Applied Sciences 9, no. 9: 1722. https://doi.org/10.3390/app9091722
APA StyleGuo, Z., Chen, T., Wang, X., Zhang, L., Wang, L., Dong, D., & Hua, X. (2019). Addition of Carbonaceous Material to Aquatic Sediments for Sorption of Lindane and p,p’-Dichlorodiphenyldichloroethylene. Applied Sciences, 9(9), 1722. https://doi.org/10.3390/app9091722