Antihypertensive Peptide Activity in Dutch-Type Cheese Models Prepared with Different Additional Strains of Lactobacillus Genus Bacteria
Abstract
1. Introduction
2. Material and Methods
2.1. Determination of Proteolytic Activity of Lactobacillus Strains
2.2. Preparation of Cheese Models
- C—a control cheese model consisting of milk, 2% of basic CHN-19 starter, and a coagulant;
- Lba 2499—a cheese model consisting of milk, 2% of CHN-19 starter, 1.5% of Lb. acidophilus 2499 culture, and a coagulant;
- Lbr 489—a cheese model consisting of milk, 2% of CHN-19 starter, 1.5% of Lb. rhamnosus 489 culture, and a coagulant;
- Lbd 490—a cheese model consisting of milk, 2% of CHN-19 starter, 1.5% of Lb. delbrueckii 490 culture, and a coagulant;
- Lbc 2639—a cheese model consisting of milk, 2% of CHN-19 starter, 1.5% of Lb. casei 2639 culture, and a coagulant.
2.3. Measurement of the ACE-Inhibitory Activity of Dutch-Type Cheese Models
2.4. Determination of Proteolysis of Dutch-Type Cheese Models
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proteolytic Activity of Lactobacillus Strains
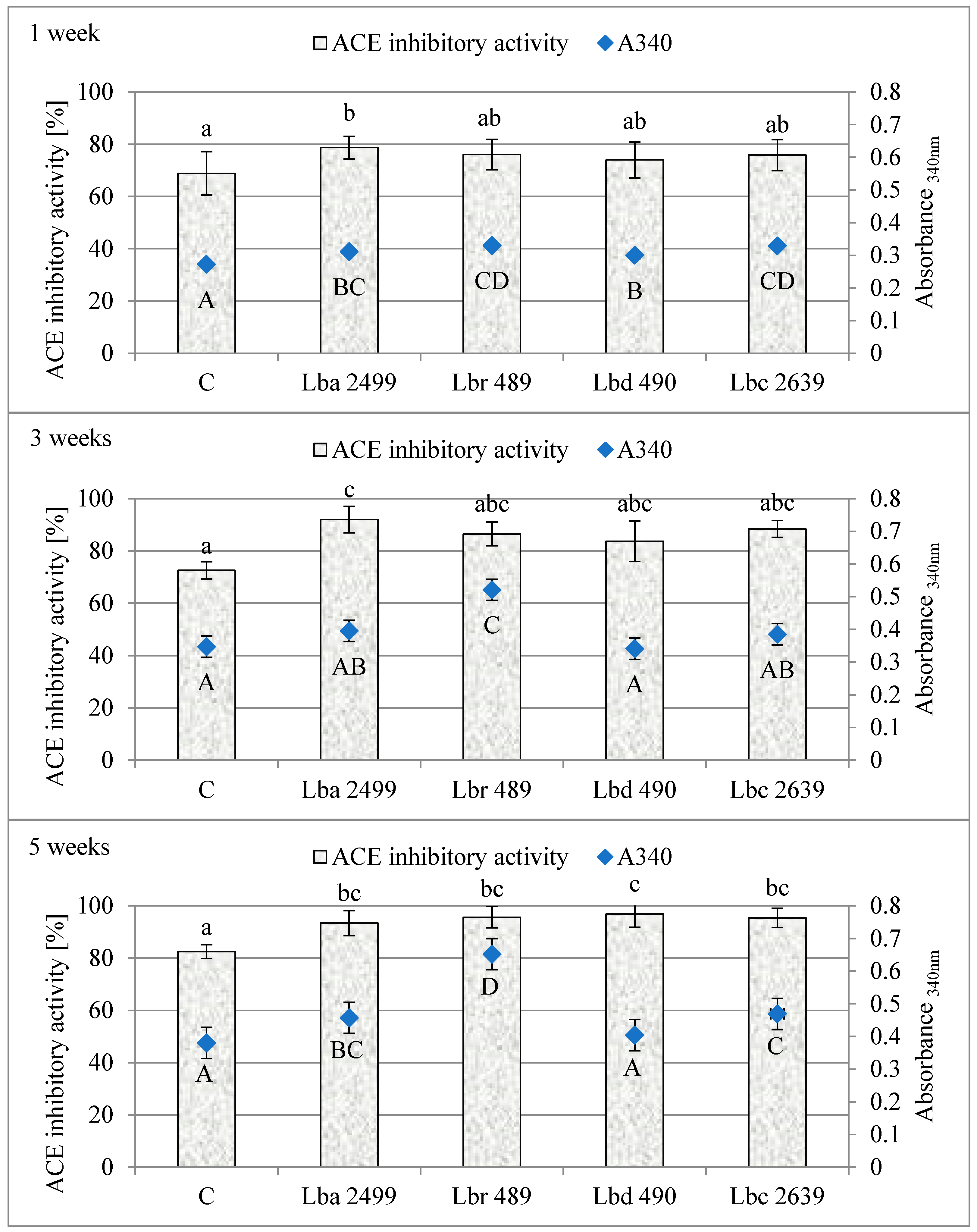
3.2. Proteolytic and ACE-Inhibitory Activity (%) During Ripening of Dutch-Type Cheese Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ryhänen, E.L.; Pihlanto-Leppälä, A.; Pahkala, E. A new type of ripened, low-fat cheese with bioactive properties. Int. Dairy J. 2001, 11, 441–447. [Google Scholar] [CrossRef]
- Dziuba, B.; Dziuba, M. Milk proteins-derived bioactive peptides in dairy products: Molecular, biological and methodological aspects. Acta Sci. Pol. Technol. Aliment. 2014, 13, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on human health of microorganisms present in fermented dairy products: An overview. Biomed. Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.; Rana, S. Bioactive peptides: A review. Int. J. Bioautom. 2011, 15, 223–250. [Google Scholar]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Sieber, R.; Bütikofer, U.; Egger, C.; Portmann, R.; Walther, B.; Wechsler, D. ACE-inhibitory activity and ACE-inhibiting peptides in different cheese varieties. Dairy Sci. Technol. 2010, 90, 47–73. [Google Scholar] [CrossRef]
- Baptista, D.P.; Galli, B.D.; Cavalheiro, F.G.; Negrāo, F.; Eberlin, M.N.; Gigante, M.L. Lactobacillus helveticus LH-B02 favours the release of bioactive peptide during Prato cheese ripening. Int. Dairy J. 2018, 87, 75–83. [Google Scholar]
- Reale, A.; Ianniello, R.G.; Ciocia, F.; Di Renzo, T.; Boscaino, F.; Ricciardi, A.; Coppola, R.; Parente, E.; Zotta, T.; McSweeney, P.L.H. Effect of respirative and catalase-positive Lactobacillus casei adjuncts on the production and quality of Cheddar-type cheese. Int. Dairy J. 2016, 63, 78–87. [Google Scholar] [CrossRef]
- Addeo, F.; Chianese, L.; Salzano, A.; Sacchi, R.; Cappuccio, U.; Ferranti, P.; Malorni, A. Characterization of the 12% trichloroacetic acid-insoluble oligopeptides of Parmigiano-Reggiano cheese. J. Dairy Res. 1992, 59, 401–411. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Bioactive peptides in dairy products: Synthesis and interaction with proteolytic enzymes. Food Microbiol. 2000, 17, 129–141. [Google Scholar] [CrossRef]
- EFSA. Evolus and reduce arterial stiffness—Scientific substantiation of a health claim related to Lactobacillus helveticus fermented Evolus® low-fat milk products and reduction of arterial stiffness pursuant to article 14 of the regulation (EC) No 1924/2006—Scientific opinion of the panel on dietetic products, nutrition and allergies; question number EFSA-Q-2008-218. EFSA J. 2008, 6, 824. [Google Scholar] [CrossRef][Green Version]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Rev. Artic. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Hajirostamloo, B. Bioactive component in milk and dairy product. Int. Sci. Index Agric. Biosyst. Eng. 2010, 4, 870–874. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Hynes, E.; Ogier, J.C.; Delacroix-Buchet, A. Protocol for the manufacture of miniature washed-curd cheeses under controlled microbiological conditions. Int. Dairy J. 2000, 10, 733–737. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Ramchandran, L.; Shah, N.P. Influence of addition of Raftiline HP® on the growth, proteolytic, ACE-and α-glucosidase inhibitory activities of selected lactic acid bacteria and Bifidobacterium. LWT Food Sci. Technol. 2010, 43, 146–152. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Le Lait 2007, 87, 21–38. [Google Scholar] [CrossRef]
- Ong, L.; Henriksson, A.; Shah, N.P. Angiotensin converting enzyme-inhibitory activity in Cheddar cheeses made with the addition of probiotic Lactobacillus casei sp. Le Lait 2007, 87, 149–165. [Google Scholar] [CrossRef]
- Ong, L.; Shah, N.P. Release and identification of angiotensin-converting enzyme-inhibitory peptides as influenced by ripening temperatures and probiotic adjuncts in Cheddar cheeses. LWT Food Sci. Technol. 2008, 41, 1555–1566. [Google Scholar] [CrossRef]
- Pripp, A.H.; Sørensen, R.; Stepaniak, L.; Sørhaug, T. Relationship between proteolysis and angiotensin-I-converting enzyme inhibition in different cheeses. LWT Food Sci. Technol. 2006, 39, 677–683. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Peptides from several Italian cheeses inhibitory to proteolytic enzymes of lactic acid bacteria, Pseudomonas fluorescens ATCC 948 and to the angiotensin I-converting enzyme. Enzym. Microb. Technol. 1998, 22, 687–694. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Daroit, D.J.; Helfer, V.E.; Corrêa, A.P.F.; Segalin, J.; Carro, S.; Brandelli, A. Bioactive peptides in water-soluble extracts of ovine cheeses from Southern Brazil and Uruguay. Food Res. Int. 2012, 48, 322–329. [Google Scholar] [CrossRef]
- Meisel, H.; Goepfert, A.; Gunther, S. ACE-inhibitory activities in milk products. Milchwissenschaft 1997, 52, 307–311. [Google Scholar]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and structural analysis of antihypertensive peptides that exist naturally in Gouda cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef]
- Ong, L.; Shah, N.P. Influence of probiotic Lactobacillus acidophilus and L. helveticus on proteolysis, organic acid profiles, and ACE inhibitory activity of Cheddar cheeses ripened at 4, 8, and 12 °C. J. Food Sci. 2008, 73, M111–M120. [Google Scholar] [CrossRef]
- Stuknytė, M.; Cattaneo, S.; Masotti, F.; De Noni, I. Occurrence and fate of ACE-inhibitor peptides in cheeses and in their digestates following in vitro static gastrointestinal digestion. Food Chem. 2015, 168, 27–33. [Google Scholar] [CrossRef]
- Bernabucci, U.; Catalani, E.; Basiricò, L.; Morera, P. In vitro ACE-inhibitory activity and in vivo antihypertensive effects of water-soluble extract by Parmigiano Reggiano and Grana Padano cheeses. Int. Dairy J. 2014, 37, 16–19. [Google Scholar] [CrossRef]

| Cheese Model | |||||
|---|---|---|---|---|---|
| C | Lba 2499 | Lbr 489 | Lbd 490 | Lbc 2639 | |
| 1 day | 0.84 ± 0.01 d, D | 0.71 ± 0.02 ab, D | 0.69 ± 0.03 a, D | 0.75 ± 0.06 bc, D | 0.75 ± 0.02 bc, D |
| 1 week | 0.69 ± 0.05 bc, C | 0.67 ± 0.03 abc, C | 0.65 ± 0.02 ab, C | 0.63 ± 0.06 a, C | 0.67 ± 0.05 abc, C |
| 3 weeks | 0.59 ± 0.06 ab, B | 0.57 ± 0.03 ab, B | 0.55 ± 0.03 a, B | 0.55 ± 0.06 a, B | 0.57 ± 0.03 a, B |
| 5 weeks | 0.49 ± 0.05 b, A | 0.45 ± 0.08 ab, A | 0.45 ± 0.03 ab, A | 0.39 ± 0.02 a, A | 0.47 ± 0.02 ab, A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbowska, M.; Pluta, A.; Berthold-Pluta, A. Antihypertensive Peptide Activity in Dutch-Type Cheese Models Prepared with Different Additional Strains of Lactobacillus Genus Bacteria. Appl. Sci. 2019, 9, 1674. https://doi.org/10.3390/app9081674
Garbowska M, Pluta A, Berthold-Pluta A. Antihypertensive Peptide Activity in Dutch-Type Cheese Models Prepared with Different Additional Strains of Lactobacillus Genus Bacteria. Applied Sciences. 2019; 9(8):1674. https://doi.org/10.3390/app9081674
Chicago/Turabian StyleGarbowska, Monika, Antoni Pluta, and Anna Berthold-Pluta. 2019. "Antihypertensive Peptide Activity in Dutch-Type Cheese Models Prepared with Different Additional Strains of Lactobacillus Genus Bacteria" Applied Sciences 9, no. 8: 1674. https://doi.org/10.3390/app9081674
APA StyleGarbowska, M., Pluta, A., & Berthold-Pluta, A. (2019). Antihypertensive Peptide Activity in Dutch-Type Cheese Models Prepared with Different Additional Strains of Lactobacillus Genus Bacteria. Applied Sciences, 9(8), 1674. https://doi.org/10.3390/app9081674





