Interhemispheric Brain Switching Correlates with Severity of Sleep-Disordered Breathing for Obstructive Sleep Apnea Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
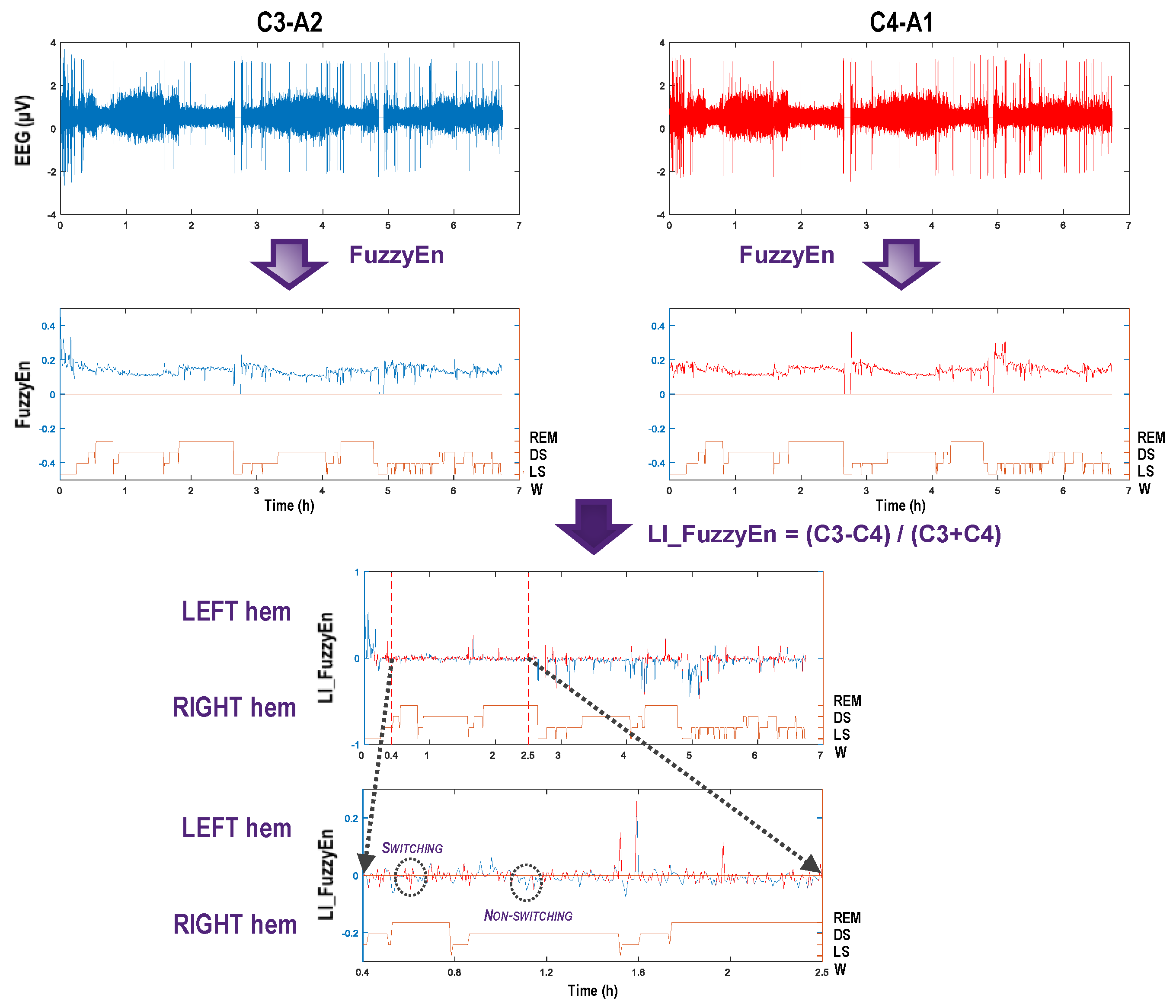
2.1.1. Fuzzy Entropy
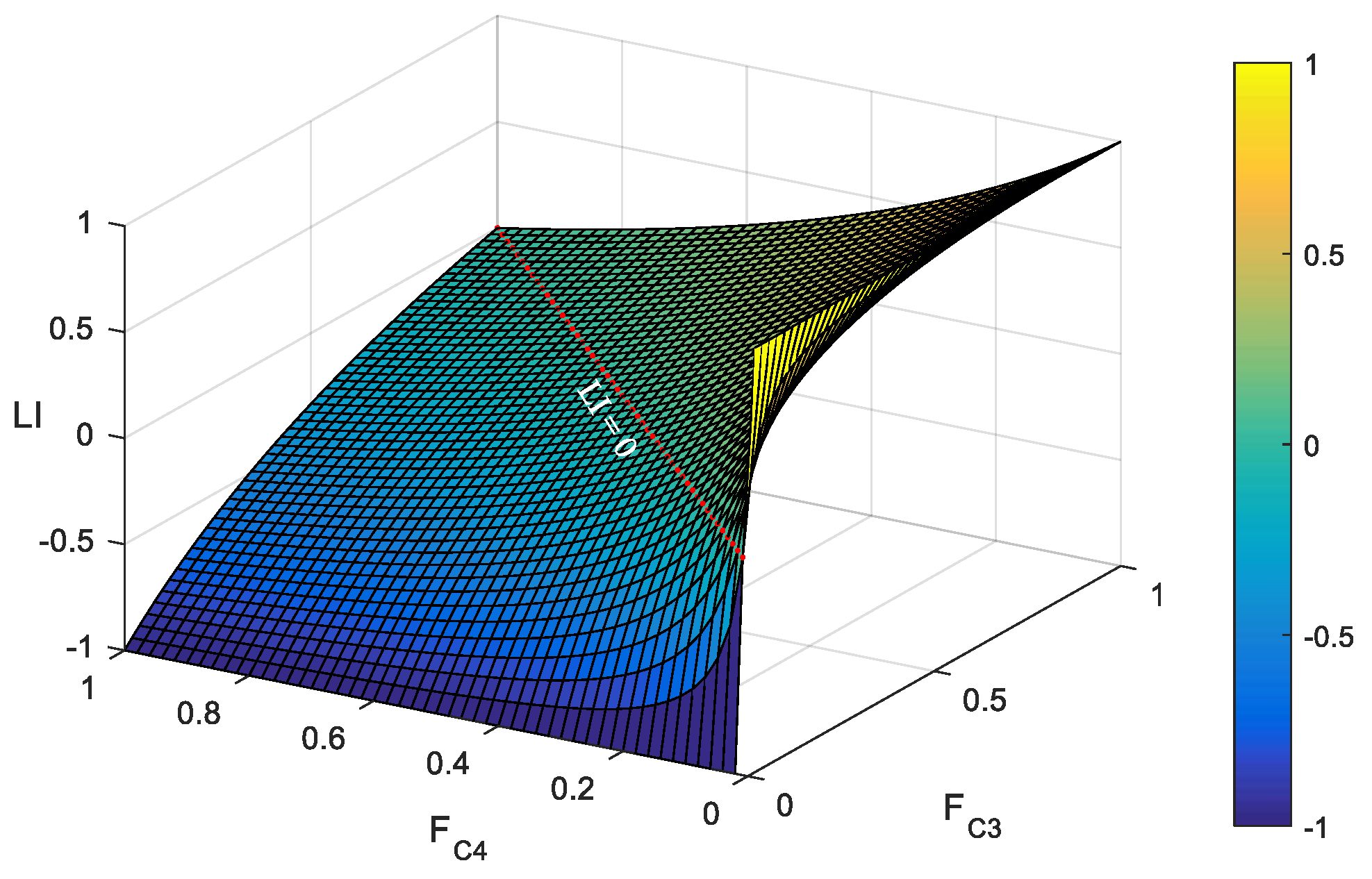
2.1.2. Laterality Index and Its Distribution
3. Results
3.1. Fuzzy Entropy Extraction
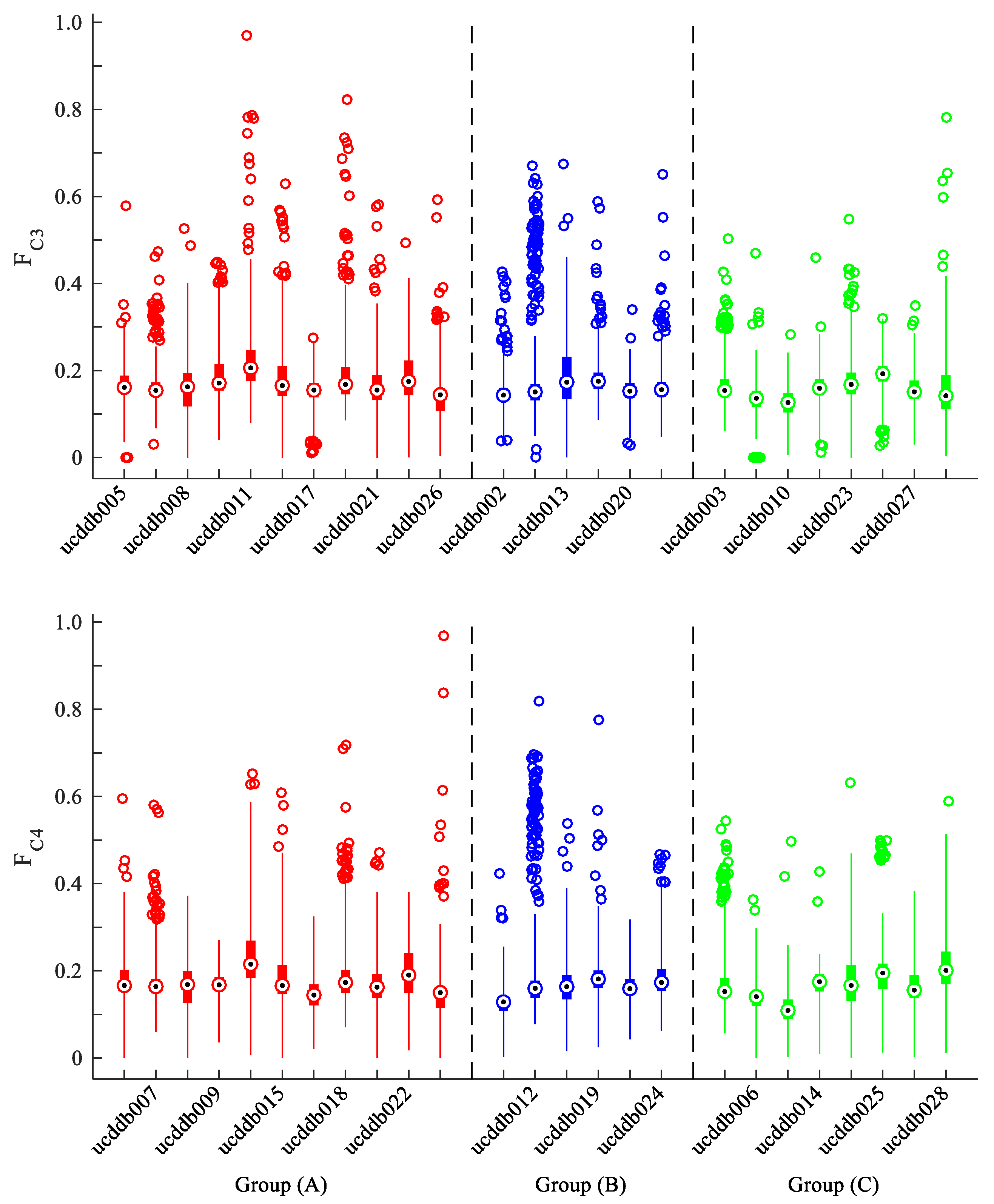
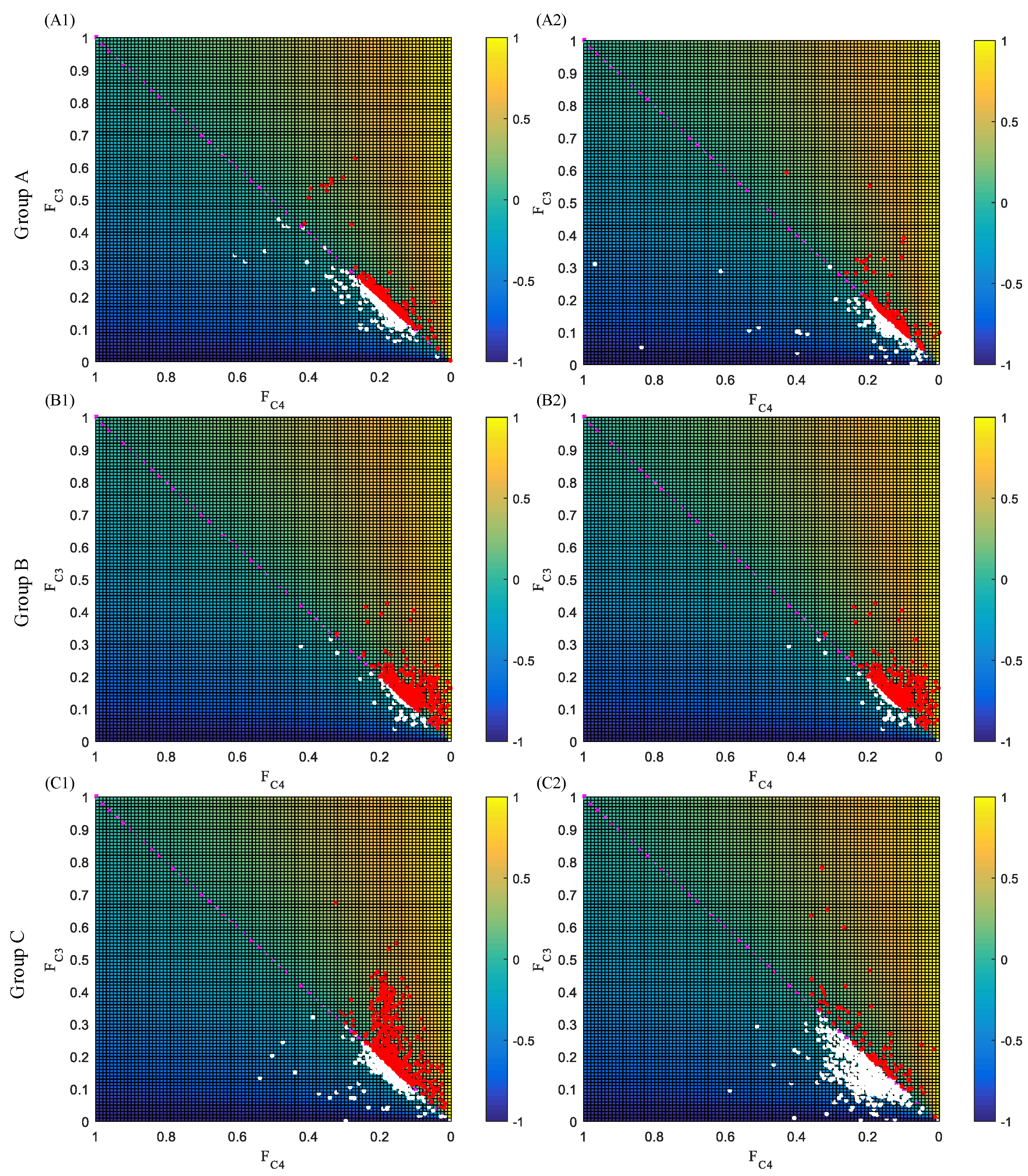
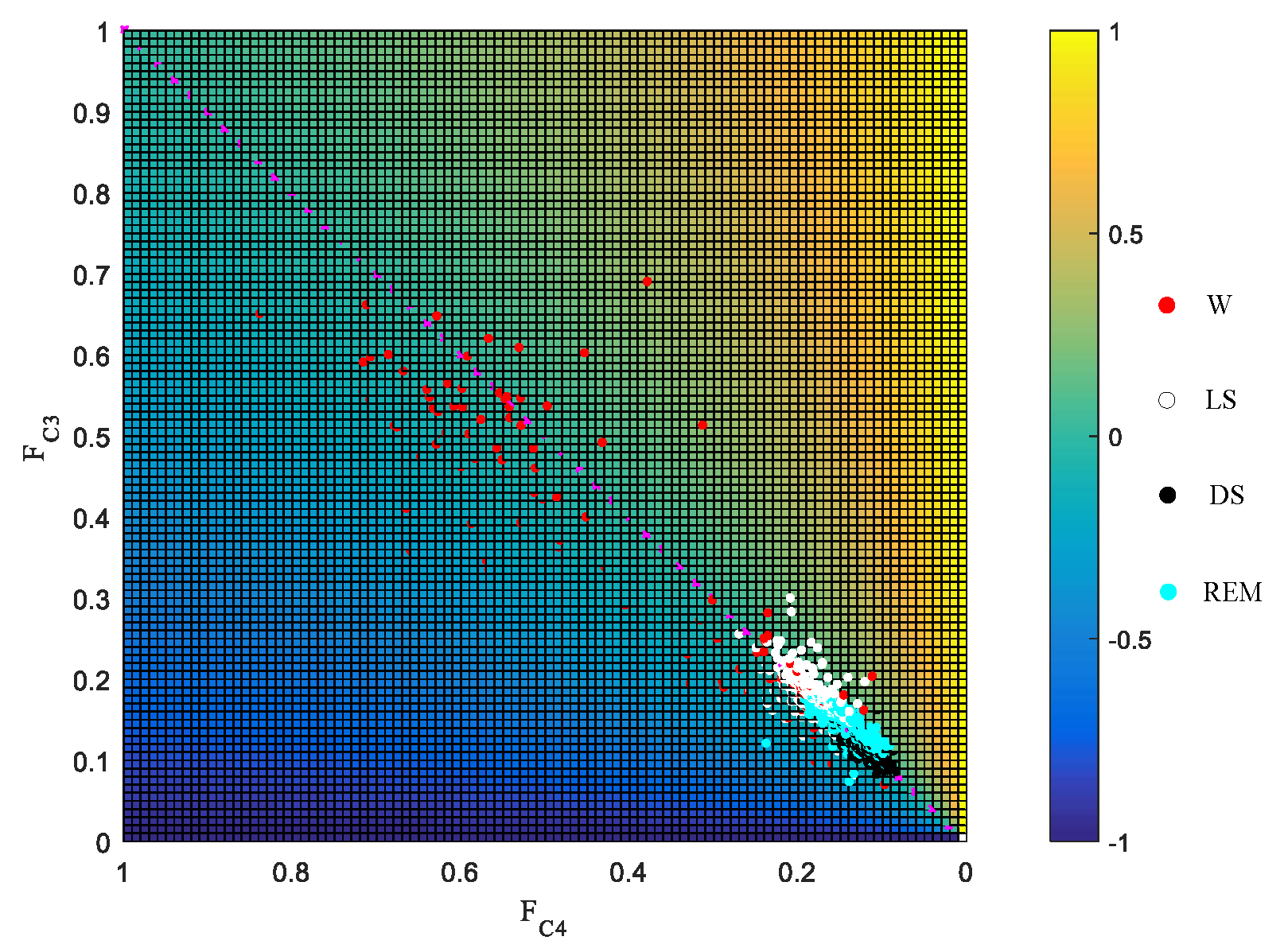
3.2. Laterality Index Distribution
3.3. Statistical Test of EEG Switching Rate
3.3.1. ANOVA Test
- Test of data normality and variance homogeneityIn order to perform the ANOVA test, two conditions need to be satisfied: (1) data normality and (2) variance homogeneity. Thus, we reported the test results of these two conditions for Groups and Sleep Stages, respectively. For normality test, p-value is the probability of observing a test statistic as extreme as, or more extreme than, the observed value under the null hypothesis. Small values of p cast doubt on the validity of the null hypothesis. Also, h = 1 indicates the rejection of the null hypothesis, i.e., the data in vector x comes from a distribution in the normal family, while h = 0 means a failure to reject the null hypothesis. Our experiments reported that the switching rate for both Groups and Sleep Stages are normally distributed, with h = 0 and p > 0.05 for both considerations. Besides, since the p-value for homogeneity test is also bigger than 0.05, with p = 0.9896 for Groups test and p = 0.2204 for Sleep Stages test, it also reveals that the data satisfies variance homogeneity.
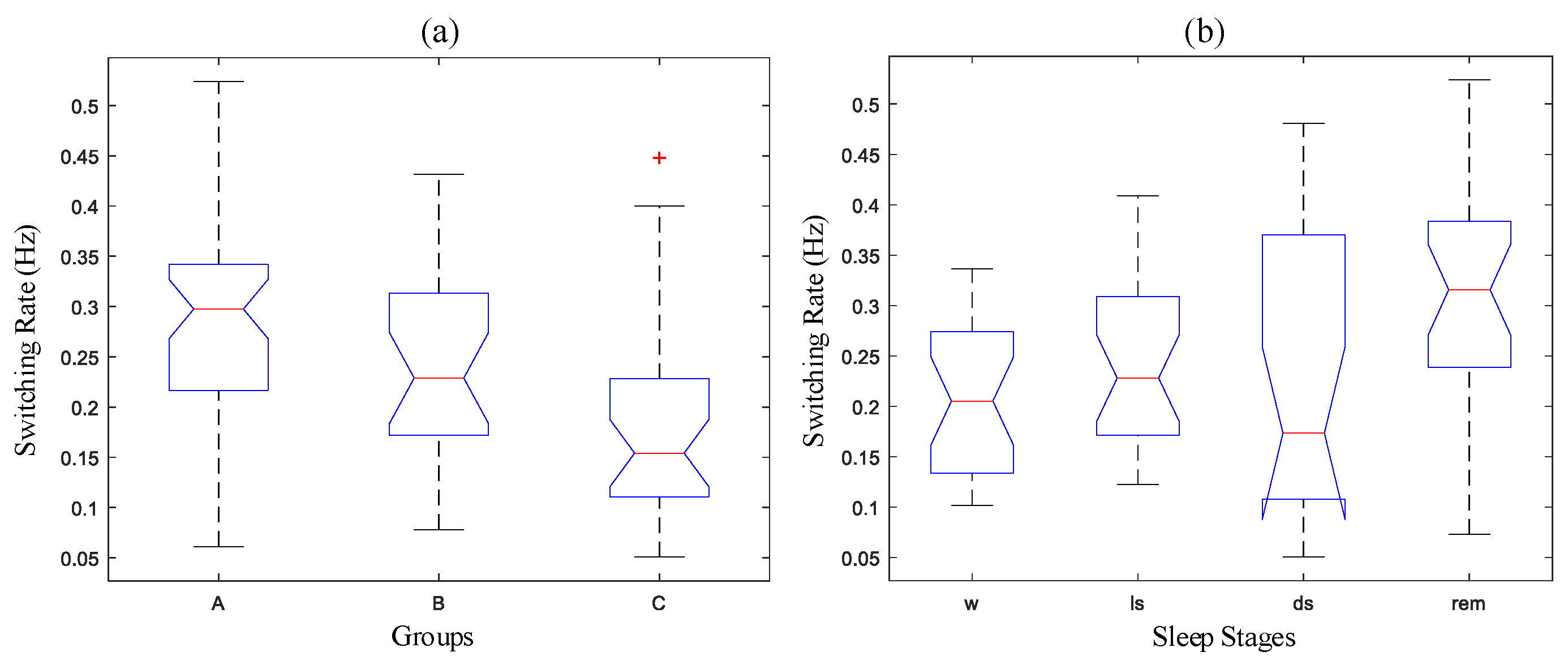
- ANOVA testBased on the test results of data normality and variance homogeneity, the ANOVA test for three groups and four sleep stages were given in this section. First of all, the one-way ANOVA test was given to test the inter-differences among groups or sleep stages. Results showed that the p-values for both tests were 0.0012 (p1 < 0.05) and 0.0026 (p2 < 0 .05), respectively, which indicates that the differences among groups or sleep stages were significant. Figure 7 illustrates the boxplot of data distributions for Figure 7a Groups and Figure 7b Sleep Stages. It also clearly reveals the inter-differences in each subfigure. Also, since we were considering two factors, i.e., groups (AHI) and sleep stages that may affect the switching rate, we also performed the two-way ANOVA to give a more comprehensive difference test for both factors. The results were given in Table 3. The p-values for both factors also agrees with the previous one-way ANOVA test, but it also reveals that there is no evidence of an interaction effect of the two.
3.3.2. Independent Samples t-Test
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kales, A.; Caldwell, A.B.; Cadieux, R.J.; Vela-Bueno, A.; Ruch, L.G.; Mayes, S.D. Severe obstructive sleep apnea—II: Associated psychopathology and psychosocial consequences. J. Dis. 1985, 38, 427–434. [Google Scholar] [CrossRef]
- Ahmad, M.; Makati, D.; Akbar, S. Review of and Updates on Hypertension in Obstructive Sleep Apnea. Int. J. Hypertens. 2017, 2017, 1848375. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Zizi, F.; Clark, L.T.; Brown, C.D.; McFarlane, S.I. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J. Clin. Sleep Med. 2008, 4, 261–272. [Google Scholar] [PubMed]
- Gurubhagavatula, I.; Sullivan, S.; Meoli, A.; Patil, S.; Olson, R.; Berneking, M.; Watson, N.F. Management of Obstructive Sleep Apnea in Commercial Motor Vehicle Operators: Recommendations of the AASM Sleep and Transportation Safety Awareness Task Force. J. Clin. Sleep Med. 2017, 13, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, K.K.; Mcclary, A.C.; Amedee, R.G. Obstructive sleep apnea: A growing problem. Ochsner J. 2009, 9, 149–153. [Google Scholar] [PubMed]
- Lucia, S.; Daniela, C.; Giuseppe, D.M. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic Dis. 2015, 6, 273–285. [Google Scholar]
- Ruehland, W.R.; Rochford, P.D.; O’Donoghue, F.J.; Pierce, R.J.; Parmjit, S.; Thornton, A.T. The new AASM criteria for scoring hypopneas: Impact on the apnea hypopnea index. Sleep 2009, 32, 150–157. [Google Scholar] [CrossRef]
- Bloch, K.E. Polysomnography: A systematic review. Technol. Health Care 1997, 5, 285. [Google Scholar]
- Chesson, A.L.; Ferber, R.A.; Fry, J.M.; Grigg-Damberger, M.; Hartse, K.M.; Hurwitz, T.D.; Johnson, S.; Kader, G.A.; Littner, M.; Rosen, G.; et al. The Indications for Polysomnography and Related Procedures. Sleep 1997, 20, 423–487. [Google Scholar] [CrossRef]
- Wu, C.T.; Dillon, D.G.; Hsu, H.C.; Huang, S.; Barrick, E.; Liu, Y.H. Depression detection using relative EEG power induced by emotionally positive images and a conformal kernel support vector machine. Appl. Sci. 2018, 8, 1244. [Google Scholar] [CrossRef]
- Kose, U. An Ant-Lion Optimizer-Trained Artificial Neural Network System for Chaotic Electroencephalogram (EEG) Prediction. Appl. Sci. 2018, 8, 1613. [Google Scholar] [CrossRef]
- Al-Nafjan, A.; Hosny, M.; Al-Ohali, Y.; Al-Wabil, A. Review and classification of emotion recognition based on EEG brain-computer interface system research: A systematic review. Appl. Sci. 2017, 7, 1239. [Google Scholar] [CrossRef]
- Li, Y.J.; Huang, J.J.; Zhou, H.Y.; Zhong, N. Human Emotion Recognition with Electroencephalographic Multidimensional Features by Hybrid Deep Neural Networks. Appl. Sci. 2017, 7, 1060. [Google Scholar] [CrossRef]
- Anwar, S.M.; Saeed, S.M.U.; Majid, M.; Usman, S.; Mehmood, C.A.M.; Liu, W. A game player expertise level classification system using electroencephalography (EEG). Appl. Sci. 2018, 8, 18. [Google Scholar] [CrossRef]
- Chowdhury, M.E.H.; Khandakar, A.; Hossain, B.; Alzoubi, K. Effects of the phantom shape on the gradient artefact of electroencephalography (EEG) data in simultaneous EEG–fMRI. Appl. Sci. 2018, 8, 1969. [Google Scholar] [CrossRef]
- Ren, H.; Qu, J.F.; Chai, Y.; Huang, L.; Tang, Q. Cepstrum Coefficient Analysis from Low-Frequency to High-Frequency Applied to Automatic Epileptic Seizure Detection with Bio-Electrical Signals. Appl. Sci. 2018, 8, 1528. [Google Scholar] [CrossRef]
- Coben, R.; Clarke, A.R.; Hudspeth, W.; Barry, R.J. EEG power and coherence in autistic spectrum disorder. Clin. Neurophysiol. 2008, 119, 1002–1009. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Guevara, M.A.; Arce, C.; Ramos, J. Inter and intrahemispheric EEG correlation as a function of sleep cycles. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1996, 20, 387–405. [Google Scholar] [CrossRef]
- Leocani, L.; Comi, G. EEG coherence in pathological conditions. J. Clin. Neurophysiol. 1999, 16, 548. [Google Scholar] [CrossRef]
- Nielsen, T.; Abel, A.; Lorrain, D.; Montplaisir, J. Interhemispheric EEG coherence during sleep and wakefulness in left- and right-handed subjects. Brain Cogn. 1990, 14, 113–125. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Zhang, X.; Yao, L.; Yue, L.; Qian, B.; Li, X. EEG-based motion intention recognition via multi-task RNNs. In Proceedings of the 2018 SIAM International Conference on Data Mining, San Diego, CA, USA, 3–5 May 2018; pp. 279–287. [Google Scholar]
- Zhang, D.; Yao, L.; Xiang, Z.; Wang, S.; Chen, W.; Boots, R. EEG-based intention recognition from spatio-temporal representations via cascade and parallel convolutional recurrent neural networks. arXiv, 2017; arXiv:1708.06578. [Google Scholar]
- Abeyratne, U.R.; Vinayak, S.; Craig, H.; Duce, B. Interhemispheric asynchrony correlates with severity of respiratory disturbance index in patients with sleep apnea. IEEE Trans. Biomed. Eng. 2010, 57, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Rial, R.; Gené, L.; Akaârir, M.; Esteban, S.; Nicolau, C.; Gonzalez, J.; Gamundí, A.; Barceló, P. Asymmetric sleep in apneic human patients. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R232–R237. [Google Scholar] [CrossRef] [PubMed]
- Long, M.A.; Fee, M.S. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 2008, 456, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lily, Y.; Foley, N.C.; Bobula, J.M.; Kriegsfeld, L.J.; Rae, S. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J. Neurosci. 2005, 25, 9017–9026. [Google Scholar]
- Butler, M.P.; Rainbow, M.N.; Elizabeth, R.; Lyon, S.M.; Rae, S. Twelve-hour days in the brain and behavior of split hamsters. Eur. J. Neurosci. 2012, 36, 2556–2566. [Google Scholar] [CrossRef]
- Bojsen-Moller, F.; Fahrenkrug, J. Nasal swell-bodies and cyclic changes in the air passage of the rat and rabbit nose. J. Anat. 1971, 110, 25–37. [Google Scholar] [PubMed]
- Campbell, W.M.; Kern, E.B. The nasal cycle in swine. Rhinology 1981, 19, 127. [Google Scholar]
- Bamford, O.S.; Eccles, R. The central reciprocal control of nasal vasomotor oscillations. Pflügers Archiv. 1982, 394, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Friling, L.; Nyman, H.T.; Johnson, V. Asymmetric nasal mucosal thickening in healthy dogs consistent with the nasal cycle as demonstrated by MRI and CT. Vet. Radiol. Ultrasound 2014, 55, 159–165. [Google Scholar] [CrossRef]
- Kahana-Zweig, R.; Geva-Sagiv, M.; Weissbrod, A.; Secundo, L.; Soroker, N.; Sobel, N. Measuring and Characterizing the Human Nasal Cycle. PLoS ONE 2016, 11, e0162918. [Google Scholar] [CrossRef]
- Rattenborg, N.C.; Lima, S.L.; Amlaner, C.J. Half-awake to the risk of predation. Nature 1999, 397, 397–398. [Google Scholar] [CrossRef]
- Lyamin, O.I.; Manger, P.R.; Ridgway, S.H.; Mukhametov, L.M.; Siegel, J.M. Cetacean sleep: An unusual form of mammalian sleep. Neurosci. Biobehav. Rev. 2008, 32, 1451–1484. [Google Scholar] [CrossRef]
- Imbach, L.L.; Esther, W.; Ulf, K.; Johannes, S.; Scammell, T.E.; Baumann, C.R. Inter-hemispheric oscillations in human sleep. PLoS ONE 2012, 7, e48660. [Google Scholar] [CrossRef]
- Heneghan, C.; Chua, C.P.; Garvey, J.F.; De Chazal, P.; Shouldice, R.; Boyle, P.; McNicholas, W.T. A portable automated assessment tool for sleep apnea using a combined Holter-oximeter. Sleep 2008, 31, 1432–1439. [Google Scholar]
- Inouye, T.; Shinosaki, K.; Sakamoto, H.; Toi, S.; Ukai, S.; Iyama, A.; Katsuda, Y.; Hirano, M. Quantification of EEG irregularity by use of the entropy of the power spectrum. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 204–210. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, S.; Zhu, G.; Liu, F.; Li, Y.; Dong, X.; Liu, C.; Liu, F. Efficient sleep classification based on entropy features and a support vector machine classifier. Physiol. Meas. 2018, 39, 115005. [Google Scholar] [CrossRef]
- Seghier, M.L. Laterality index in functional MRI: Methodological issues. Magn. Resonance Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef]
- Binder, J.R.; Swanson, S.J.; Hammeke, T.A.; Morris, G.L.; Mueller, W.M.; Fischer, M.; Benbadis, S.; Frost, J.; Rao, S.M.; Haughton, V.M. Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 1996, 46, 978–984. [Google Scholar] [CrossRef]
- Fernandez, B.; Cardebat, D.; Demonet, J.F.; Joseph, P.A.; Mazaux, J.M.; Barat, M.; Allard, M. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke 2004, 35, 2171–2176. [Google Scholar] [CrossRef]
- Benson, R.R.; Fitzgerald, D.B.; Lesueur, L.L.; Kennedy, D.N.; Kwong, K.K.; Buchbinder, B.R.; Davis, T.L.; Weisskoff, R.M.; Talavage, T.M.; Logan, W.J.; et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 1999, 52, 798. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Fernández, A.; Escudero, J. Refined multiscale fuzzy entropy based on standard deviation for biomedical signal analysis. Med. Biol. Eng. Comput. 2017, 55, 2037–2052. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.M.; Kumar, R.; Ogren, J.A.; Macey, P.M. Sleep-disordered breathing: Effects on brain structure and function. Respir. Physiol. Neurobiol. 2013, 188, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Mascetti, G.G. Unihemispheric sleep and asymmetrical sleep: Behavioral, neurophysiological, and functional perspectives. Nat. Sci. Sleep 2016, 8, 221–238. [Google Scholar] [CrossRef]
- Zhu, G.; Ngo, T. Characterising a putative EEG switching biomarker in chronobiology. J. Sleep Res. 2018, 27, e53_12766. [Google Scholar]

| (a) Group A (AHI < 15) | |||||||||
| Subject ID | Age | Sex | AHI | Sleep Efficiency (%) | PSG Duration (s) | Percentage (%) | |||
| W | LS | DS | REM | ||||||
| ucddb005 | 65 | M | 13 | 83 | 24798 | 37.41 | 41.16 | 6.30 | 15.13 |
| ucddb007 | 47 | M | 12 | 90 | 24405 | 10.33 | 57.81 | 15.62 | 16.24 |
| ucddb008 | 63 | F | 5 | 64 | 23041 | 34.74 | 49.93 | 10.96 | 4.36 |
| ucddb009 | 52 | M | 12 | 80 | 27759 | 20.54 | 54.59 | 15.24 | 9.62 |
| ucddb011 | 51 | M | 8 | 60 | 27030 | 39.56 | 42.67 | 13.11 | 4.67 |
| ucddb015 | 28 | M | 6 | 77 | 27488 | 22.71 | 53.93 | 15.94 | 7.42 |
| ucddb017 | 53 | M | 12 | 87 | 23684 | 13.05 | 54.25 | 8.24 | 24.46 |
| ucddb018 | 35 | M | 2 | 60 | 24685 | 39.90 | 41.12 | 16.67 | 2.31 |
| ucddb021 | 41 | F | 13 | 82 | 27409 | 18.18 | 53.89 | 13.91 | 14.02 |
| ucddb022 | 34 | M | 7 | 58 | 23640 | 41.55 | 36.21 | 16.65 | 5.59 |
| ucddb026 | 49 | M | 14 | 87 | 25160 | 13.37 | 40.69 | 16.59 | 29.36 |
| (b) Group B (15 ≤ AHI < 30) | |||||||||
| Subject ID | Age | Sex | AHI | Sleep Efficiency (%) | PSG Duration (s) | Percentage (%) | |||
| W | LS | DS | REM | ||||||
| ucddb002 | 54 | M | 23 | 84 | 22470 | 16.18 | 51.47 | 11.63 | 20.72 |
| ucddb012 | 51 | M | 25 | 85 | 25941 | 14.93 | 45.72 | 16.90 | 22.45 |
| ucddb013 | 62 | F | 16 | 61 | 24333 | 39.09 | 38.96 | 13.69 | 8.26 |
| ucddb019 | 49 | M | 16 | 92 | 25573 | 8.33 | 46.83 | 23.00 | 21.83 |
| ucddb020 | 52 | M | 15 | 78 | 22586 | 22.47 | 48.27 | 8.51 | 20.74 |
| ucddb024 | 54 | M | 24 | 83 | 27250 | 17.15 | 49.12 | 15.15 | 18.58 |
| (c) Group C (AHI ≥ 30) | |||||||||
| Subject ID | Age | Sex | AHI | Sleep Efficiency (%) | PSG Duration (s) | Percentage (%) | |||
| W | LS | DS | REM | ||||||
| ucddb003 | 48 | M | 51 | 81 | 26478 | 19.05 | 40.82 | 18.59 | 21.54 |
| ucddb006 | 52 | M | 31 | 89 | 24267 | 11.39 | 34.16 | 30.69 | 23.76 |
| ucddb010 | 38 | M | 34 | 92 | 27211 | 8.16 | 64.50 | 8.71 | 18.63 |
| ucddb014 | 56 | M | 36 | 79 | 23239 | 21.32 | 68.09 | 0 | 10.59 |
| ucddb023 | 68 | F | 39 | 67 | 25850 | 32.75 | 52.61 | 8.01 | 6.62 |
| ucddb025 | 52 | M | 91 | 77 | 21350 | 22.93 | 69.34 | 1.27 | 6.47 |
| ucddb027 | 45 | M | 55 | 86 | 26791 | 14.22 | 66.29 | 3.92 | 15.57 |
| ucddb028 | 50 | M | 46 | 68 | 21660 | 31.62 | 47.85 | 7.21 | 13.31 |
| Wake | Light Sleep | Deep Sleep | REM Sleep | |
|---|---|---|---|---|
| Group A | 0.2497 ± 0.0041 | 0.3009 ± 0.0041 | 0.2316 ± 0.0198 | 0.3537 ± 0.0077 |
| Group B | 0.2093 ± 0.0024 | 0.2319 ± 0.0035 | 0.1785 ± 0.0178 | 0.3293 ± 0.0086 |
| Group C | 0.1505 ± 0.0049 | 0.1800 ± 0.0081 | 0.1541 ± 0.0232 | 0.2267 ± 0.0180 |
| Factors | p |
|---|---|
| Sleep Stages | 0.0012 |
| Groups | 0.0006 |
| Sleep Stages * Groups | 0.1106 |
| Pairs | Groups | Pairs | Sleep Stages | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A-B | A-C | B-C | W-LS | W-DS | W-REM | LS-DS | LS-REM | DS-REM | ||
| p | 0.135 | 0.000 | 0.063 | p | 0.129 | 0.582 | 0.000 | 0.612 | 0.010 | 0.017 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Z.; Zhu, G.; Gan, H.; Liu, D.; Weng, W.; Wei, S. Interhemispheric Brain Switching Correlates with Severity of Sleep-Disordered Breathing for Obstructive Sleep Apnea Patients. Appl. Sci. 2019, 9, 1568. https://doi.org/10.3390/app9081568
Li Y, Zhang Z, Zhu G, Gan H, Liu D, Weng W, Wei S. Interhemispheric Brain Switching Correlates with Severity of Sleep-Disordered Breathing for Obstructive Sleep Apnea Patients. Applied Sciences. 2019; 9(8):1568. https://doi.org/10.3390/app9081568
Chicago/Turabian StyleLi, Yuwen, Zhimin Zhang, Guohun Zhu, Hongping Gan, Deyin Liu, Wei Weng, and Shoushui Wei. 2019. "Interhemispheric Brain Switching Correlates with Severity of Sleep-Disordered Breathing for Obstructive Sleep Apnea Patients" Applied Sciences 9, no. 8: 1568. https://doi.org/10.3390/app9081568
APA StyleLi, Y., Zhang, Z., Zhu, G., Gan, H., Liu, D., Weng, W., & Wei, S. (2019). Interhemispheric Brain Switching Correlates with Severity of Sleep-Disordered Breathing for Obstructive Sleep Apnea Patients. Applied Sciences, 9(8), 1568. https://doi.org/10.3390/app9081568





