Study on the Fabrication of Porous TiAl Alloy via Non-Aqueous Gel Casting of a TiH2 and Al Powder Mixture
Abstract
:1. Introduction
2. Materials and Methods
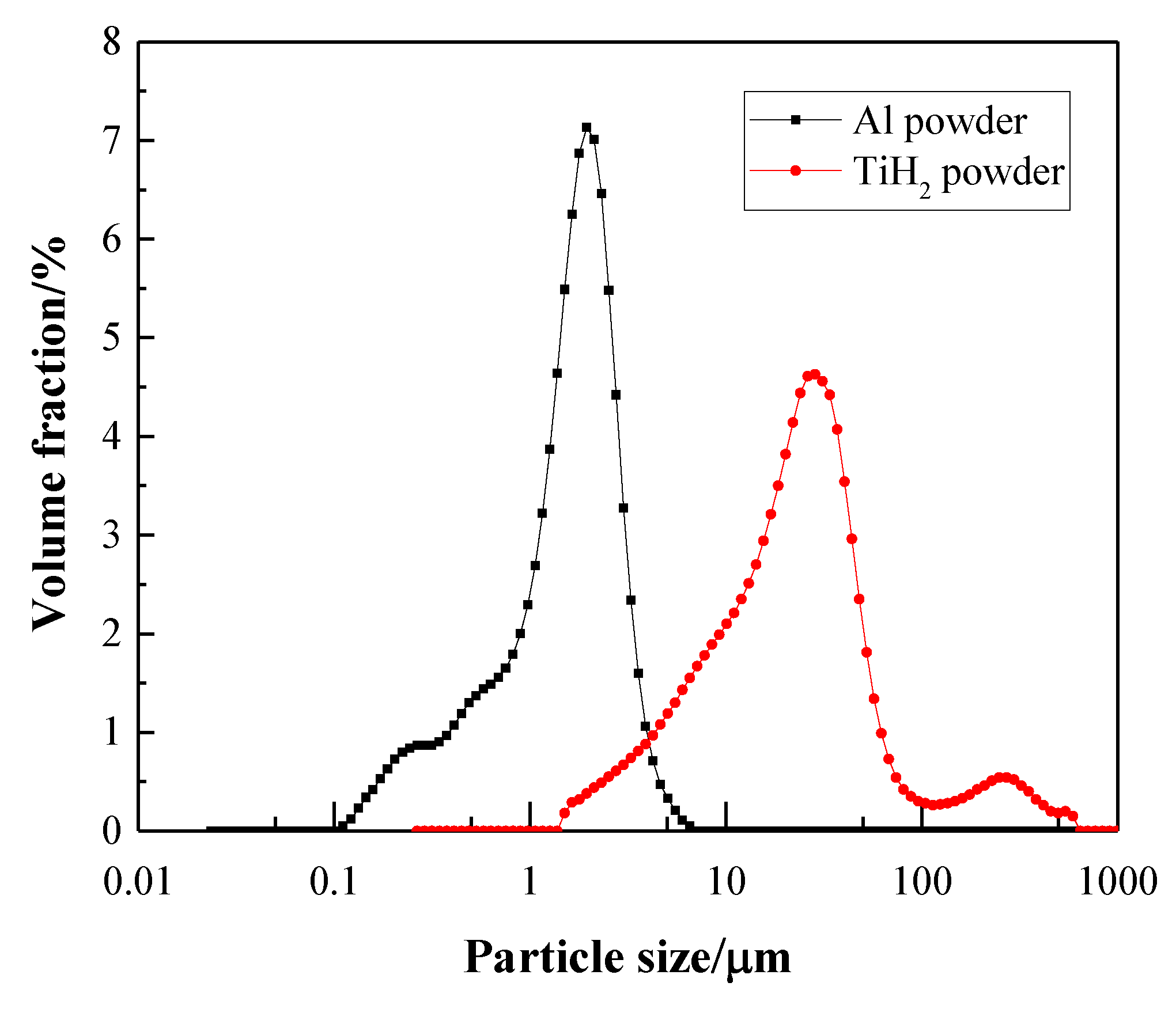
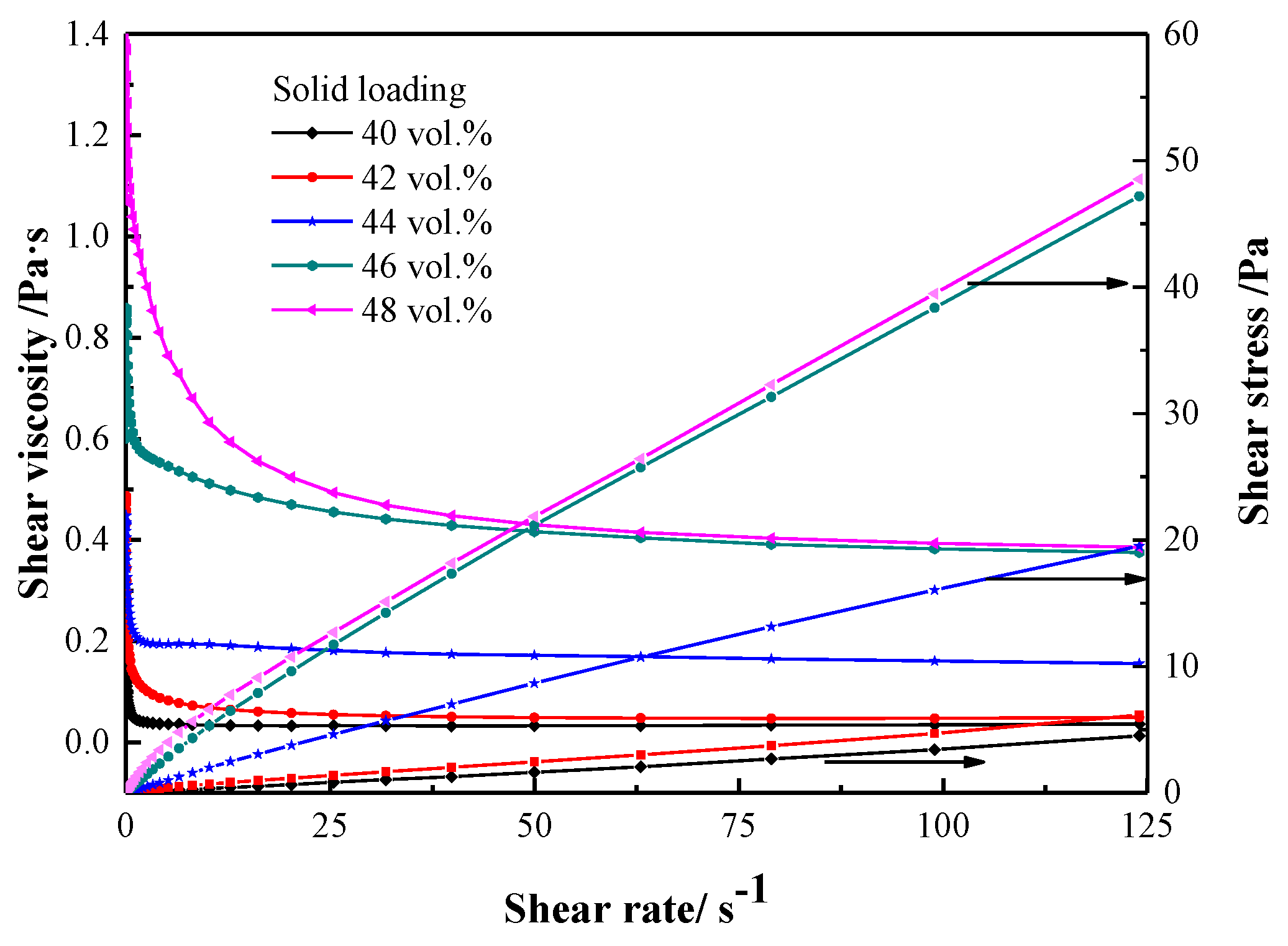
3. Results
4. Conclusions
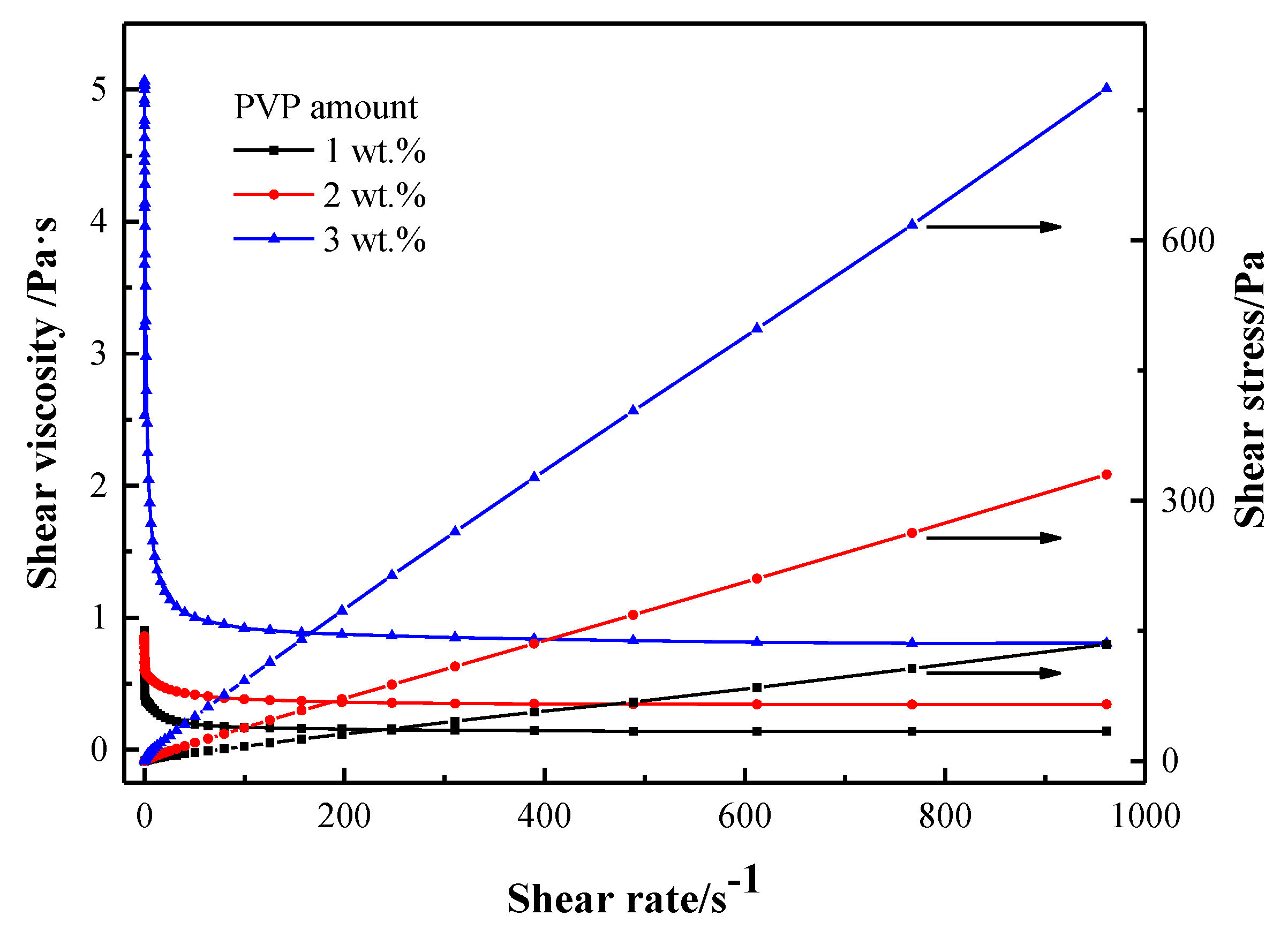
- A non-aqueous TiH2/Al slurry system for gel casting was developed, which exhibited shear-thinning behavior and was favorable for the gel casting process of TiH2/Al green bodies with a complex shape. The optimum amount of PVP addition to the slurry was 1 wt % relative to the TiH2/Al powder mixture.
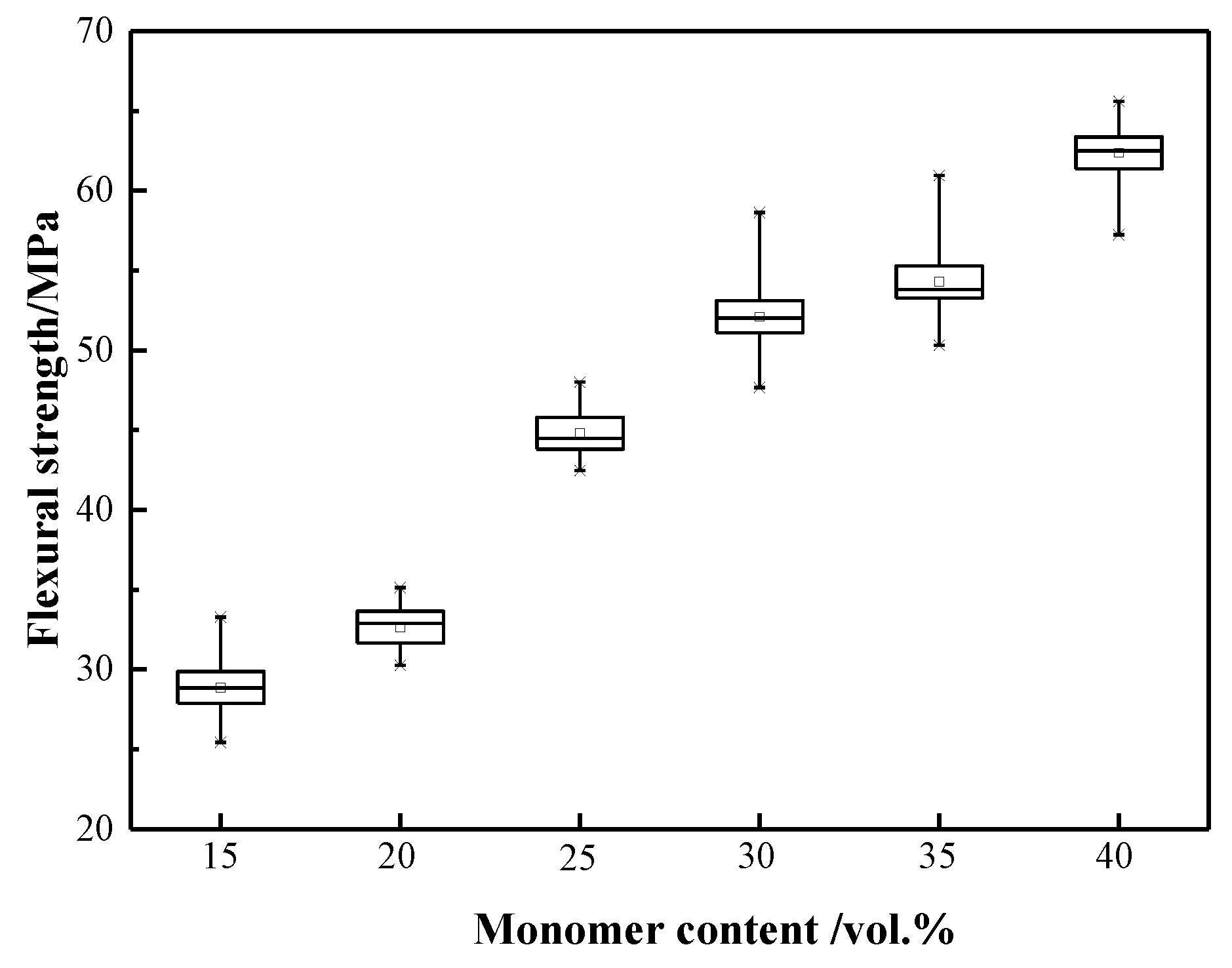
- The monomer contents have a great effect on the mechanical properties of the TiH2/Al green bodies. The flexural strength of the green bodies increased from 28.86 to 62.36 MPa as the monomer contents in the premix solution increased from 15 to 40 vol % relative to the premix solution. The suitable monomer dosage determined was 30 vol % relative to the volume of the solutions.
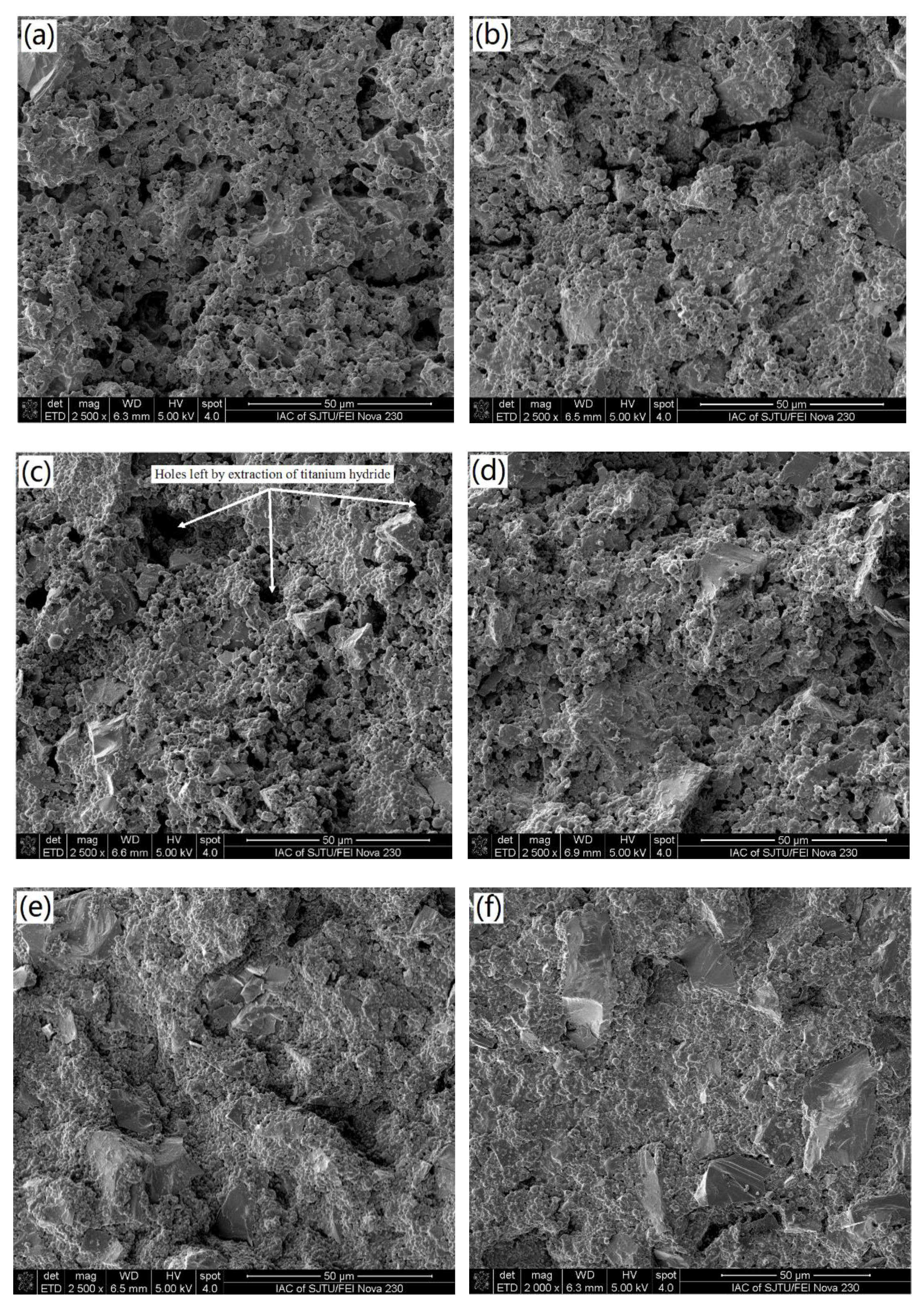
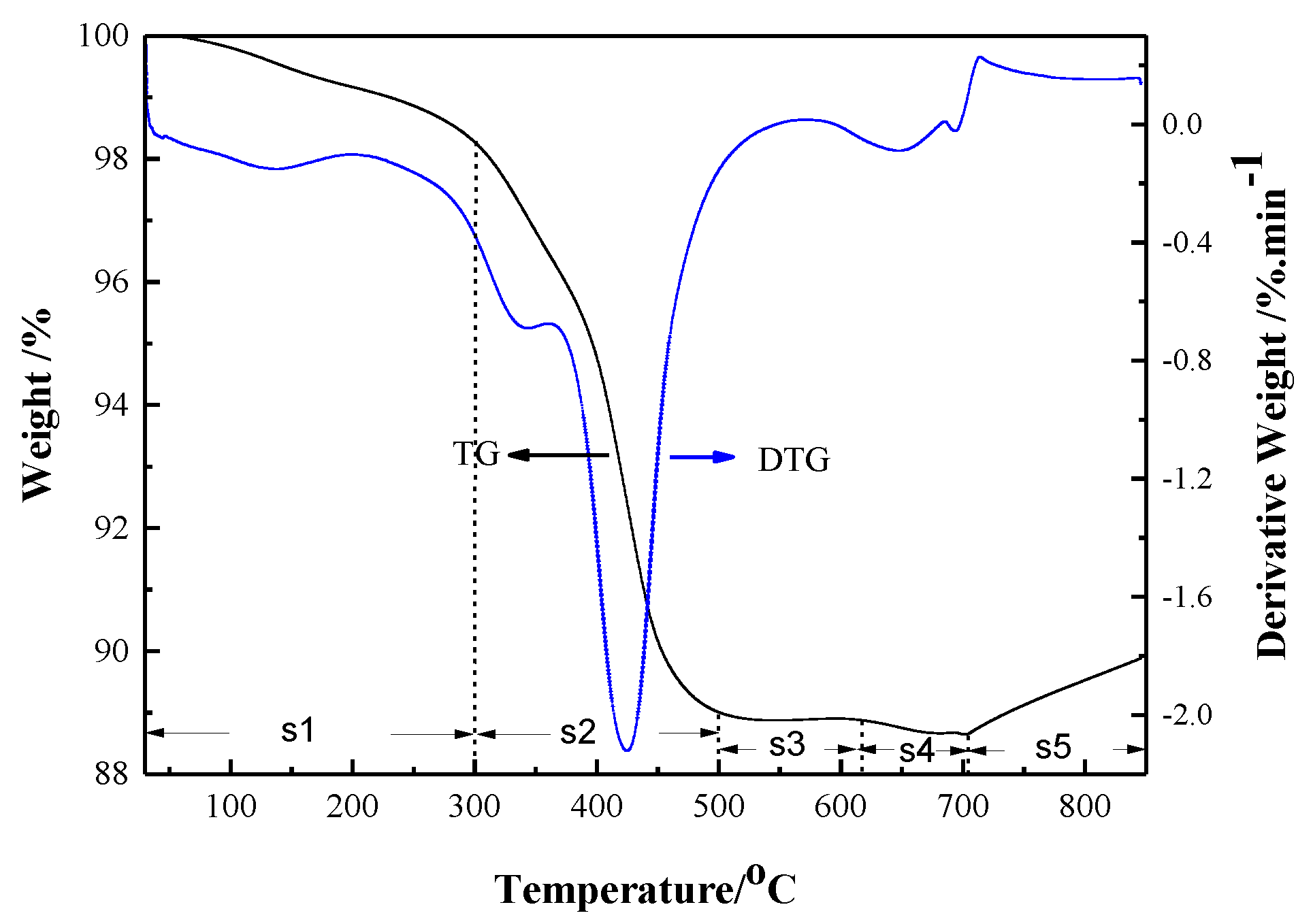
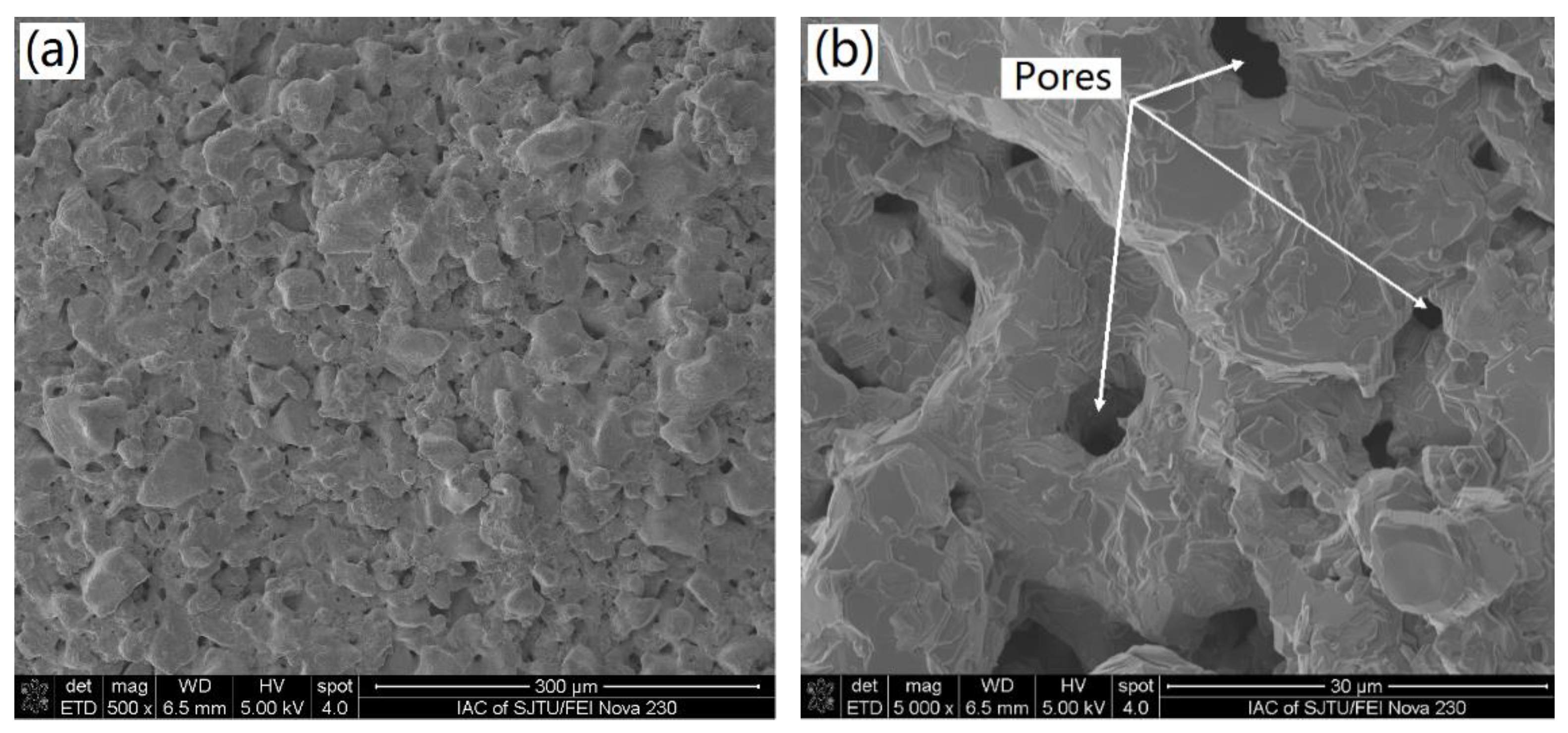
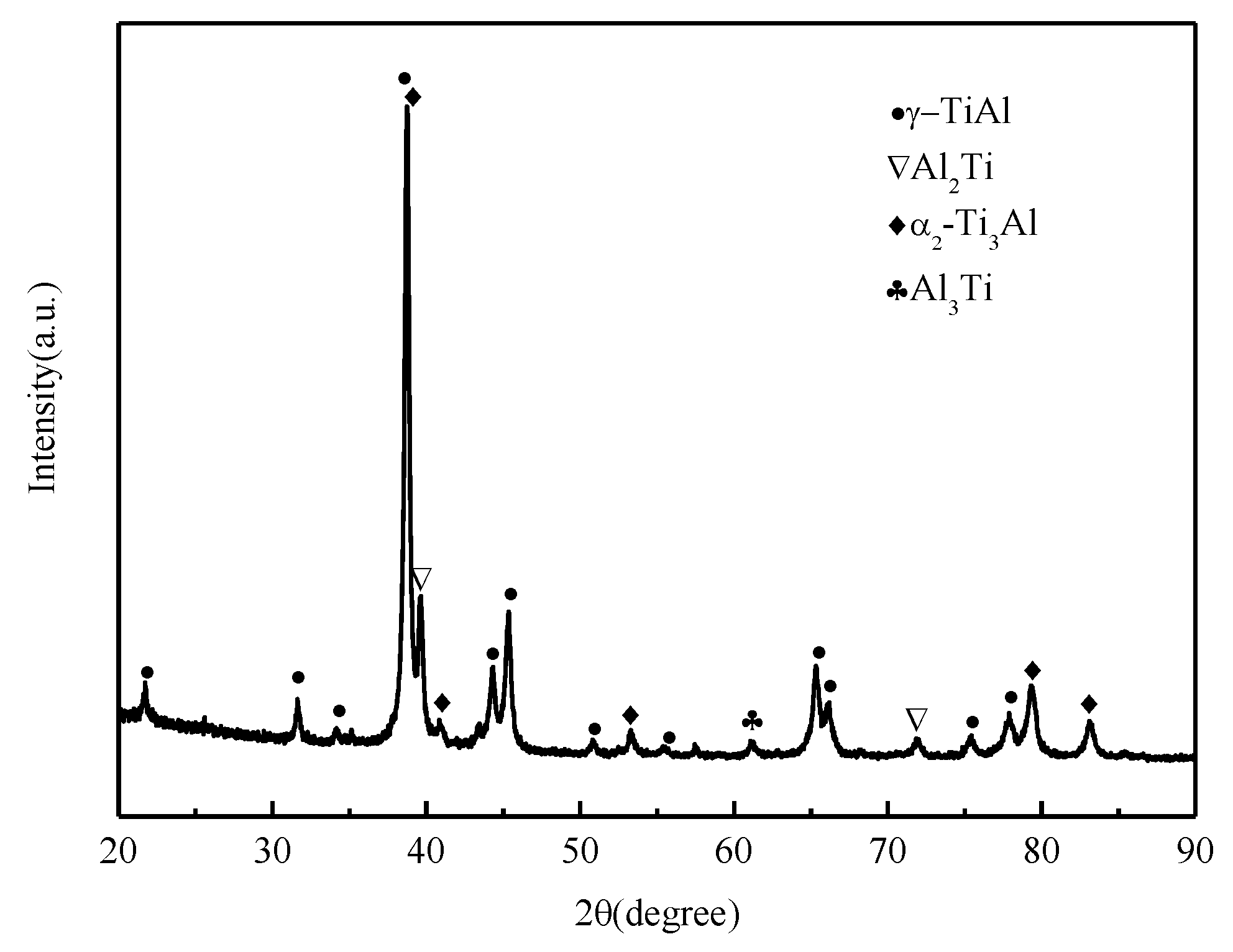
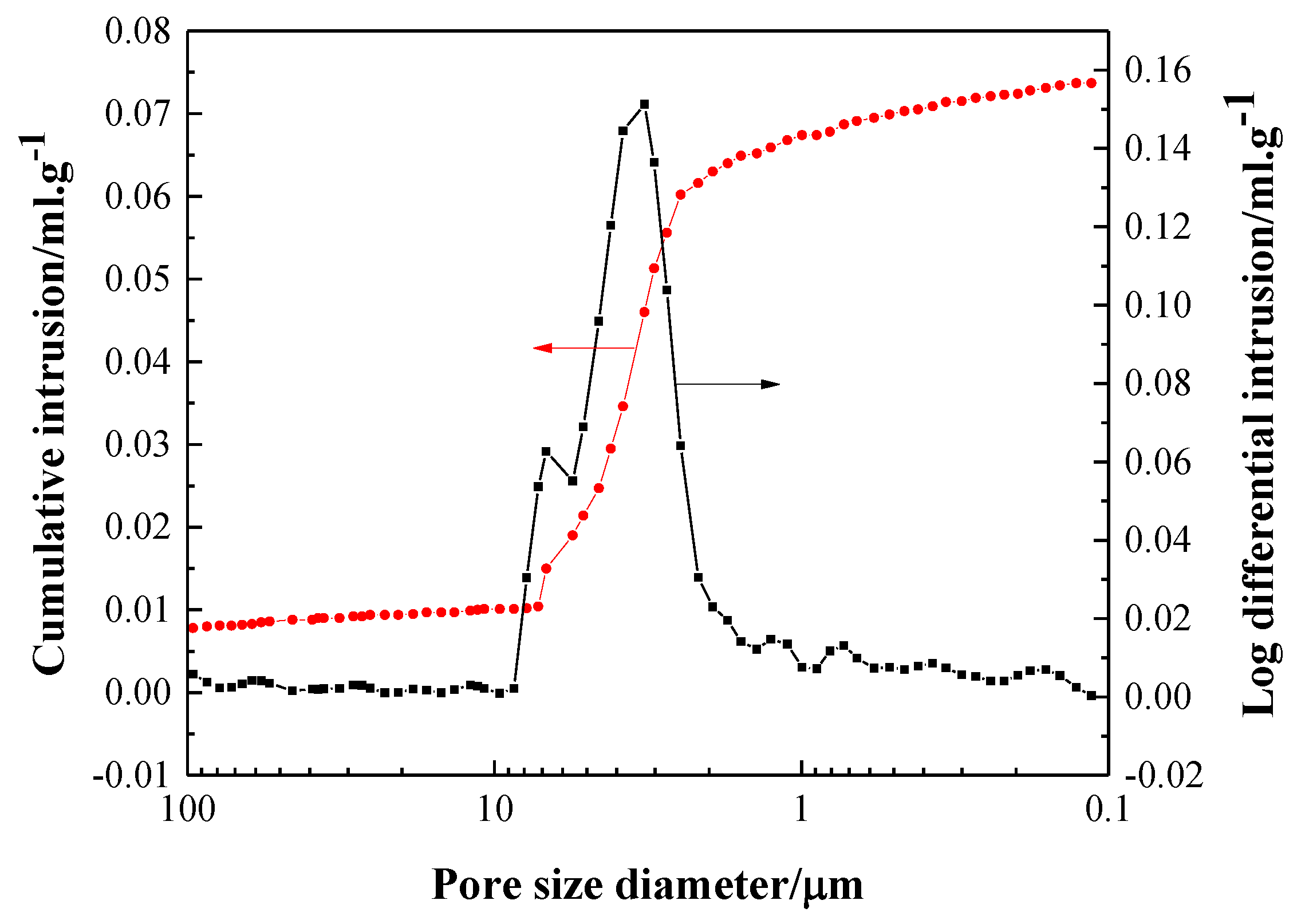
- The sintered TiAl alloy exhibited a typical fracture cleavage surface; meanwhile, its pore size distribution was 2 μm to 8 μm and its porosity was 23.78%, which indicates that the gel casting route is a suitable way to prepare porous TiAl alloy components. The result of the XRD analysis shows that γ-TiAl and α2-Ti3Al were the main phases and no contaminated carbide phase was formed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamaguchi, M.; Inui, H.; ITO, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 115–122. [Google Scholar] [CrossRef]
- Imayev, R.M.; Imayev, V.M.; Oehring, M.; Appel, F. Alloy design concepts for refined gamma titanium aluminide based alloys. Intermetallics 2007, 15, 451–460. [Google Scholar] [CrossRef]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium alloys for aerospace applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Coro, A.; Abasolo, M.; Aguirrebeitia, J.; Lo´pez de Lacalle, L.N. Inspection scheduling based on reliability updating of gas turbine welded structures. Adv. Mech. Eng. 2019, 11, 1–20. [Google Scholar] [CrossRef]
- Rak, Z.S.; Walter, J. Porous titanium foil by tape casting technique. J. Mater. Process. Technol. 2006, 175, 358–363. [Google Scholar] [CrossRef]
- He, Y.H.; Jiang, Y.; Xu, Y.N.P.; Zou, J.; Huang, B.Y.; Liu, C.T.; Peter, K.L. Fabrication of Ti-Al micro/nanometer-sized porous alloys through the Kirkendall effects. Adv. Mater. 2007, 19, 2102–2106. [Google Scholar] [CrossRef]
- Qin, P.; Yang, B.; Liu, L.B.; Song, C.J.; Friedrich, B. Porous TiAl alloys fabricated by sintering of TiH2 and Al powder mixtures. J. Alloys Compd. 2016, 656, 530–538. [Google Scholar]
- Hao, G.L.; Xu, Q.P.; Wang, H.; Li, X.Y. Effect of pore structure on mechanical properties of porous TiAl. Mater. Sci. Technol. 2016, 32, 1592–1596. [Google Scholar] [CrossRef]
- Lascano, S.; Arevalo, C.; Montealegre-Melendez, I.; Munoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous titanium for biomedical applications: Evaluation of the conventional powder metallurgy frontier and space-holder technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef]
- Jovanovic, M.T.; Dimcic, B.; Bobic, I.; Zec, S.; Maksimovic, V. Microstructure and mechanical properties of precision cast TiAl turbocharger wheel. J. Mater. Process. Technol. 2005, 167, 14–21. [Google Scholar] [CrossRef]
- Tetsui, T.; Shindo, K.; Kaji, S.; Kobayashi, S.; Takeyama, M. Fabrication of TiAl Components by means of hot forging and machining. Intermetallics 2005, 13, 971–978. [Google Scholar] [CrossRef]
- Clemens, H.; Kestler, H. Processing and applications of intermetallic γ-TiAl-based alloys. Adv. Eng. Mater. 2000, 9, 551–570. [Google Scholar] [CrossRef]
- Kothari, K.; Radhakrishnan, R.; Wereley, N.M. Advances in gamma titanium aluminides and their manufacturing techniques. Prog. Aerosp. Sci. 2012, 55, 1–16. [Google Scholar] [CrossRef]
- Young, A.C.; Omatete, O.O.; Janney, M.A.; Menchhofer, P.A. Gelcasting of alumina. J. Am. Ceram. Soc. 1991, 74, 612–618. [Google Scholar] [CrossRef]
- Omatete, O.O.; Janney, M.A.; Nunn, S.D. Gelcasting: From laboratory development towards industrial production. J. Eur. Ceram. Soc. 1997, 17, 407–413. [Google Scholar] [CrossRef]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Franks, G.V.; Tallon, C.; Studart, A.R.; Sesso, M.L.; Leo, S. Colloidal processing: Enabling complex shaped ceramics with unique multiscale structures. J. Am. Ceram. Soc. 2017, 100, 1–33. [Google Scholar] [CrossRef]
- Hernandez, N.; Sanchez-Herencia, A.J.; Moreno, R. Forming of nickel compacts by a colloidal filtration route. Acta Mater. 2005, 53, 919–925. [Google Scholar] [CrossRef]
- Lu, Z.L.; Cao, J.W.; Bai, S.Z.; Wang, M.Y.; Li, D.C. Microstructure and mechanical properties of TiAl-based composites prepared by stereolithography and gelcasting technologies. J. Alloys Compd. 2015, 633, 280–287. [Google Scholar] [CrossRef]
- Biasetto, L.; Moraes, E.G.; Colombo, P.; Bonollo, F. Ovalbumin as foaming agent for Ti6Al4V foams produced by gelcasting. J. Alloys Compd. 2016, 687, 839–844. [Google Scholar] [CrossRef]
- Lux, J.; Moraes, E.G.; Maire, E.; Adrien, J.; Biasetto, L. Gas permeability of Ti6Al4V foams prepared via gelcasting, experiments and modelling. Comp. Mater. Sci. 2018, 152, 363–373. [Google Scholar] [CrossRef]
- Azevedo, C.R.F.; Rodrigues, D.; Neto, F.B. Ti-Al-V powder metallurgy (PM) via the hydrogenation-dehydrogenation (HDH) process. J. Alloys Compd. 2003, 353, 217–227. [Google Scholar] [CrossRef]
- Erk, K.A.; Dunand, D.C.; Shull, K.R. Titanium with controllable pore fractions by thermoreversible gelcasting of TiH2. Acta Meter. 2008, 56, 5147–5157. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.M.; Hao, J.J.; Ren, S.B. Porosity and mechanical properties of porous titanium fabricated by gelcasting. Rare Met. 2008, 27, 282–286. [Google Scholar] [CrossRef]
- Ye, Q.; Guo, Z.M.; Bai, J.L.; Lu, B.X.; Lin, J.P.; Hao, J.J.; Luo, L.; Shao, H.P. Gelcasting of titanium hybride to fabricate low-cost titanium. Rare Met. 2015, 34, 351–356. [Google Scholar] [CrossRef]
- Studart, A.R.; Amstad, E.; Gauckler, L.J. Colloidal stabilization of nanoparticles in concentrated suspensions. Langmuir 2008, 23, 1081–1090. [Google Scholar] [CrossRef]
- Lin, T.R.; Lin, J.F. The elastohydrodynamic lubrication of line contacts with pseudoplastic fluids. Wear 1990, 140, 235–249. [Google Scholar]
- Jimenez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Klaus, M.; Wollgarten, M.; Zizak, I.; Schumacher, G.; Tovar, M.; Banhart, J. Decomposition of TiH2 studied in situ by synchrotron X-ray and neutron diffraction. Acta Mater. 2011, 59, 6318–6330. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhang, X.; Jiang, Y.; Yang, L.; Qi, C.; Sun, B. Study on the Fabrication of Porous TiAl Alloy via Non-Aqueous Gel Casting of a TiH2 and Al Powder Mixture. Appl. Sci. 2019, 9, 1569. https://doi.org/10.3390/app9081569
Li F, Zhang X, Jiang Y, Yang L, Qi C, Sun B. Study on the Fabrication of Porous TiAl Alloy via Non-Aqueous Gel Casting of a TiH2 and Al Powder Mixture. Applied Sciences. 2019; 9(8):1569. https://doi.org/10.3390/app9081569
Chicago/Turabian StyleLi, Fei, Xiao Zhang, Yi Jiang, Lixiang Yang, Chengkang Qi, and Baode Sun. 2019. "Study on the Fabrication of Porous TiAl Alloy via Non-Aqueous Gel Casting of a TiH2 and Al Powder Mixture" Applied Sciences 9, no. 8: 1569. https://doi.org/10.3390/app9081569
APA StyleLi, F., Zhang, X., Jiang, Y., Yang, L., Qi, C., & Sun, B. (2019). Study on the Fabrication of Porous TiAl Alloy via Non-Aqueous Gel Casting of a TiH2 and Al Powder Mixture. Applied Sciences, 9(8), 1569. https://doi.org/10.3390/app9081569





