Can Mathematical Models of Body Heat Exchanges Accurately Predict Thermal Stress in Premature Neonates?
Abstract
Featured Application
Abstract
1. Introduction
2. Why Are Mathematical Models of Value?
3. Limitations of Mathematical Models
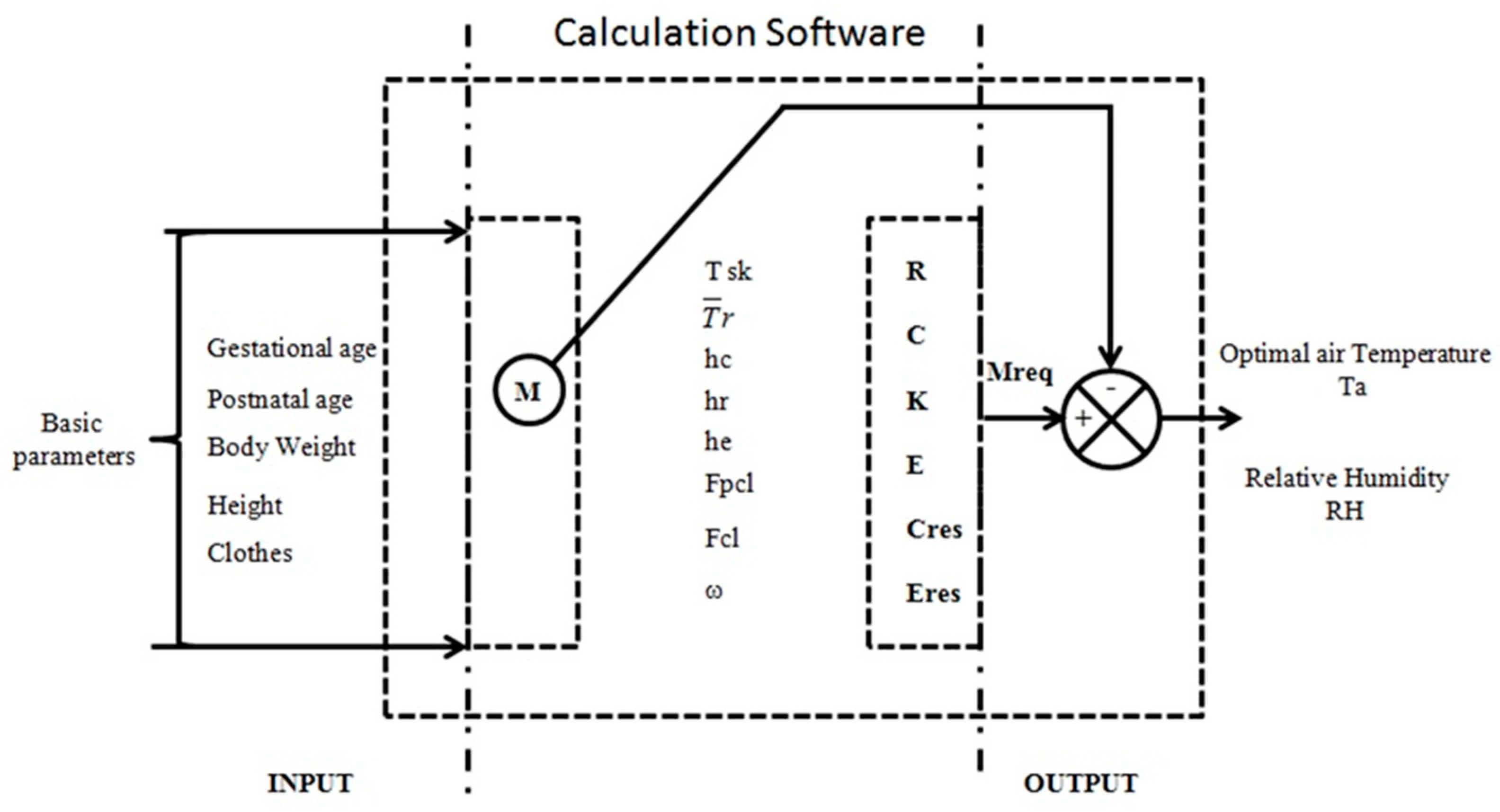
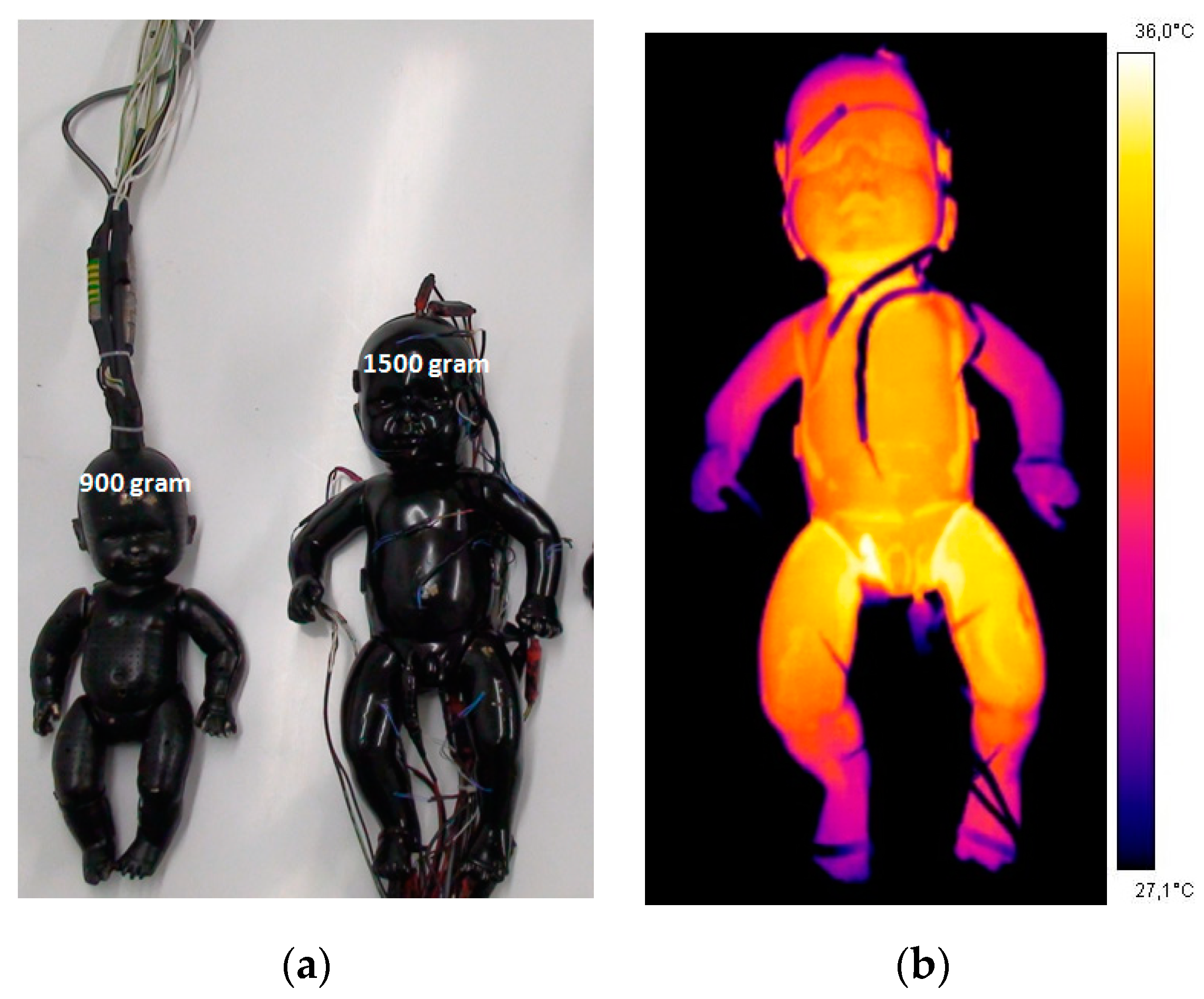
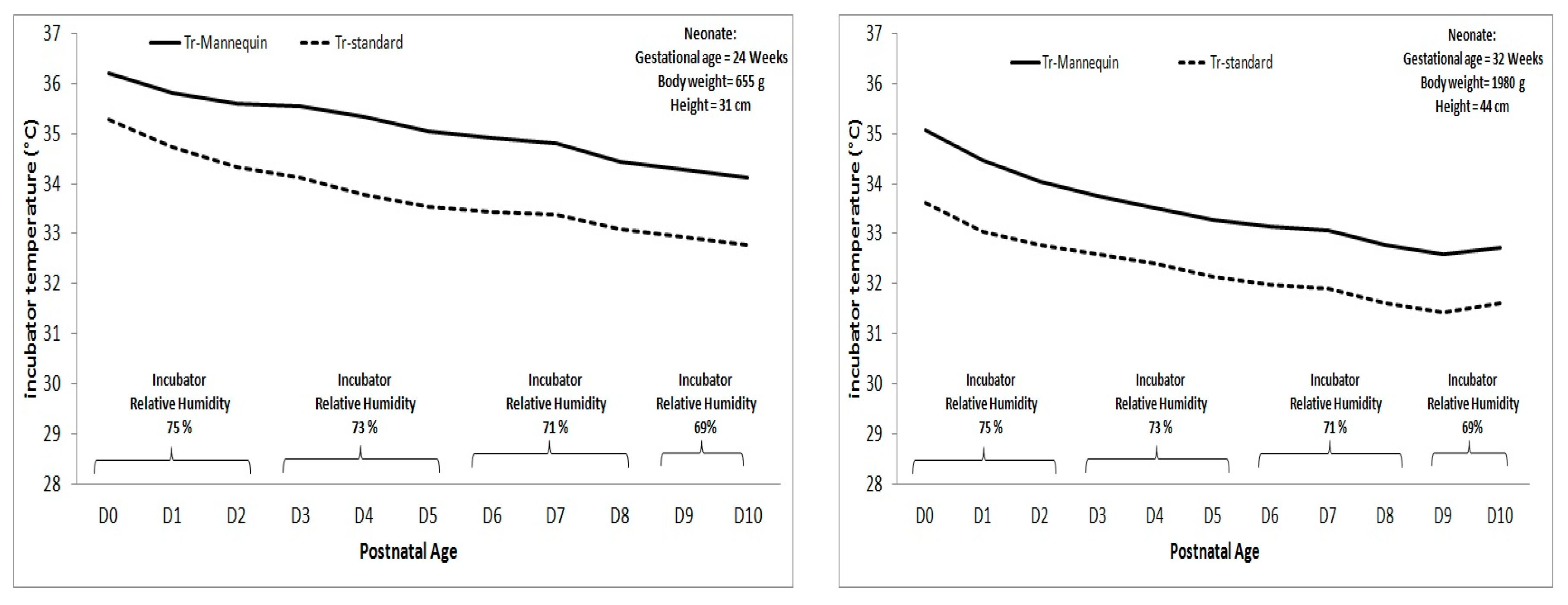
4. How to Solve (at Least in Part) These Problems
Applications and Research Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foster, K.G.; Hey, E.N.; Katz, G. The response of the sweat glands of the new-born baby to thermal stimuli and to intradermal acetylcholine. J. Physiol. 1969, 203, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kroth, J.; Weidlich, K.; Hiedl, S.; Nussbaum, C.; Christ, F.; Genzel-Boroviczény, O.; Genzel-Borovicz, O. Functional Vessel Density in the First Month of Life in Preterm Neonates. Pediatr. Res. 2008, 64, 567–571. [Google Scholar] [CrossRef]
- Knobel, R.B.; Holditch-Davis, D.; Schwartz, T.A.; Wimmer, J.E.; Wimmer, J.E., Jr. Extremely low birth weight preterm infants lack vasomotor response in relationship to cold body temperatures at birth. J. Perinatol. 2009, 29, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Department of Reproductive Health and Research. World Health Organisation Thermal Protection of the Newborn: A Practical Guide; Department of Reproductive Health and Research: Geneva, Switzerland, 1997. [Google Scholar]
- Laptook, A.R.; Salhab, W.; Bhaskar, B.; Neonatal Research Network. Admission Temperature of Low Birth Weight Infants: Predictors and Associated Morbidities. Pediatrics 2007, 119, e643–e649. [Google Scholar] [CrossRef]
- Bredemeyer, S.; Reid, S.; Wallace, M. Thermal management for premature births. J. Adv. Nurs. 2005, 52, 482–489. [Google Scholar] [CrossRef]
- Baumgart, S.; Engle, W.D.; Fox, W.W.; Polin, R.A. Effect of heat shielding on convective and evaporative heat losses and on radiant heat transfer in the premature infant. J. Pediatr. 1981, 99, 948–956. [Google Scholar] [CrossRef]
- Soll, R.F. Heat loss prevention in neonates. J. Perinatol. 2008, 28, S57–S59. [Google Scholar] [CrossRef]
- Sofer, S.; Phillip, M. Hemorrhagic Shock and Encephalopathy Syndrome. AJDC 1986, 140, 1252–1254. [Google Scholar] [CrossRef]
- Frank, J.; Brown, S. Thermometers and rectal perforations in the neonate. Arch. Dis. Child. 1978, 53, 824–825. [Google Scholar] [CrossRef]
- Lenzi, P.; Libert, J.; Franzini, C.; Cianci, T.; Guidalotti, P. Short-term thermoregulatory adjustments involving opposite regional temperature changes. J. Therm. Biol. 1986, 11, 151–156. [Google Scholar] [CrossRef]
- Cross, K.; Stratton, D. Aural temperature of the newborn infant. Lancet 1974, 304, 1179–1180. [Google Scholar] [CrossRef]
- Hammarlund, K.; Sedin, G.; Strömberg, B. Transepidermal Water Loss in Newborn Infants. Acta Paediatr. 1982, 71, 369–374. [Google Scholar] [CrossRef]
- Chessex, P.; Reichman, B.L.; Verellen, G.J.; Putet, G.; Smith, J.M.; Heim, T.; Swyer, P.R. Influence of postnatal age, energy intake, and weight gain on energy metabolism in the very low-birth-weight infant. J. Pediatr. 1981, 99, 761–766. [Google Scholar] [CrossRef]
- Adams, A.K.; Nelson, R.A.; Bell, E.F.; Egoavil, C.A. Use of infrared thermographic calorimetry to determine energy expenditure in preterm infants. Am. J. Clin. Nutr. 2000, 71, 969–977. [Google Scholar] [CrossRef]
- Museux, N.; Cardot, V.; Bach, V.; Delanaud, S.; Degrugilliers, L.; Agourram, B.; Elabbassi, E.B.; Libert, J.-P. A reproducible means of assessing the metabolic heat status of preterm neonates. Med. Phys. 2008, 35, 89–100. [Google Scholar] [CrossRef]
- Konecny, E.; Frankenberger, R.T.; Bussmann, O.; Nahm, W. Modell zur Simulation der Wärmeabgabe von Frühgeborenen. Simulation Model of Heat Loss in Preterm Infants. Biomed. Tech. Eng. 1998, 43, 137–143. [Google Scholar]
- Elabbassi, E.B.; Bach, V.; Makki, M.; Delanaud, S.; Telliez, F.; Léké, A.; Libert, J.-P. Assessment of dry heat exchanges in newborns: Influence of body position and clothing in SIDS. J. Appl. Physiol. 2001, 91, 51–56. [Google Scholar] [CrossRef]
- ISO 9920 (1995). Ergonomie des ambiances thermiques: Détermination de l’isolement vestimentaire et de la résistance à l’évaporation d’une tenue vestimentaire. AFNOR 2002, 313–372, ISBN (tome2):2-12-213562-X. [Google Scholar]
- Jardine, D.S. A mathematical model of life-threatening hyperthermia during infancy. J. Appl. Physiol. 1992, 73, 329–339. [Google Scholar] [CrossRef]
- Wheldon, A.E. Energy balance in the newborn baby: Use of a manikin to estimate radiant and convective heat loss. Phys. Med. Biol. 1982, 27, 285–296. [Google Scholar] [CrossRef]
- Ultman, J.S.; Berman, S.; Kirlin, P.; Vreslovic, J.M.; Baer, C.B.; Marks, K.H. Electrically heated simulator for relative evaluation of alternative infant incubator environments. Med. Instrum. 1988, 22, 33–38. [Google Scholar]
- Décima, P.; Stéphan-Blanchard, E.; Pelletier, A.; Ghyselen, L.; Delanaud, S.; Dégrugilliers, L.; Telliez, F.; Bach, V.; Libert, J.-P. Assessment of radiant temperature in a closed incubator. Eur. J. Appl. Physiol. 2012, 112, 2957–2968. [Google Scholar] [CrossRef]
- Sinclair, A. The measurement of radiant temperature in neonatal thermal environments. Biomed. Instrum. Technol. 1992, 26, 400–407. [Google Scholar]
- Sarman, I.; Bolin, D.; Holmér, I.; Tunell, R. Assessment of Thermal Conditions in Neonatal Care: Use of a Manikin of Premature Baby Size. Am. J. Perinatol. 1992, 9, 239–246. [Google Scholar] [CrossRef]
- Elabbassi, E.B.; Chardon, K.; Telliez, F.; Bach, V.; Libert, J.-P. Influence of head position on thermal stress in newborns: Simulation using a thermal mannequin. J. Appl. Physiol. 2002, 93, 1275–1279. [Google Scholar] [CrossRef][Green Version]
- Belghazi, K.; Tourneux, P.; Elabbassi, E.B.; Ghyselen, L.; Delanaud, S.; Libert, J.-P. Effect of posture on the thermal efficiency of a plastic bag wrapping in neonate: Assessment using a thermal “sweating” mannequin. Med. Phys. 2006, 33, 637–644. [Google Scholar] [CrossRef]
- Wheldon, A.E.; Hull, D. Incubation of very immature infants. Arch. Dis. Child. 1983, 58, 504–508. [Google Scholar] [CrossRef]
- Helder, O.K.; Mulder, P.G.H.; Van Goudoever, J.B. Computer-Generated Versus Nurse-Determined Strategy for Incubator Humidity and Time to Regain Birthweight. J. Obstet. Gynecol. Neonatal Nurs. 2008, 37, 255–261. [Google Scholar] [CrossRef]
- Lyon, A.; Oxley, C. HeatBalance, a computer program to determine optimum incubator air temperature and humidity. A comparison against nurse settings for infants less than 29 weeks gestation. Early Hum. Dev. 2001, 62, 33–41. [Google Scholar] [CrossRef]
- Décima, P.; Dégrugilliers, L.; Delanaud, S.; Stéphan-Blanchard, E.; Vanhée, J.-L.; Libert, J.-P. Conception d’un logiciel de calcul de la thermoneutralité dans les incubateurs fermés pour nouveau-nés prématurés (projet Pretherm®). IRBM 2012, 33, 48–54. [Google Scholar] [CrossRef]
- Delanaud, S.; Decima, P.; Pelletier, A.; Libert, J.-P.; Durand, E.; Stephan-Blanchard, E.; Bach, V.; Tourneux, P. Thermal management in closed incubators: New software for assessing the impact of humidity on the optimal incubator air temperature. Med. Eng. Phys. 2017, 46, 89–95. [Google Scholar] [CrossRef]
- Bolton, D.P.; Nelson, E.A.; Taylor, B.J.; Weatherall, I.L. Thermal balance in infants. J. Appl. Physiol. 1996, 80, 2234–2242. [Google Scholar] [CrossRef]
- Agourram, B.; Bach, V.; Tourneux, P.; Krim, G.; Delanaud, S.; Libert, J.-P. Why wrapping premature neonates to prevent hypothermia can predispose to overheating. J. Appl. Physiol. 2010, 108, 1674–1681. [Google Scholar] [CrossRef]
- Vohra, S.; Roberts, R.S.; Zhang, B.; Janes, M.; Schmidt, B. Heat Loss Prevention (HeLP) in the delivery room: A randomized controlled trial of polyethylene occlusive skin wrapping in very preterm infants. J. Pediatr. 2004, 145, 750–753. [Google Scholar] [CrossRef]
- Berardi, A.; Lugli, L.; Baronciani, D.; Creti, R.; Rossi, K.; Ciccia, M.; Gambini, L.; Mariani, S.; Papa, I.; Serra, L.; et al. Group B Streptococcal Infections in a Northern Region of Italy. PEDIATRICS 2007, 120, e487–e493. [Google Scholar] [CrossRef]
- Mank, A.; van Zanten, H.A.; Meyer, M.P.; Pauws, S.; Lopriore, E.; Te Pas, A.B. Hypothermia in Preterm Infants in the First Hours after Birth: Occurrence, Course and Risk Factors. PLoS ONE 2016, 11, e0164817. [Google Scholar] [CrossRef]
- Elabbassi, E.B.; Belghazi, K.; Libert, J.-P. Dry heat loss in incubator: Comparison of two premature newborn sized manikins. Eur. J. Appl. Physiol. 2004, 92, 679–682. [Google Scholar] [CrossRef]
- De Dear, R.J.; Arens, E.; Hui, Z.; Oguro, M.C. Convective and radiative heat transfer coefficients for individual human body segments. Int. J. Biometeorol. 1997, 40, 141–156. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delanaud, S.; Chahin Yassin, F.; Durand, E.; Tourneux, P.; Libert, J.-P. Can Mathematical Models of Body Heat Exchanges Accurately Predict Thermal Stress in Premature Neonates? Appl. Sci. 2019, 9, 1541. https://doi.org/10.3390/app9081541
Delanaud S, Chahin Yassin F, Durand E, Tourneux P, Libert J-P. Can Mathematical Models of Body Heat Exchanges Accurately Predict Thermal Stress in Premature Neonates? Applied Sciences. 2019; 9(8):1541. https://doi.org/10.3390/app9081541
Chicago/Turabian StyleDelanaud, Stéphane, Fatima Chahin Yassin, Estelle Durand, Pierre Tourneux, and Jean-Pierre Libert. 2019. "Can Mathematical Models of Body Heat Exchanges Accurately Predict Thermal Stress in Premature Neonates?" Applied Sciences 9, no. 8: 1541. https://doi.org/10.3390/app9081541
APA StyleDelanaud, S., Chahin Yassin, F., Durand, E., Tourneux, P., & Libert, J.-P. (2019). Can Mathematical Models of Body Heat Exchanges Accurately Predict Thermal Stress in Premature Neonates? Applied Sciences, 9(8), 1541. https://doi.org/10.3390/app9081541




