Technologies for the Production of Fertilizable Mammalian Oocytes
Abstract
1. Introduction
1.1. Infertility: What Is It?
1.2. Main Causes of Infertility in the Female
1.2.1. Disease-Related Infertility
1.2.2. Cancer and Anticancer Treatments
1.2.3. Age-Related Infertility
1.2.4. Environment-Related Infertility
1.2.5. Lifestyle-Related Infertility
1.3. Strategies for Contrasting Infertility in the Female
2. The Production of Fertilizable Oocytes
2.1. Folliculogenesis
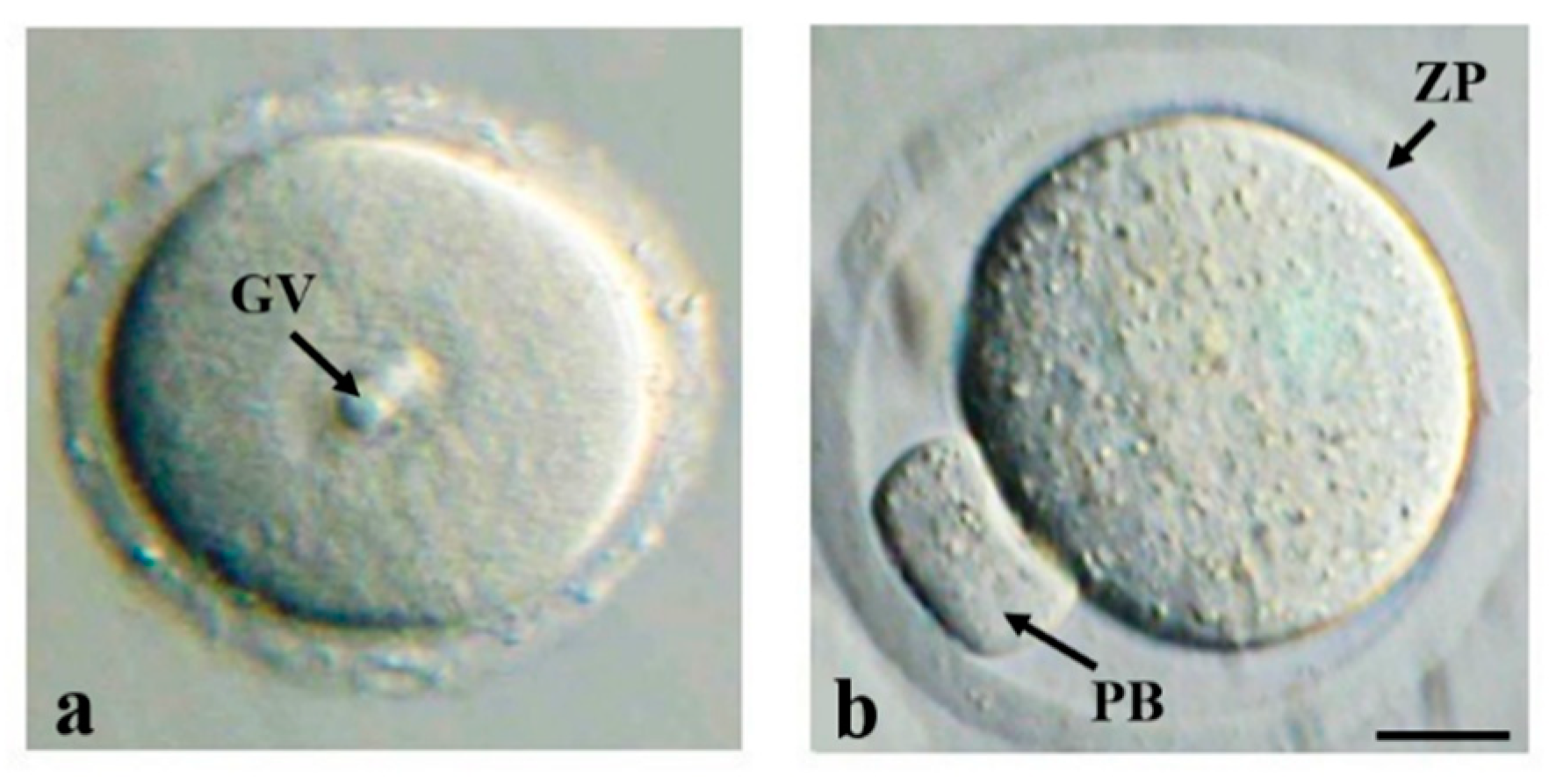
2.2. Oocyte Maturation
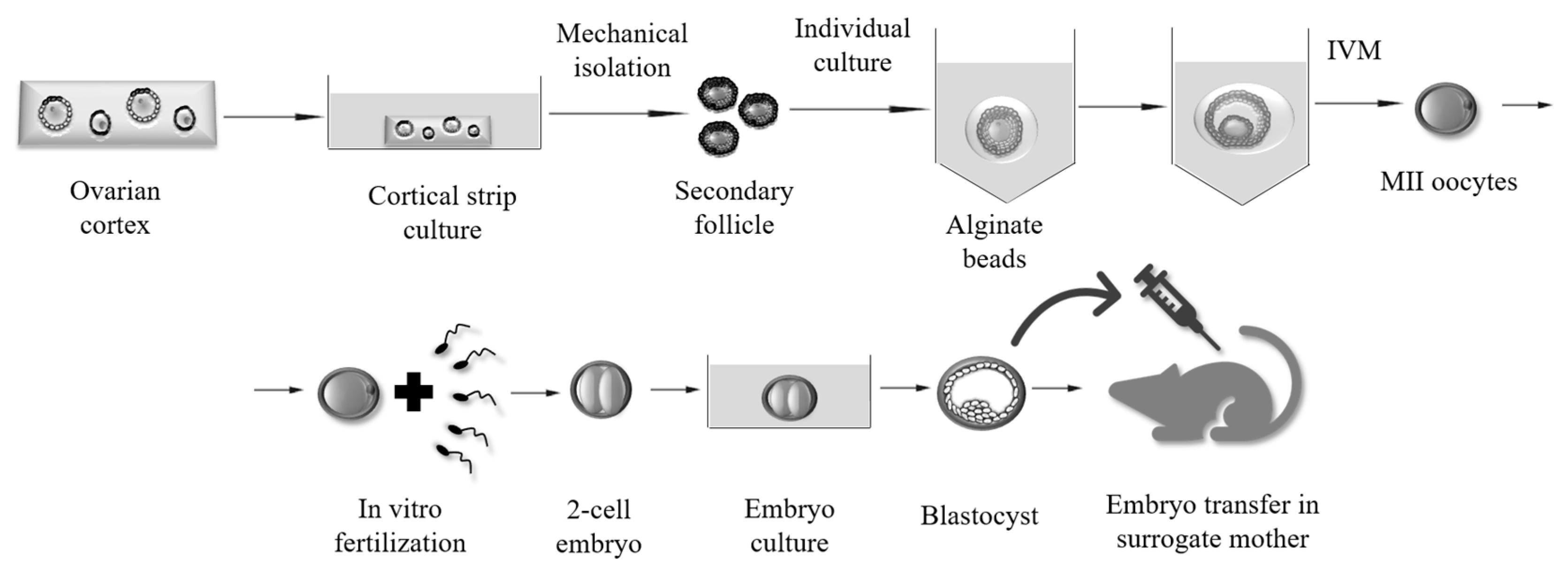
3. Methods for Obtaining Fertilizable Oocytes from In Vitro Grown (IVG) Mammalian Follicles
2D and 3D Culture Systems
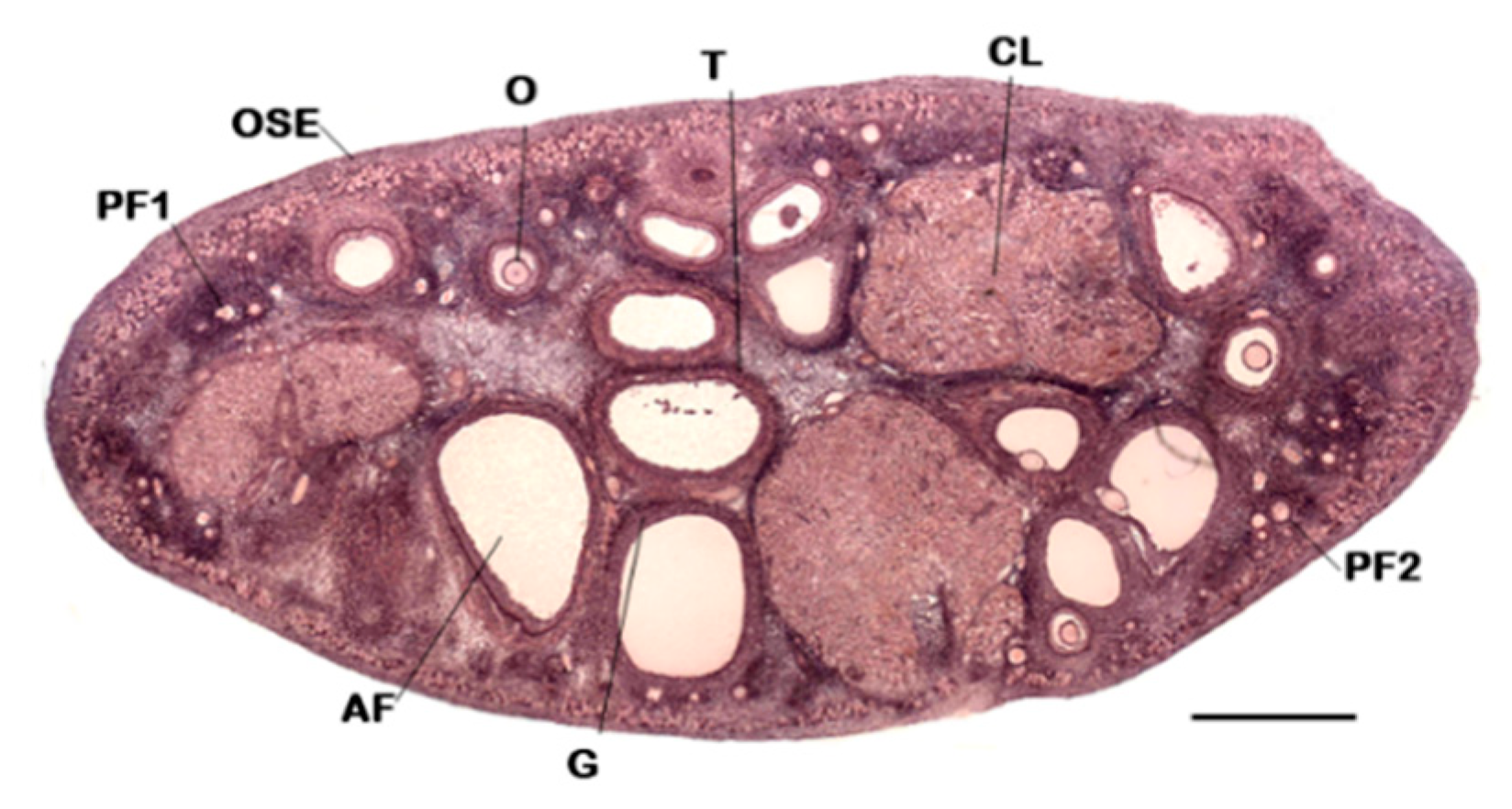
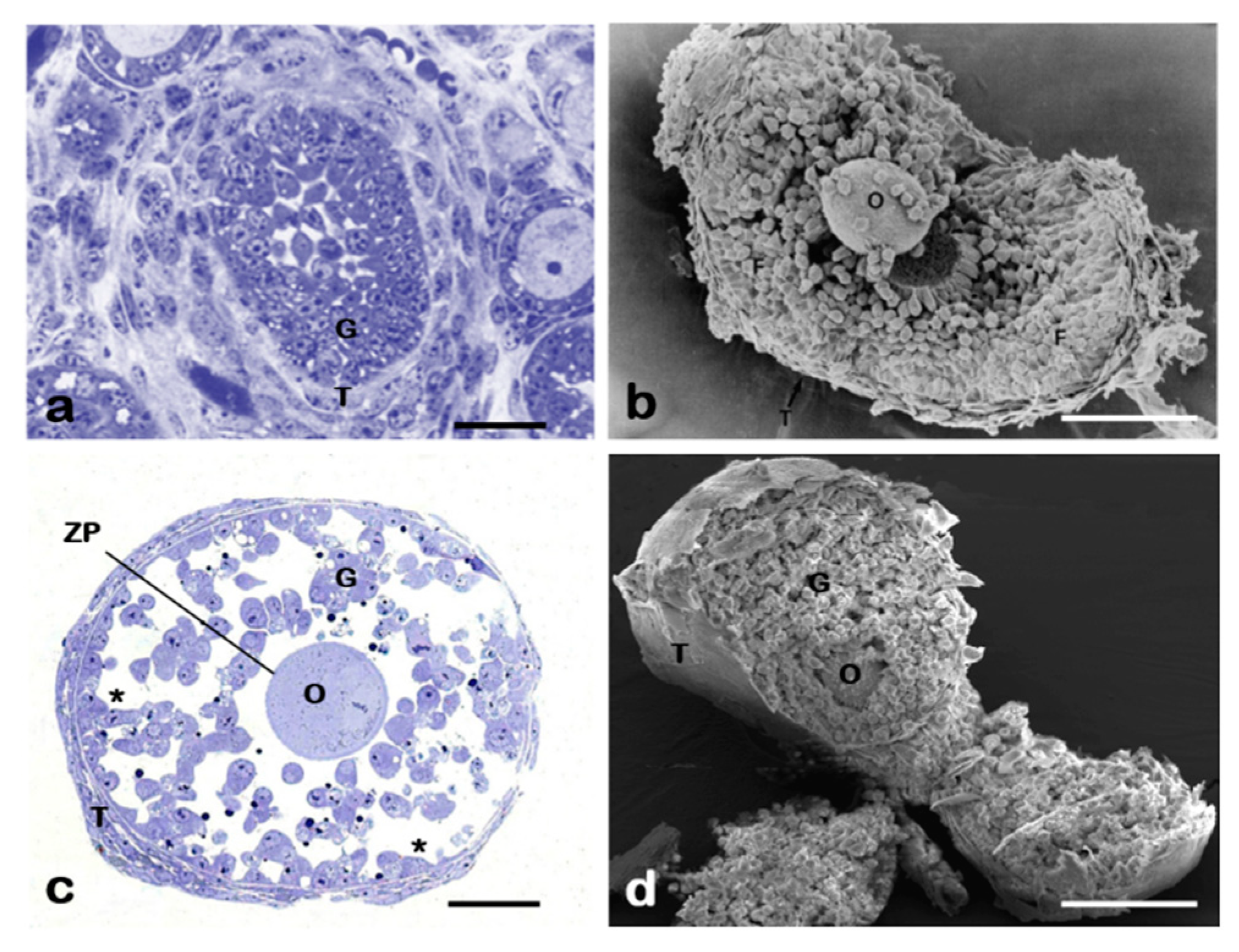
4. Focus on the Ultrastructural of 2D Ovarian Follicles
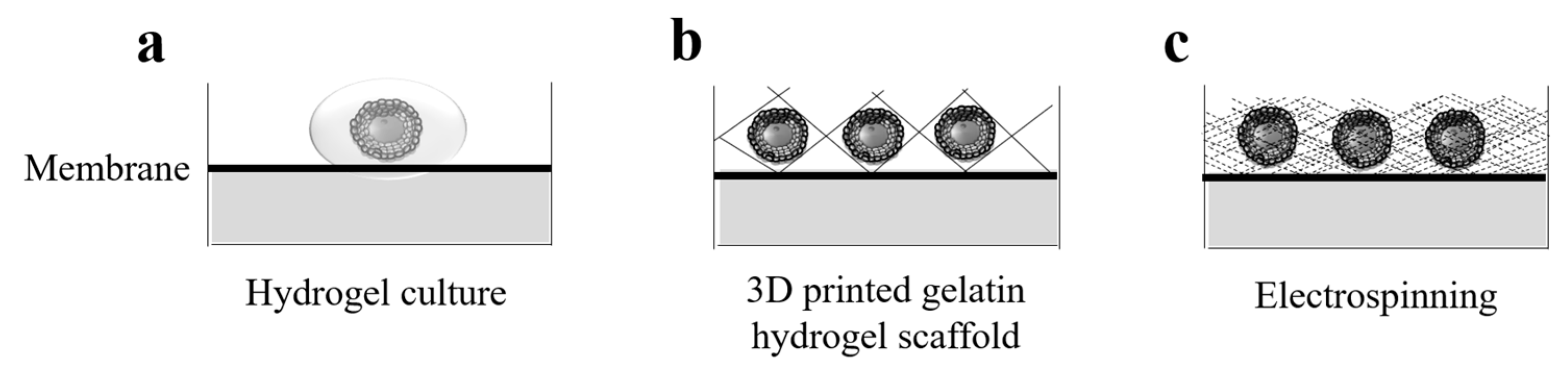
5. Towards a Biosynthetic Mammalian Ovary
5.1. 3D Bioprinting
5.2. The New Ovaries
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Weiss, R.V.; Clapauch, R. Female infertility of endocrine origin. Arq. Bras. Endocrinol. Metabol. 2014, 58, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Kubba, A.; Aguilar, A.; Mueck, A.O. Use of cyproterone acetate/ethinylestradiol in polycystic ovary syndrome: Rationale and practical aspects. Eur. J. Contracept. Reprod. Health Care 2017, 22, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar]
- Tomassetti, C.; D’Hooghe, T. Endometriosis and infertility: Insights into the causal link and management strategies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Shehata, F.; Moria, A.; Holzer, H.; Son, W.Y.; Tulandi, T. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil. Steril. 2011, 96, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Mirallié, S.; Leperlier, F.; Reignier, A.; Barrière, P.; Fréour, T. Ovarian reserve and response to stimulation in women undergoing fertility preservation according to malignancy type. Reprod. BioMed. Online 2018, 37, 201–207. [Google Scholar] [CrossRef]
- Salama, M.; Woodruff, T.K. Anticancer treatments and female fertility: Clinical concerns and role of oncologists in oncofertility practice. Expert Rev. Anticancer Ther. 2017, 17, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Kelvin, J.F.; Quinn, G.P.; Gracia, C.R. Infertility in reproductive-age female cancer survivors. Cancer 2015, 121, 1532–1539. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Sanfilippo, J.S.; Valli-Pulaski, H. Female fertility preservation in the pediatric and adolescent cancer patient population. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 147–157. [Google Scholar] [CrossRef]
- Bianchi, S.; Macchiarelli, G.; Micara, G.; Linari, A.; Boninsegna, C.; Aragona, C.; Rossi, G.; Cecconi, S.; Nottola, S.A. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: A comparison between reproductive and in vitro aging. J. Assist. Reprod. Genet. 2015, 32, 1343–1358. [Google Scholar] [CrossRef]
- Lockwood, G.M. Social egg freezing: The prospect of reproductive ‘immortality’ or a dangerous delusion? Reprod. Biomed. Online 2011, 23, 334–340. [Google Scholar] [CrossRef]
- Fritz, R.; Jindal, S. Reproductive aging and elective fertility preservation. J. Ovarian Res. 2018, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.; Mascia, M.; Cioffi, G.; De Angelis, C.; Coppola, G.; De Rosa, P.; Pivonello, R.; Alviggi, C.; De Placido, G. Air pollution and female fertility: A systematic review of literature. Reprod. Biol. Endocrinol. 2018, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Rashtian, J.; Chavkin, D.E.; Merhi, Z. Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod. Biol. Endocrinol. 2019, 17, 5. [Google Scholar] [CrossRef]
- Rossi, G.; Buccione, R.; Baldassarre, M.; Macchiarelli, G.; Palmerini, M.G.; Cecconi, S. Mancozeb exposure in vivo impairs mouse oocyte fertilizability. Reprod. Toxicol. 2006, 21, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Palmerini, M.G.; Macchiarelli, G.; Buccione, R.; Cecconi, S. Mancozeb adversely affects meiotic spindle organization and fertilization in mouse oocytes. Reprod. Toxicol. 2006, 22, 51–55. [Google Scholar] [CrossRef]
- Cecconi, S.; Paro, R.; Rossi, G.; Macchiarelli, G. The effects of the endocrine disruptors dithiocarbamates on the mammalian ovary with particular regard to mancozeb. Curr. Pharm. Des. 2007, 13, 2989–3004. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, M.G.; Zhurabekova, G.; Balmagambetova, A.; Nottola, S.A.; Miglietta, S.; Belli, M.; Bianchi, S.; Cecconi, S.; Di Nisio, V.; Familiari, G.; et al. The pesticide Lindane induces dose-dependent damage to granulosa cells in an in vitro culture. Reprod. Biol. 2017, 17, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, M.G.; Belli, M.; Nottola, S.A.; Miglietta, S.; Bianchi, S.; Bernardi, S.; Antonouli, S.; Cecconi, S.; Familiari, G.; Macchiarelli, G. Mancozeb impairs the ultrastructure of mouse granulosa cells in a dose-dependent manner. J. Reprod. Dev. 2018, 64, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S. Do some addictions interfere with fertility? Fertil. Steril. 2015, 103, 22–26. [Google Scholar] [CrossRef]
- Medrano, J.V.; Andrés, M.D.M.; García, S.; Herraiz, S.; Vilanova-Pérez, T.; Goossens, E.; Pellicer, A. Basic and Clinical Approaches for Fertility Preservation and Restoration in Cancer Patients. Trends Biotechnol. 2018, 36, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Madrid, B.; Camboni, A.; Dolmans, M.M.; Nottola, S.; Van Langendonckt, A.; Donnez, J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil. Steril. 2007, 87, 1153–1165. [Google Scholar] [CrossRef]
- Camboni, A.; Martinez-Madrid, B.; Dolmans, M.M.; Amorim, C.A.; Nottola, S.A.; Donnez, J.; Van Langendonckt, A. Preservation of fertility in young cancer patients: Contribution of transmission electron microscopy. Reprod. BioMed. Online 2008, 17, 136–150. [Google Scholar] [CrossRef]
- Camboni, A.; Martinez-Madrid, B.; Dolmans, M.M.; Nottola, S.; Van Langendonckt, A.; Donnez, J. Autotransplantation of frozen-thawed ovarian tissue in a young woman: Ultrastructure and viability of grafted tissue. Fertil. Steril. 2008, 90, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Nottola, S.A.; Camboni, A.; Van Langendonckt, A.; Demylle, D.; Macchiarelli, G.; Dolmans, M.M.; Martinez-Madrid, B.; Correr, S.; Donnez, J. Cryopreservation and xenotransplantation of human ovarian tissue: An ultrastructural study. Fertil. Steril. 2008, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nottola, S.A.; Cecconi, S.; Bianchi, S.; Motta, C.; Rossi, G.; Continenza, M.A.; Macchiarelli, G. Ultrastructure of isolated mouse ovarian follicles cultured in vitro. Reprod. Biol. Endocrinol. 2011, 9, 3. [Google Scholar] [CrossRef]
- Khalili, M.A.; Shahedi, A.; Ashourzadeh, S.; Nottola, S.A.; Macchiarelli, G.; Palmerini, M.G. Vitrification of human immature oocytes before and after in vitro maturation: A review. J. Assist. Reprod. Genet. 2017, 34, 1413–1426. [Google Scholar] [CrossRef]
- Fisch, B.; Abir, R. Female fertility preservation: Past, present and future. Reproduction 2018, 156, F11–F27. [Google Scholar] [CrossRef]
- Luconi, M.; Torcia, S.; Grillo, D.; Fiorenza, M.T.; Forti, G.; Mangia, F.; Baldi, E. Enhancement of mouse sperm motility by the PI3-kinase inhibitor LY294002 does not result in toxic effects on preimplantation embryo development. Hum. Reprod. 2005, 20, 3500–3504. [Google Scholar] [CrossRef]
- Tardif, S.; Madamidola, O.A.; Brown, S.G.; Frame, L.; Lefièvre, L.; Wyatt, P.G.; Barratt, C.L.; Martins Da Silva, S.J. Clinically relevant enhancement of human sperm motility using compounds with reported phosphodiesterase inhibitor activity. Hum. Reprod. 2014, 29, 2123–2135. [Google Scholar] [CrossRef]
- De Rooij, D.G. The nature and dynamics of spermatogonial stem cells. Development 2017, 144, 3022–3030. [Google Scholar] [CrossRef]
- Ginsburg, M.; Snow, M.H.A.M. Primordial germ cells in the mouse embryo during gastrulation. Development 1990, 110, 521–528. [Google Scholar]
- Gondos, B. Comparative studies of normal and neoplastic ovarian germ cells: 1. Ultrastructure of oogonia and intercellular bridges in the fetal ovary. Int. J. Gynecol. Pathol. 1987, 6, 114–123. [Google Scholar] [CrossRef]
- Hunt, P.A.; Hassold, T.J. Human female meiosis: What makes a good egg go bad? Trends Genet. 2008, 24, 86–93. [Google Scholar] [CrossRef]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145. [Google Scholar] [CrossRef]
- Bhartiya, D.; Parte, S.; Patel, H.; Sriraman, K.; Zaveri, K.; Hinduja, I. Novel action of FSH on stem cells in adult mammalian ovary induces postnatal oogenesis and primordial follicle assembly. Stem Cells Int. 2016, 2016, 5096596. [Google Scholar] [CrossRef]
- Porras-Gómez, T.J.; Moreno-Mendoza, N. Neo-oogenesis in mammals. Zygote 2017, 25, 404–422. [Google Scholar] [CrossRef]
- Wandji, S.A.; Srsen, V.; Voss, A.K.; Eppig, J.J.; Fortune, J.E. Initiation in vitro of growth of bovine primordial follicles. Biol. Reprod. 1996, 55, 942–948. [Google Scholar] [CrossRef]
- Macchiarelli, G.; Vizza, E.; Nottola, S.A.; Familiari, G.; Motta, P.M. Cellular and microvascular changes of the ovarian follicle during folliculogenesis: A scanning electron microscopic study. Arch. Histol. Cytol. 1992, 55, 191–204. [Google Scholar] [CrossRef]
- Eppig, J.J.; O’Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996, 54, 197–207. [Google Scholar] [CrossRef]
- Sánchez, F.; Smitz, J. Molecular control of oogenesis. Biochim. Biophys. Acta 2012, 1822, 1896–1912. [Google Scholar] [CrossRef]
- Fair, T. Mammalian oocyte development: Checkpoints for competence. Reprod. Fertil. Dev. 2009, 22, 13–20. [Google Scholar] [CrossRef]
- Richards, J.S.; Jahnsen, T.; Hedin, L.; Lifka, J.; Ratoosh, S.; Durica, J.M.; Goldring, N.B. Ovarian follicular development: From physiology to molecular biology. Recent Prog. Horm. Res. 1987, 43, 231–276. [Google Scholar]
- Bao, B.; Garverick, H.A. Expression of steroidogenic enzyme and gonadotropin receptor genes in bovine follicles during ovarian follicular waves: A review. J. Anim. Sci. 1998, 76, 1903–1921. [Google Scholar] [CrossRef]
- Fortune, J.; Eppig, J. Effects of gonadotropins on steroid secretion by infantile and juvenile mouse ovaries in vitro. Endocrinology 1979, 105, 760–768. [Google Scholar] [CrossRef]
- Oktay, K.; Briggs, D.; Gosden, R.G. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J. Clin. Endocrinol. Metab. 1997, 82, 3748–3751. [Google Scholar] [CrossRef]
- Roy, S.K.; Albee, L. Requirement for follicle-stimulating hormone action in the formation of primordial follicles during perinatal ovarian development in the hamster. Endocrinology 2000, 141, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.M.; Wang, Y.; Jimenez, M.; Marshan, B.; Spaliviero, J.; Illingworth, P.; Handelsman, D.J. Follicle-stimulating hormone increases primordial follicle reserve in mature female hypogonadal mice. J. Endocrinol. 2006, 188, 549–557. [Google Scholar] [CrossRef][Green Version]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- Cecconi, S.; Ciccarelli, C.; Barberi, M.; Macchiarelli, G.; Canipari, R. Granulosa cell-oocyte interactions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, M.; Merico, V.; Cecconi, S.; Redi, C.A.; Garagna, S. What does it take to make a evelopmentally competent mammalian egg? Hum. Reprod. Update 2011, 17, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.; Dahl, K.D.; Vaughan, J.; Tucker, E.; Rivier, J.; Bardin, C.W.; Vale, W. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc. Natl. Acad. Sci. USA 1987, 84, 5082–5086. [Google Scholar] [CrossRef]
- Alak, B.M.; Smith, G.D.; Woodruff, T.K.; Stouffer, R.L.; Wolf, D.P. Enhancement of primate oocyte maturation and fertilization in vitro by inhibin A and activin A. Fertil. Steril. 1996, 66, 646–653. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Galactose at the nonreducing terminus of O-linked ligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein’s sperm receptor activity. Proc. Natl. Acad. Sci. USA 1988, 85, 6778–6782. [Google Scholar] [CrossRef]
- Sinowatz, F.; Kölle, S.; Töpfer-Petersen, E. Biosynthesis and expression of zona pellucida glycoproteins in mammals. Cells Tissues Organs 2001, 168, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Van Santbrink, E.J.; Hop, W.C.; van Dessel, T.J.; de Jong, F.H.; Fauser, B.C. Decremental follicle-stimulating hormone and dominant follicle development during the normal menstrual cycle. Fertil. Steril. 1995, 64, 37–43. [Google Scholar] [CrossRef]
- Tesfaye, D.; Gebremedhn, S.; Salilew-Wondim, D.; Hailay, T.; Hoelker, M.; Grosse-Brinkhaus, C.; Schellander, K. MicroRNAs: Tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction 2018, 155, R121–R135. [Google Scholar] [CrossRef]
- Liu, W.; Xin, Q.; Wang, X.; Wang, S.; Wang, H.; Zhang, W.; Yang, Y.; Zhang, Y.; Zhang, Z.; Wang, C.; et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 2017, 8, e2662. [Google Scholar] [CrossRef]
- Richards, J.S.; Ascoli, M. Endocrine, Paracrine, and Autocrine Signaling Pathways That Regulate Ovulation. Trends Endocrinol. Metab. 2018, 29, 313–325. [Google Scholar] [CrossRef]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef]
- Eppig, J.J.; Schroeder, A.C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol. Reprod. 1989, 41, 268–276. [Google Scholar] [CrossRef]
- Guzel, Y.; Oktem, O. Understanding follicle growth in vitro: Are we getting closer to obtaining mature oocytes from in vitro-grown follicles in human? Mol. Reprod. Dev. 2017, 84, 544–559. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 2003, 68, 1682–1686. [Google Scholar] [CrossRef]
- Hasegawa, A.; Mochida, N.; Ogasawara, T.; Koyama, K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil. Steril. 2006, 86, 1182–1192. [Google Scholar] [CrossRef]
- Mochida, N.; Akatani-Hasegawa, A.; Saka, K.; Ogino, M.; Hosoda, Y.; Wada, R.; Sawai, H.; Shibahara, H. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 2013, 146, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, E.J.; Kong, H.S.; Youm, H.W.; Kim, S.K.; Lee, J.R.; Suh, C.S.; Kim, S.H. Comparison of the oocyte quality derived from two-dimensional follicle culture methods and developmental competence of in vitro grown and matured oocytes. BioMed Res. Int. 2018, 7907092. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S. Growth and differentiation of small ovarian follicles in mammals: Problems and future perspectives. J. Reprod. Dev. 2002, 48, 431–435. [Google Scholar] [CrossRef]
- Cecconi, S.; D’Aurizio, R.; Colonna, R. Role of antral follicle development and cumulus cells on in vitro fertilization of mouse oocytes. J. Reprod. Fertil. 1996, 107, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Barboni, B.; Coccia, M.; Mattioli, M. In vitro development of sheep preantral follicles. Biol. Reprod. 1999, 60, 594–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cecconi, S.; Capacchietti, G.; Russo, V.; Berardinelli, P.; Mattioli, M.; Barboni, B. In vitro growth of preantral follicles isolated from cryopreserved ovine ovarian tissue. Biol. Reprod. 2004, 70, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Srividya, D.; Praveen Chakravarthi, V.; Kona, S.; Siva Kumar, A.; Brahmaiah, K.V.; Rao, V.H. Expression of kit ligand and insulin-like growth factor binding protein 3 during in vivo or in vitro development of ovarian follicles in sheep. Reprod. Domest. Anim. 2017, 52, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Cecconi, S.; Curini, V.; Colosimo, A.; Garofalo, M.L.; Capacchietti, G.; Di Giacinto, O.; Mattioli, M. In vitro grown sheep preantral follicles yield oocytes with normal nuclear-epigenetic maturation. PLoS ONE 2011, 6, e27550. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the Ovarian Follicle Microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef]
- Higuchi, C.M.; Maeda, Y.; Horiuchi, T.; Yamazaki, Y. A Simplified method for three-dimensional (3-D) ovarian tissue culture yielding oocytes competent to produce full-term offspring in mice. PLoS ONE 2015, 10, e0143114. [Google Scholar] [CrossRef]
- Gosden, R.G.; Picton, H.M.; Nugent, D.; Rutherford, A.J. Gonadal tissue cryopreservation: Clinical objectives and practical prospects. Hum. Fertil. 1999, 2, 107–114. [Google Scholar] [CrossRef]
- Hovatta, O.; Wright, C.; Krausz, T.; Hardy, K.; Winston, R.M. Human primordial, primary and secondary ovarian follicles in long-term culture: Effect of partial isolation. Hum. Reprod. 1999, 14, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kawamura, K.; Cheng, Y.; Liu, S.; Klein, C.; Liu, S.; Duan, E.K.; Hsueh, A.J. Activation of dormant ovarian follicles to generate mature eggs. Proc. Natl. Acad. Sci. USA 2010, 107, 10280–10284. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Maidarti, M.; Clarkson, Y.L.; McLaughlin, M.; Anderson, R.A.; Telfer, E.E. Inhibition of PTEN activates bovine non-growing follicles in vitro but increases DNA damage and reduces DNA repair response. Hum. Reprod. 2019, 34, 297–307. [Google Scholar] [CrossRef]
- Laronda, M.M.; Duncan, F.E.; Hornick, J.E.; Xu, M.; Pahnke, J.E.; Whelan, K.A.; Shea, L.D.; Woodruff, T.K. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J. Assist. Reprod. Genet. 2014, 31, 1013–1028. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 2015, 5, 17323. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Palmerini, M.G.; Russo, V.; Rinaldi, C.; Bernabò, N.; Di Giacinto, O.; Berardinelli, P.; Nottola, S.A.; Macchiarelli, G.; Barboni, B. Blood vessel remodeling in pig ovarian follicles during the periovulatory period: An immunohistochemistry and SEM-corrosion casting study. Reprod. Biol. Endocrinol. 2009, 7, 72. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Nottola, S.A.; Tunjung, W.A.; Kadowaki, A.; Bianchi, S.; Cecconi, S.; Sato, E.; Macchiarelli, G. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes. Biochem. Biophys. Res. Commun. 2016, 481, 159–164. [Google Scholar] [CrossRef]
- Jimenez, C.R.; De Azevedo, J.L.; Silveira, R.G.; Penitente-Filho, J.; Carrascal-Triana, E.L.; Zolini, A.M.; Araujo, V.R.; Torres, C.; Gonçalves, W.G. Effects of IGF-1 on In Vitro Culture of Bovine Preantral Follicles are Dose-Dependent. Reprod. Domest. Anim. 2016, 51, 435–444. [Google Scholar] [CrossRef]
- Silva, A.W.B.; Ribeiro, R.P.; Menezes, V.G.; Barberino, R.S.; Passos, J.R.S.; Dau, A.M.P.; Costa, J.J.N.; Melo, L.R.F.; Bezerra, F.T.G.; Donato, M.A.M.; et al. Expression of TNF-α system members in bovine ovarian follicles and the effects of TNF-α or dexamethasone on preantral follicle survival, development and ultrastructure in vitro. Anim. Reprod. Sci. 2017, 182, 56–68. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Nottola, S.A.; Leoni, G.G.; Succu, S.; Borshi, X.; Berlinguer, F.; Naitana, S.; Bekmukhambetov, Y.; Macchiarelli, G. In vitro maturation is slowed in prepubertal lamb oocytes: Ultrastructural evidences. Reprod. Biol. Endocrinol. 2014, 12, 115. [Google Scholar] [CrossRef]
- Leoni, G.G.; Palmerini, M.G.; Satta, V.; Succu, S.; Pasciu, V.; Zinellu, A.; Carru, C.; Macchiarelli, G.; Nottola, S.A.; Naitana, S.; Berlinguer, F. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS ONE 2015, 10, e0124911. [Google Scholar] [CrossRef]
- Factsheet: The European Day for Organ Donation and Transplantation. Available online: https://www.edqm.eu/sites/default/files/factsheet_organ_tissue_cell_donation_eodd_2018.pdf (accessed on 12 January 2019).
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Sonntag, F.; Schilling, N.; Mader, K.; Gruchow, M.; Klotzbach, U.; Lindner, G.; Horland, R.; Wagner, I.; Lauster, R.; Howitz, S.; et al. Design and prototyping of a chip-based multi-micro-organoid culture system for substance testing, predictive to human (substance) exposure. J. Biotechnol. 2010, 148, 70–75. [Google Scholar] [CrossRef]
- Paulini, F.; Vilela, J.M.; Chiti, M.C.; Donnez, J.; Jadoul, P.; Dolmans, M.M.; Amorim, C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. BioMed. Online 2016, 33, 425–432. [Google Scholar] [CrossRef]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef]
- Hassanpour, A.; Talaei-Khozani, T.; Kargar-Abarghouei, E.; Razban, V.; Vojdani, Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res. Ther. 2018, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Liverani, L.; Raffel, N.; Fattahi, A.; Preis, A.; Hoffmann, I.; Boccaccini, A.R.; Beckmann, M.W.; Dittrich, R. Electrospun patterned porous scaffolds for the support of ovarian follicles growth: A feasibility study. Sci. Rep. 2019, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Antonino, D.C.; Soares, M.M.; Júnior, J.M.; de Alvarenga, P.B.; Mohallem, R.F.F.; Rocha, C.D.; Vieira, L.A.; de Souza, A.G.; Beletti, M.E.; Alves, B.G.; et al. Three-dimensional levitation culture improves in-vitro growth of secondary follicles in bovine model. Reprod. BioMed. Online 2019, 38, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Skory, R.M.; Xu, Y.; Shea, L.D.; Woodruff, T.K. Engineering the ovarian cycle using in vitro follicle culture. Hum. Reprod. 2015, 30, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; van den Hurk, R.; Figueiredo, J.R. Ovarian follicle development in vitro and oocyte competence: Advances and challenges for farm animals. Domest. Anim. Endocrinol. 2016, 55, 123–135. [Google Scholar] [CrossRef]
- Baba, T.; Ting, A.Y.; Tkachenko, O.; Xu, J.; Stouffer, R.L. Direct actions of androgen, estrogen and anti-Müllerian hormone on primate secondary follicle development in the absence of FSH in vitro. Hum. Reprod. 2017, 32, 2456–2464. [Google Scholar] [CrossRef]
- Jones, A.S.K.; Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. J. Biol. Eng. 2019, 13, 2. [Google Scholar] [CrossRef]
- Baysal, Ö.; Hamilton, J.A.M.; Hamilton, C.J.C.M.; Braat, D.D.M.; Beerendonk, C.C.M.; Nelen, W.L.D.M. Clinical practice guidelines for fertility preservation in young women undergoing gonadotoxic treatment: An overview and critical appraisal of methodological quality and content. Reprod. BioMed. Online 2018, 37, 60–70. [Google Scholar] [CrossRef]
- Woodruff, T.K. From the bench to bedside to babies: Translational medicine made possible by funding multidisciplinary team science. J. Assist. Reprod. Genet. 2013, 30, 1249–1253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Network FertiPROTEKT. Available online: www.fertiprotekt.com (accessed on 20 February 2019).
- Oncofertility Consortium Network. Available online: www.oncofertility.northwestern.edu (accessed on 20 February 2019).
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: An Ethics Committee opinion. Fertil. Steril. 2018, 110, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; Hayashi, K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, G.; Di Nisio, V.; Macchiarelli, G.; Nottola, S.A.; Halvaei, I.; De Santis, L.; Cecconi, S. Technologies for the Production of Fertilizable Mammalian Oocytes. Appl. Sci. 2019, 9, 1536. https://doi.org/10.3390/app9081536
Rossi G, Di Nisio V, Macchiarelli G, Nottola SA, Halvaei I, De Santis L, Cecconi S. Technologies for the Production of Fertilizable Mammalian Oocytes. Applied Sciences. 2019; 9(8):1536. https://doi.org/10.3390/app9081536
Chicago/Turabian StyleRossi, Gianna, Valentina Di Nisio, Guido Macchiarelli, Stefania Annarita Nottola, Iman Halvaei, Lucia De Santis, and Sandra Cecconi. 2019. "Technologies for the Production of Fertilizable Mammalian Oocytes" Applied Sciences 9, no. 8: 1536. https://doi.org/10.3390/app9081536
APA StyleRossi, G., Di Nisio, V., Macchiarelli, G., Nottola, S. A., Halvaei, I., De Santis, L., & Cecconi, S. (2019). Technologies for the Production of Fertilizable Mammalian Oocytes. Applied Sciences, 9(8), 1536. https://doi.org/10.3390/app9081536





