Estimation of Source-Based Aerosol Optical Properties for Polydisperse Aerosols from Receptor Models
Abstract
1. Introduction
2. Methods
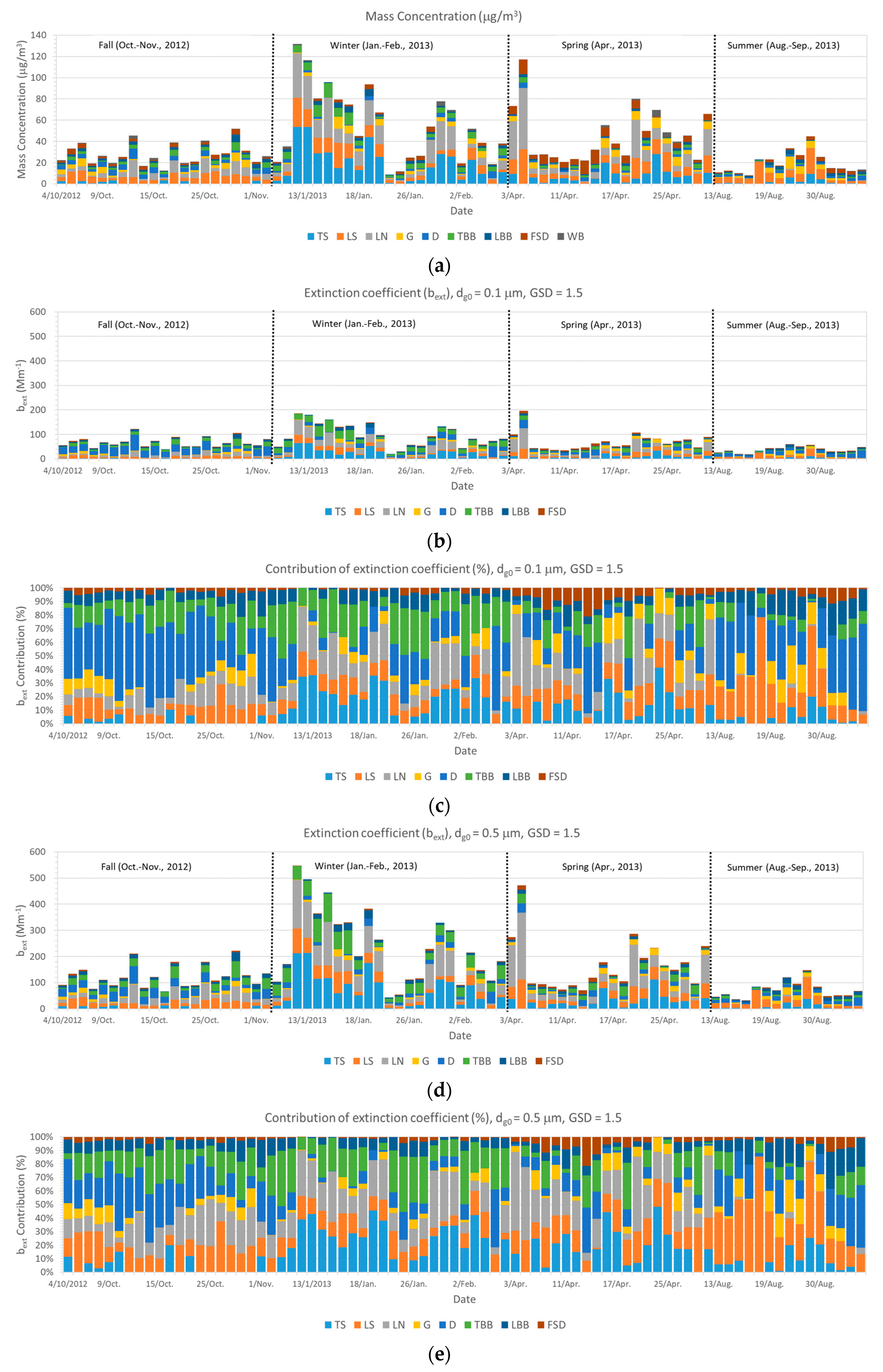
2.1. Data
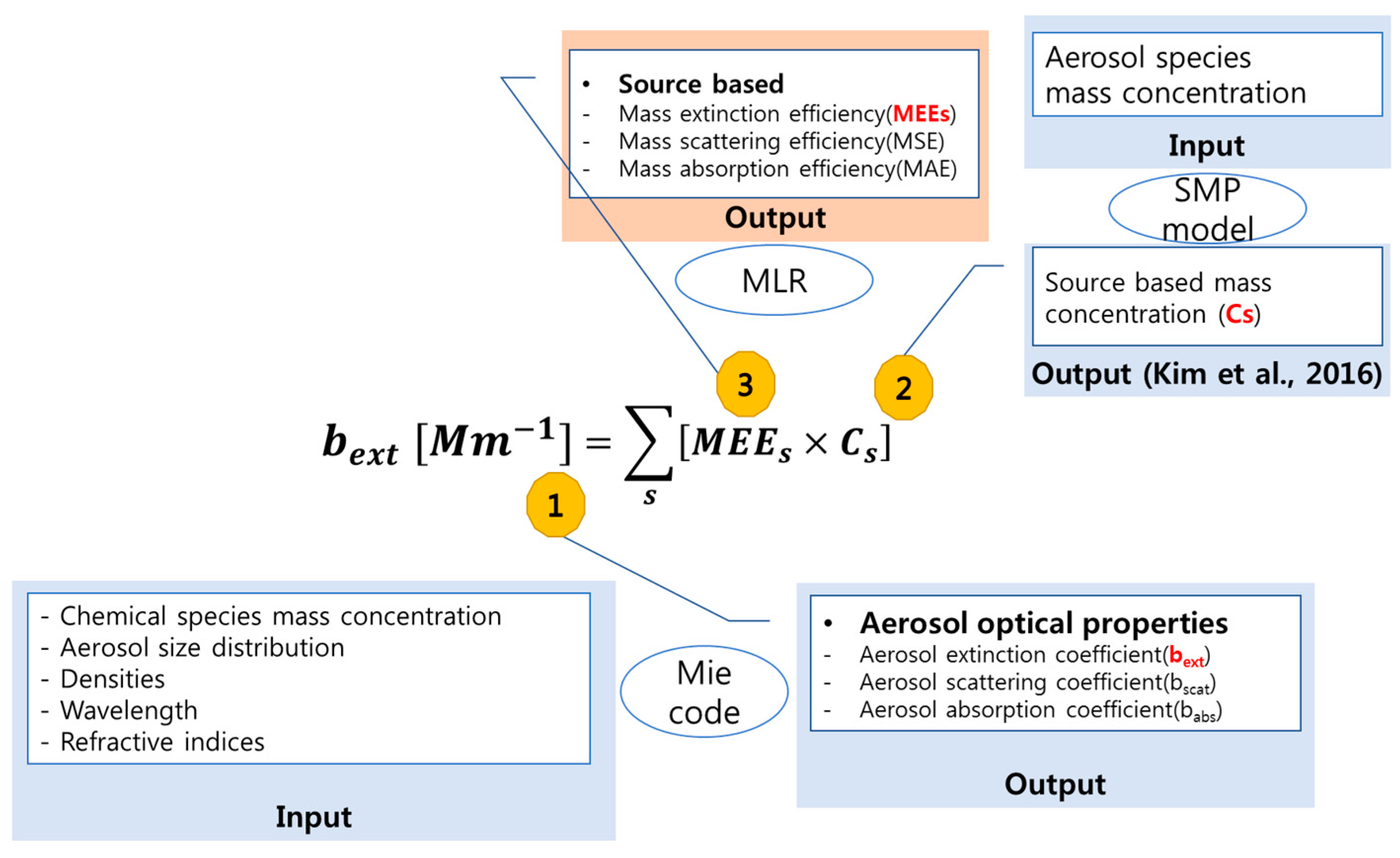
2.2. Extinction Coefficient (bext) Calculations for Polydisperse Aerosols
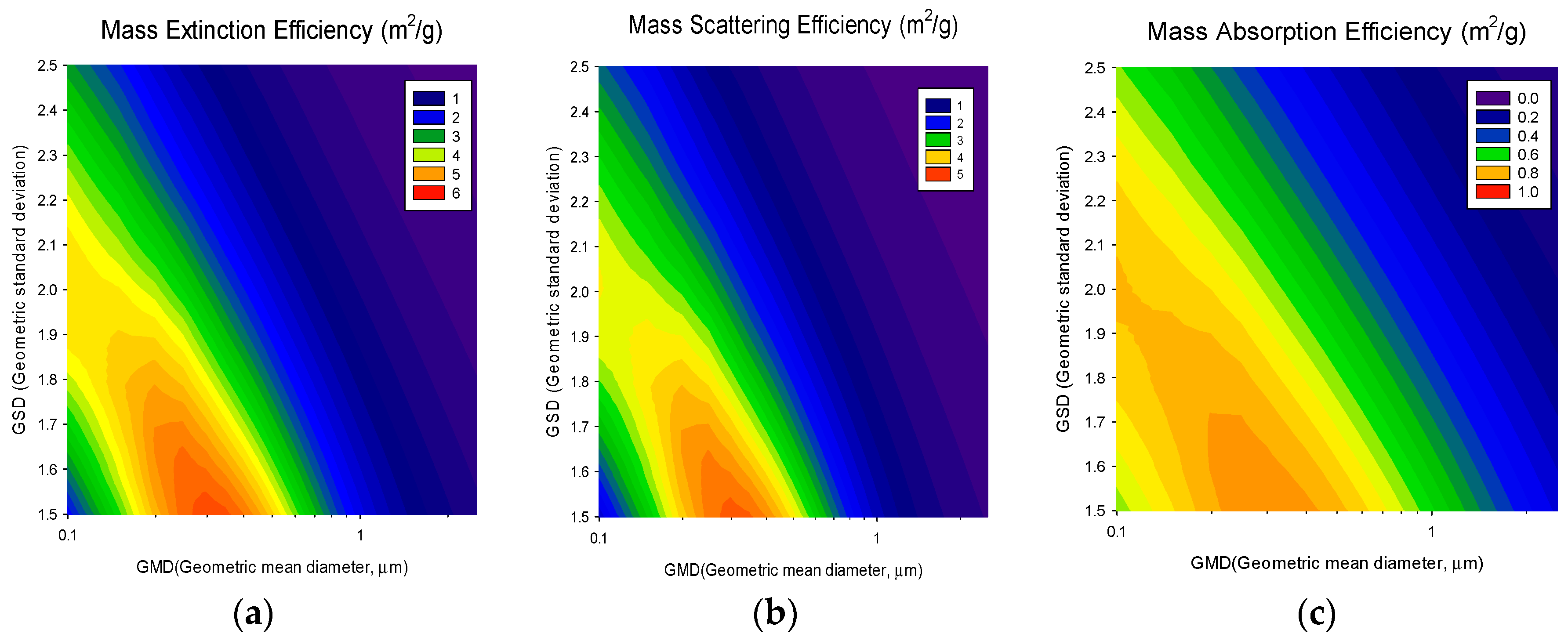
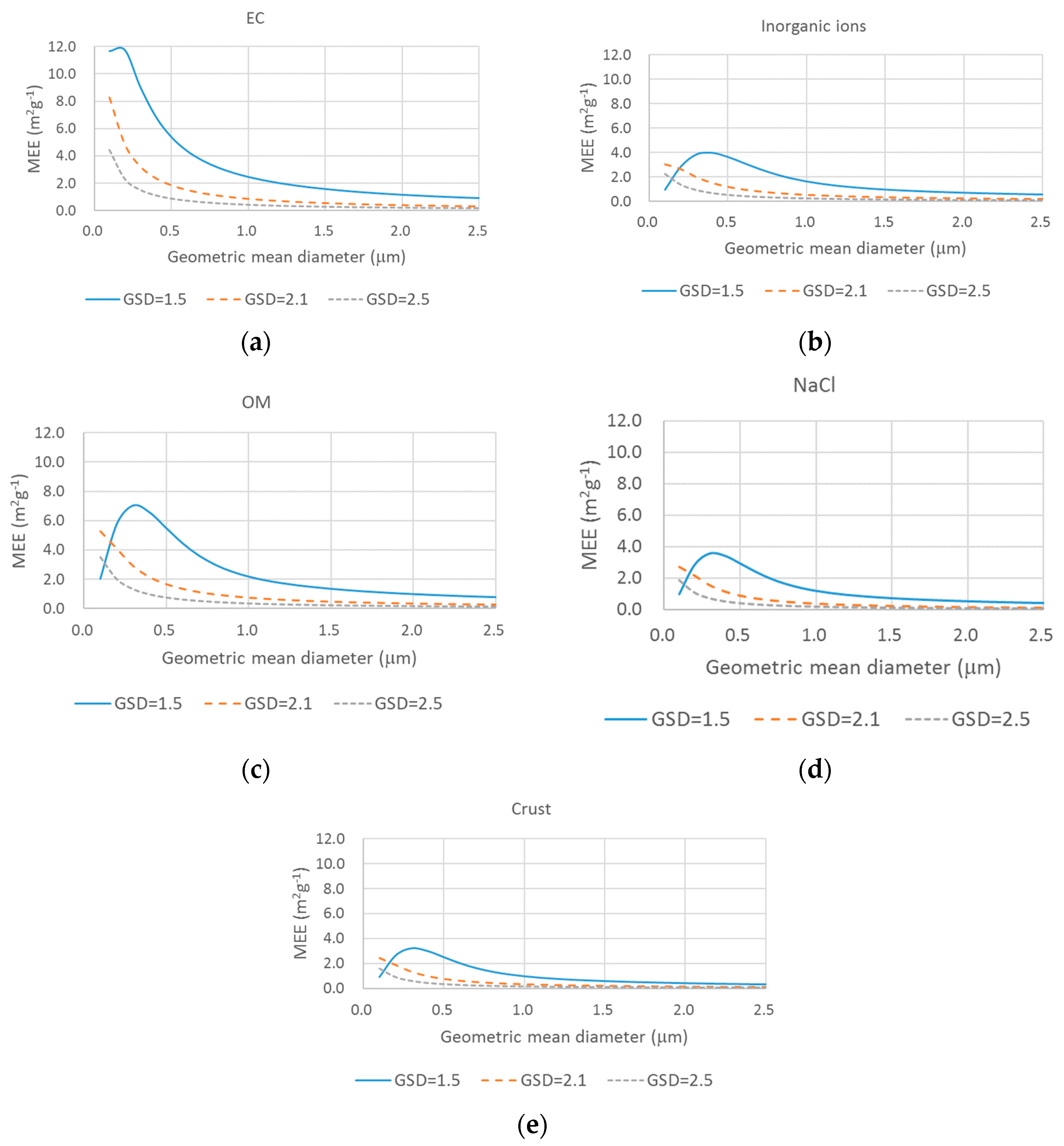
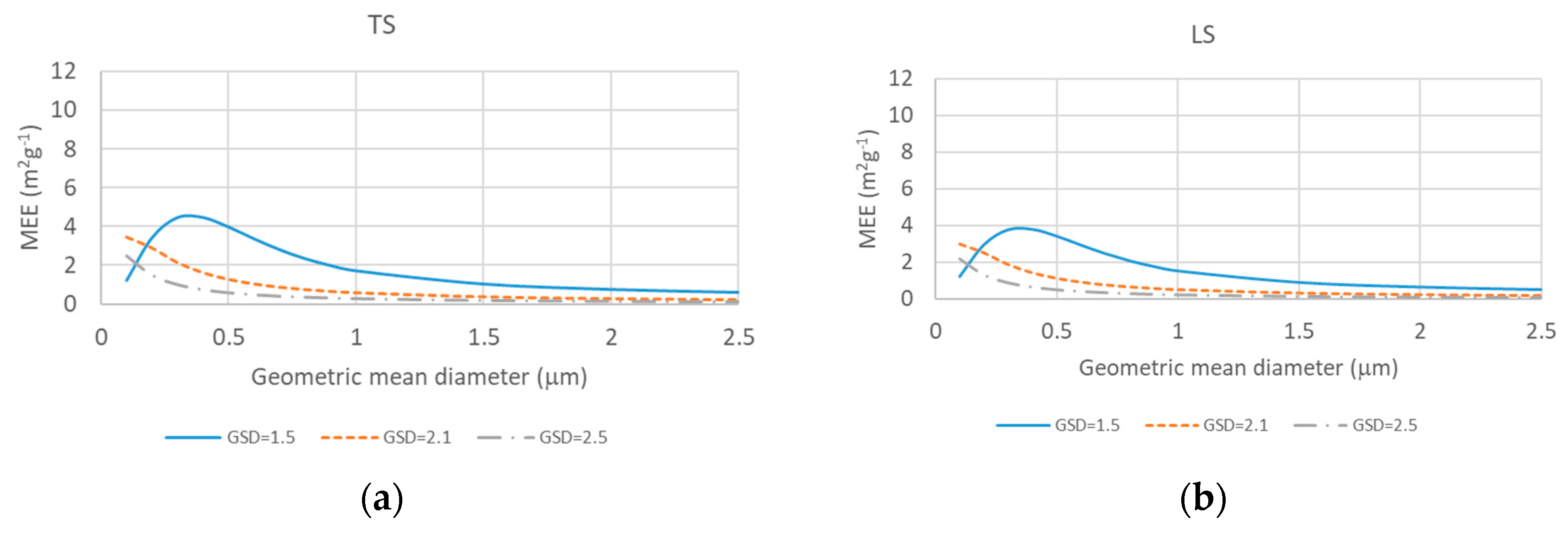
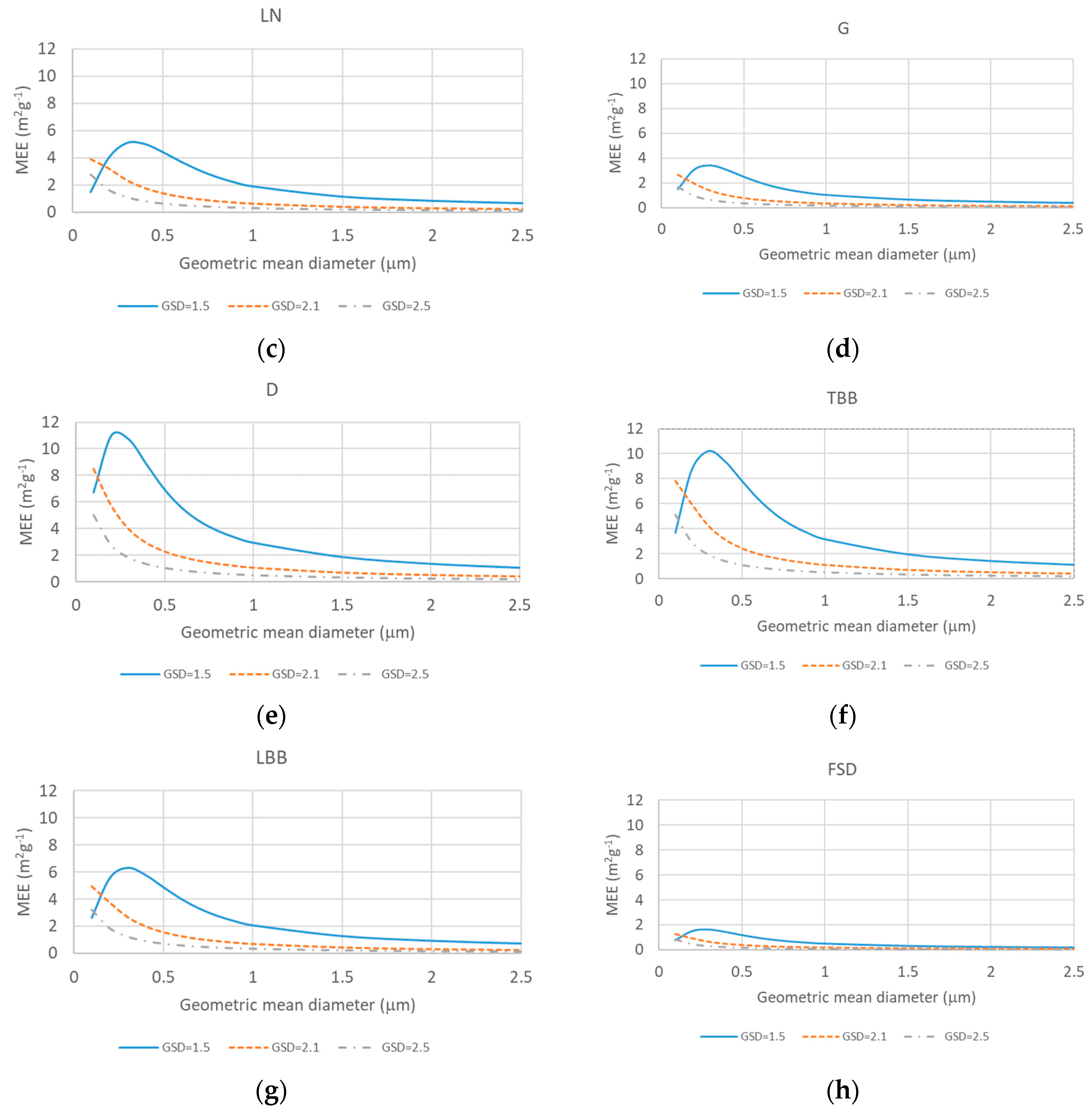
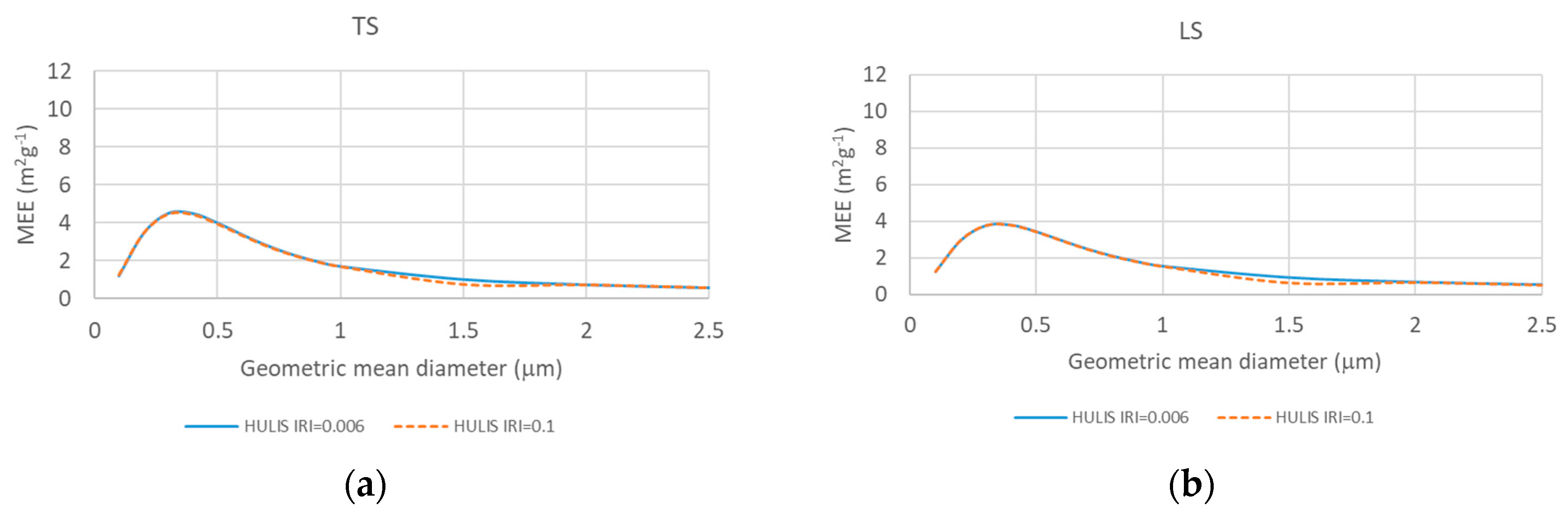
2.3. Source-Based Aerosol Mass Extinction Efficiency for Polydisperse Aerosol Particles
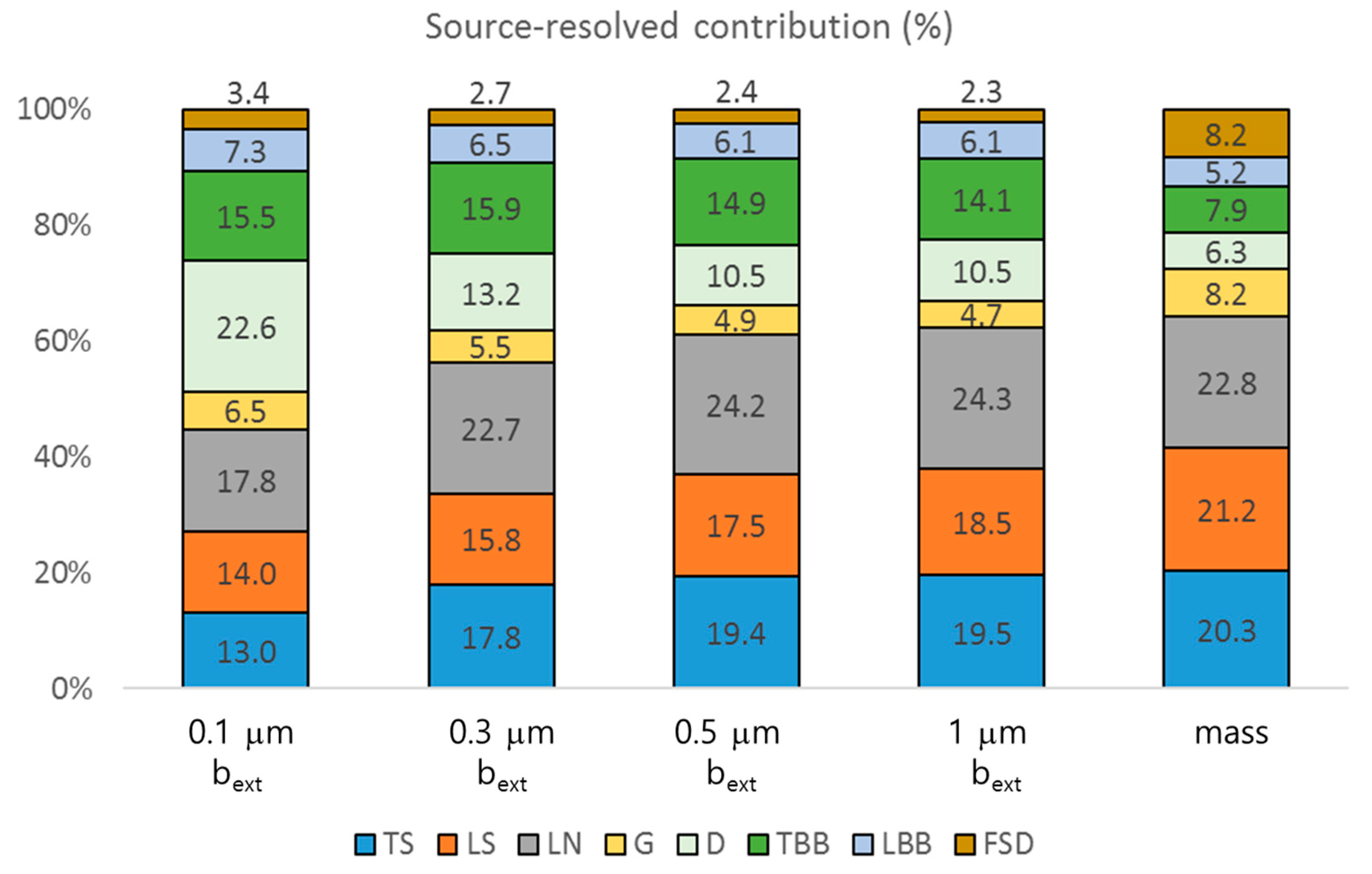
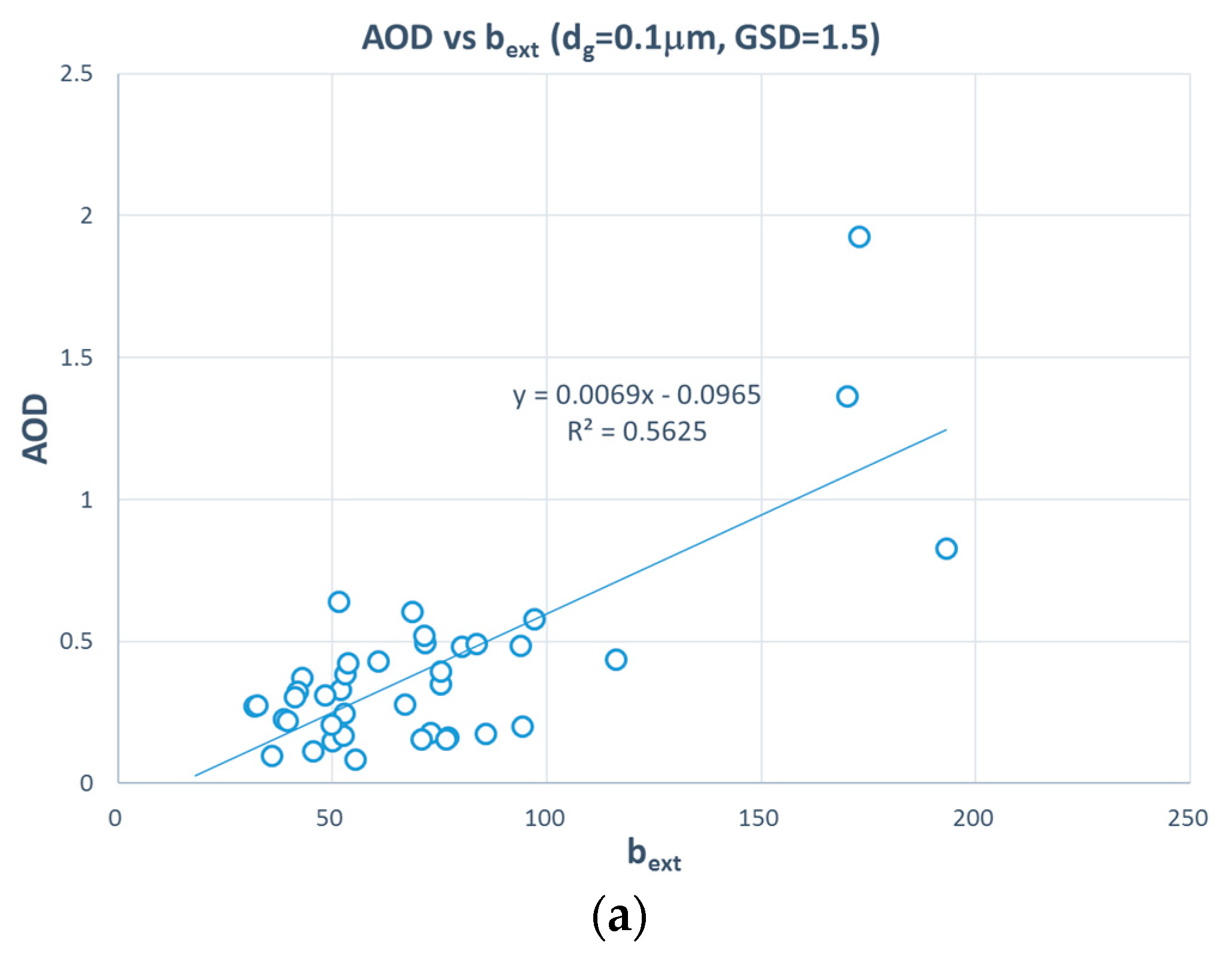
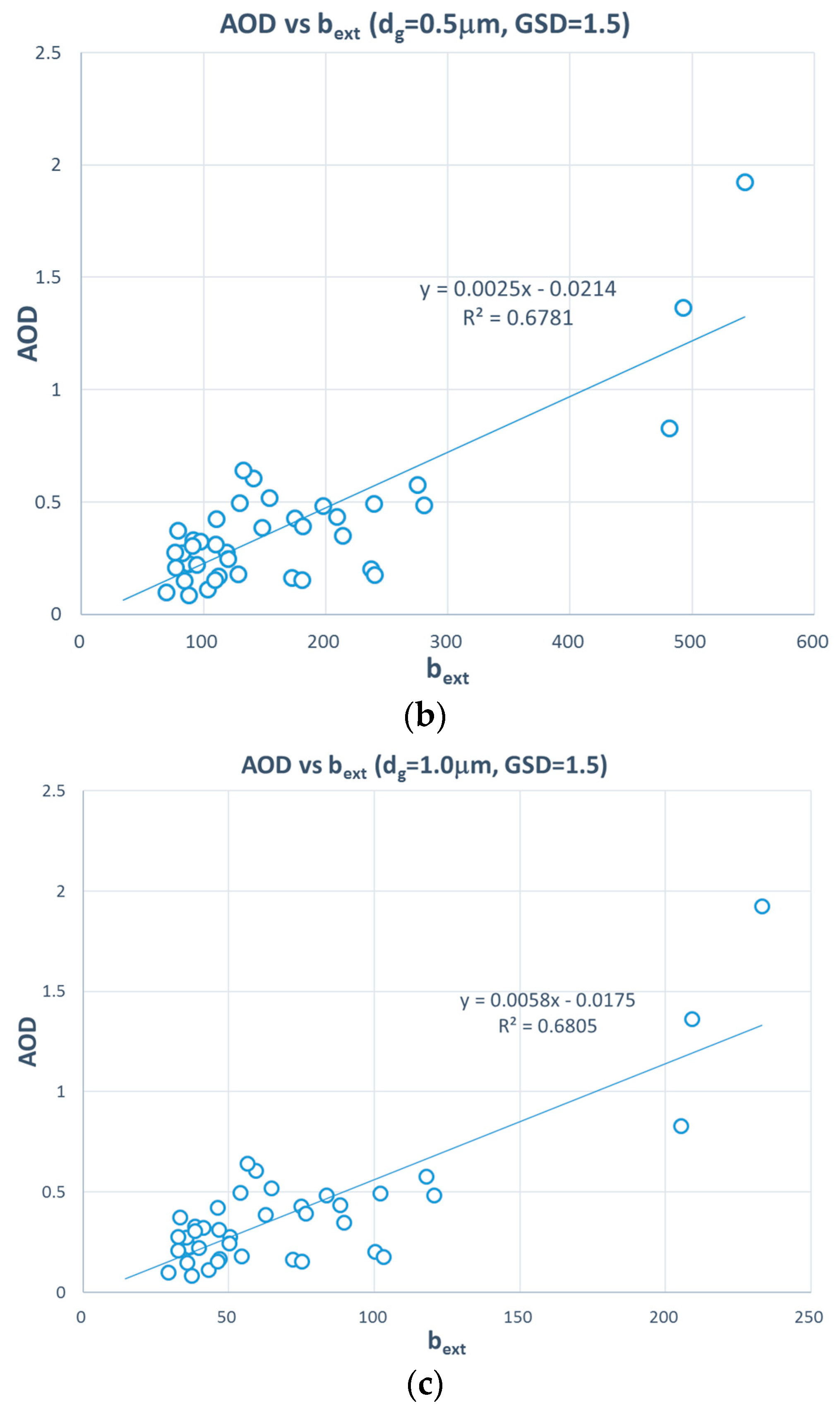
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pilinis, C.; Pandis, S.N.; Seinfeld, J.H. Sensitivity of direct climate forcing by atmospheric aerosols to aerosol size and composition. J. Geophys. Res. 1995, 100, 18739–18754. [Google Scholar] [CrossRef]
- Cheng, Z.; Ma, X.; He, Y.; Jiang, J.; Wang, X.; Wang, Y.; Sheng, L.; Hu, J.; Yan, N. Mass extinction efficiency and extinction hygroscopicity of ambient PM2.5 in urban China. Environ. Res. 2017, 156, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Um, J.; Bae, S.Y.; Yoon, Y.J.; Lee, S.S.; Kim, Y.P. Analytic expression for the aerosol mass efficiencies for polydispersed accumulation mode. Aerosol Air Qual. Res. 2018, 18, 1503–1514. [Google Scholar] [CrossRef]
- Horvath, H. Atmospheric light absorption-A review. Atmos. Environ. 1993, 27, 293–317. [Google Scholar] [CrossRef]
- Watson, J.G. Overview of receptor model principles. J. Air Pollut. Contr. Assoc. 1984, 34, 620–623. [Google Scholar] [CrossRef]
- Lowenthal, D.H.; Watson, J.G.; Saxena, P. Contributions to light extinction during project MOHAVE. Atmos. Environ. 2000, 34, 2351–2359. [Google Scholar] [CrossRef]
- Malm, W.C.; Day, D.; Kreidenweis, S. Light scattering characteristics of aerosols as a function of relative humidity: Part I—A comparison of measured scattering and aerosol concentrations using the theoretical models. J. Air Waste Manag. Assoc. 2000, 50, 686–700. [Google Scholar] [CrossRef]
- Jung, C.H.; Shin, H.J.; Lee, J.Y.; Kim, Y.P. Sensitivity and contribution of organic aerosols to aerosol optical properties based on their refractive index and hygroscopicity. Atmosphere 2016, 7, 65. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jung, C.H.; Kim, Y.P. Estimation of Optical Properties for HULIS Aerosols at Anmyeon Island, Korea. Atmosphere 2017, 8, 120. [Google Scholar] [CrossRef]
- Chan, Y.C.; Simpson, R.W.; Mctainsh, G.H.; Vowles, P.D.; Cohen, D.D.; Bailey, G.M. Source apportionment of visibility degradation problems in Brisbane (Australia) using the multiple linear regression techniques. Atmos. Environ. 1999, 33, 3237–3250. [Google Scholar] [CrossRef]
- Titos, G.; Foyo-Moreno, I.; Lyamani, H.; Querol, X.; Alastuey, A.; Alados-Arboledas, L. Optical properties and chemical composition of aerosol particles at an urban location: An estimation of the aerosol mass scattering and absorption efficiencies. J. Geophys. Res. 2012, 117, D04206. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Cao, J.; Hsu, S.C.; Xia, X.; Zhang, Z.; Lin, Z.; Cheng, T.; Zhang, R. Characterization and source apportionment of aerosol light extinction in Chengdu, southwest China. Atmos. Environ. 2014, 95, 552–562. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Kim, B.M.; Seo, J.; Kim, J.Y.; Lee, J.Y.; Kim, Y.P. Transported vs. local contributions from secondary and biomass burning sources to PM2.5. Atmos. Environ. 2016, 144, 24–36. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- Sloane, C.S. Optical properties of aerosols of mixed composition. Atmos. Environ. 1984, 18, 871–878. [Google Scholar] [CrossRef]
- Arola, A.; Schuster, G.; Myhre, G.; Kazadzis, S.; Dey, S.; Tripathi, S.N. Inferring absorbing organic carbon content from AERONET data. Atmos. Chem. Phys. 2011, 11, 215–225. [Google Scholar] [CrossRef]
- Hess, M.; Koepke, P.; Schult, I. Optical properties of aerosols and clouds: The software package OPAC. Bull. Am. Meteor. Soc. 1998, 79, 831–844. [Google Scholar] [CrossRef]
- Dillner, A.M.; Stein, C.; Larson, S.M.; Hitzenberger, R. Measuring the Mass Extinction Efficiency of Elemental Carbon in Rural Aerosol. Aerosol Sci. Technol. 2001, 35, 1009–1021. [Google Scholar] [CrossRef]
- Lesins, G.; Chylek, P.; Lohmann, U. A study of internal and external mixing scenarios and its effect on aerosol optical properties and direct radiative forcing. J. Geophys. Res. 2002, 107, 4094. [Google Scholar] [CrossRef]
- Sloane, C.S.; Watson, J.; Chow, J.; Pritchett, L.; Richards, L.W. Size-segregated fine particle measurements by chemical species and their impact on visibility impairment in Denver. Atmos. Environ. 1991, 25, 1013–1024. [Google Scholar] [CrossRef]
- Hand, J.L.; Malm, W.C. Review of aerosol mass scattering efficiencies from ground-based measurements since 1990. J. Geophys. Res. 2007, 112, D16203. [Google Scholar] [CrossRef]
- Malm, W.C.; Sisler, J.F.; Huffman, D.; Eldred, R.A.; Cahill, T.A. Spatial and seasonal trends in particle concentration and optical extinction in the United States. J. Geophys. Res. 1994, 99, 1347–1370. [Google Scholar] [CrossRef]
- Fox, J. Applied Regression Analysis, Linear Models and Related Methods; Sage Publication, Inc.: Thousand Oaks, CA, USA, 1997. [Google Scholar]
- Jung, C.H.; Kim, Y.P. Particle extinction coefficient for polydispersed aerosol using a harmonic mean type general approximated solution. Aerosol Sci. Technol. 2007, 41, 994–1001. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Holben, B.N.; Tanré, D.; Smirnov, A.; Eck, T.; Slutsker, I.; Abuhassan, N.; Newcomb, W.; Schafer, J.; Chatenet, B.; Lavenu, F.; et al. An emerging ground-based aerosol climatology: Aerosol optical depth from AERONET. J. Geophys. Res. 2001, 106, 12067–12097. [Google Scholar] [CrossRef]

| mr | mi | Density (g cm−3) | |
|---|---|---|---|
| EC | 1.75 | 0.44 | 1.00 |
| Inorganic ions | 1.43 | 0.00 | 1.7 |
| OM | 1.53 | 0.00 | 1.2 |
| NaCl | 1.5 | 1.0 × 10−8 | 2.2 |
| Crust | 1.53 | 1.1 × 10−3 | 2.6 |
| Source | Range | MEE | MAE | MSE |
|---|---|---|---|---|
| TS | min | 0.098 | 0.000 | 0.061 |
| max | 4.471 | 0.112 | 4.471 | |
| LS | min | 0.089 | 0.036 | 0.053 |
| max | 3.802 | 0.397 | 3.409 | |
| LN | min | 0.110 | 0.047 | 0.063 |
| max | 5.064 | 0.345 | 4.792 | |
| G | min | 0.062 | 0.034 | 0.028 |
| max | 3.418 | 0.750 | 2.693 | |
| D | min | 0.185 | 0.099 | 0.086 |
| max | 11.004 | 4.604 | 6.438 | |
| TBB | min | 0.191 | 0.084 | 0.107 |
| max | 10.229 | 1.143 | 9.126 | |
| LBB | min | 0.125 | 0.000 | 0.069 |
| max | 6.335 | 0.246 | 6.335 | |
| FSD | min | 0.031 | 0.019 | 0.012 |
| max | 1.655 | 0.458 | 1.197 |
| dg (µm) | TS | LS | LN | G | D | TBB | LBB | FSD |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 9.3 | 10.0 | 12.7 | 4.6 | 16.2 | 11.1 | 5.2 | 2.4 |
| 0.2 | 26.6 | 24.0 | 34.5 | 9.7 | 26.5 | 26.4 | 11.0 | 4.8 |
| 0.3 | 34.7 | 30.7 | 44.1 | 10.7 | 25.7 | 30.8 | 12.6 | 5.2 |
| 0.4 | 34.7 | 30.8 | 43.5 | 9.5 | 21.2 | 28.3 | 11.6 | 4.6 |
| 0.5 | 30.9 | 27.9 | 38.4 | 7.8 | 16.7 | 23.7 | 9.8 | 3.8 |
| 0.6 | 26.1 | 23.9 | 32.4 | 6.3 | 13.3 | 19.2 | 8.0 | 3.1 |
| 0.7 | 21.7 | 20.2 | 26.9 | 5.1 | 10.9 | 15.6 | 6.6 | 2.5 |
| 0.8 | 18.1 | 17.0 | 22.5 | 4.3 | 9.3 | 13.0 | 5.5 | 2.1 |
| 0.9 | 15.3 | 14.5 | 19.0 | 3.6 | 8.0 | 11.0 | 4.7 | 1.8 |
| 1.0 | 13.2 | 12.5 | 16.4 | 3.2 | 7.1 | 9.6 | 4.1 | 1.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, C.H.; Lee, J.Y.; Um, J.; Lee, S.S.; Yoon, Y.J.; Kim, Y.P. Estimation of Source-Based Aerosol Optical Properties for Polydisperse Aerosols from Receptor Models. Appl. Sci. 2019, 9, 1443. https://doi.org/10.3390/app9071443
Jung CH, Lee JY, Um J, Lee SS, Yoon YJ, Kim YP. Estimation of Source-Based Aerosol Optical Properties for Polydisperse Aerosols from Receptor Models. Applied Sciences. 2019; 9(7):1443. https://doi.org/10.3390/app9071443
Chicago/Turabian StyleJung, Chang Hoon, Ji Yi Lee, Junshik Um, Seoung Soo Lee, Young Jun Yoon, and Yong Pyo Kim. 2019. "Estimation of Source-Based Aerosol Optical Properties for Polydisperse Aerosols from Receptor Models" Applied Sciences 9, no. 7: 1443. https://doi.org/10.3390/app9071443
APA StyleJung, C. H., Lee, J. Y., Um, J., Lee, S. S., Yoon, Y. J., & Kim, Y. P. (2019). Estimation of Source-Based Aerosol Optical Properties for Polydisperse Aerosols from Receptor Models. Applied Sciences, 9(7), 1443. https://doi.org/10.3390/app9071443







