Efficient Characterization of Macroscopic Composite Cement Mortars with Various Contents of Phase Change Material
Abstract
:1. Introduction
- the thermal conductivity is searched for in [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] using either hot wire [31,45], hot plate [32,34,35,36,39,42,43,44,46], or laser flash [33,40] techniques.Let us mention here that the thermal contact resistances and their influence on the measured conductivity are rarely taken into account.
- the heat capacity is mainly investigated as an apparent capacity, which is supposed to integrate the latent heat and goes continuously from the solid value to the liquid one [31,33,34,36,37,38,40,44,45,47,48,49,50,51,52,53]. However, many of these studies determine this property from a differential scanning calorimetry (DSC) experiment [33,34,38,40,44,45,47,48,49,50,51,53,54,55], where the size of the sample is such that it is not very representative of the macroscopic material [5,31,36,52]. As far as real scales are concerned, one can also note the possible use of the dynamic heat flow meter method (DHFM), which resembles the isothermal mode of calorimeters [56,57]. Practically, this apparent capacity is very often obtained by directly integrating the heat-flow rate. Unfortunately, it can potentially lead to inconsistent or imprecise characterization [58,59,60,61,62] and be responsible for erroneous predictions when modeling the macroscopic material [60,63,64].
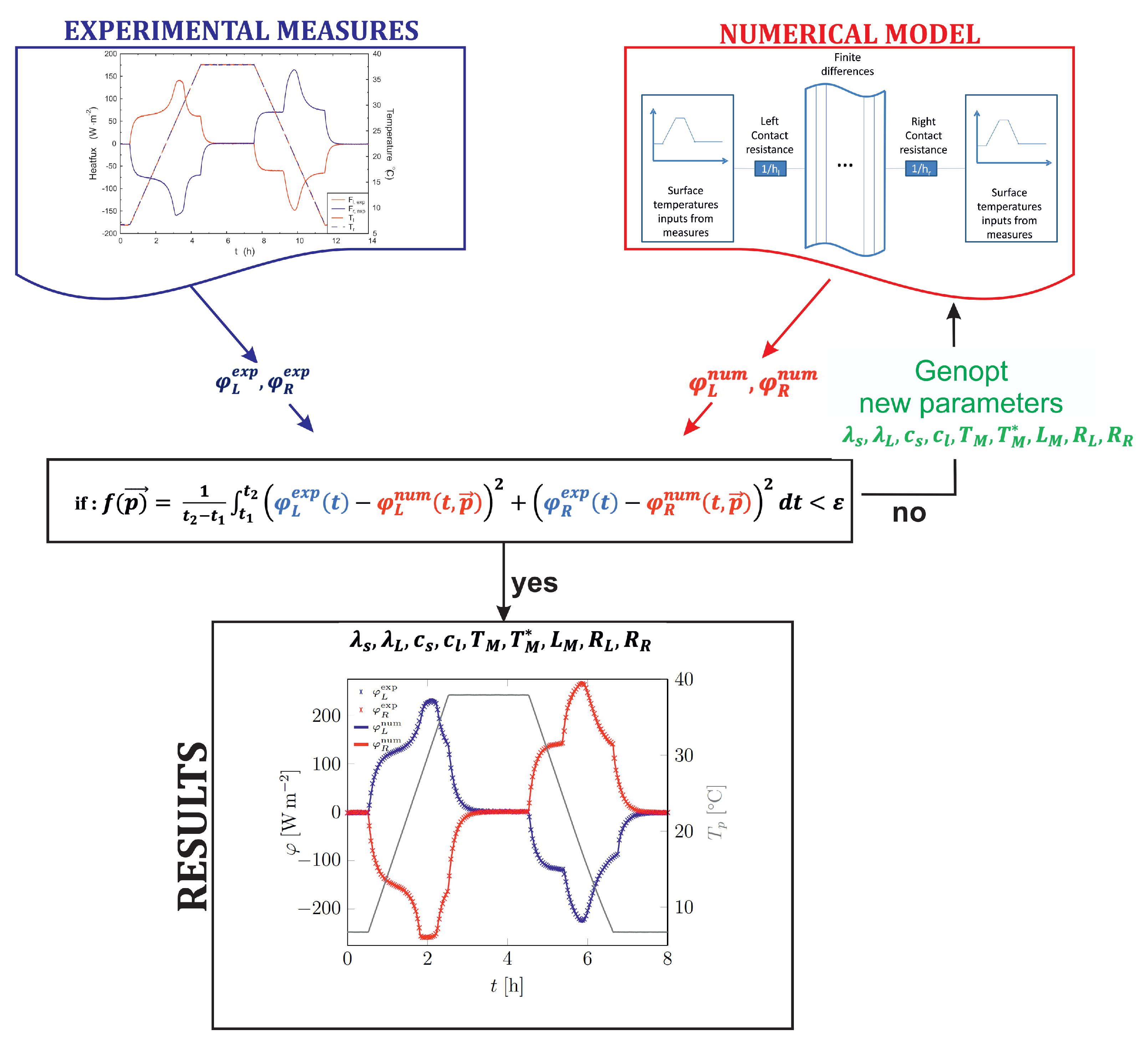
- in recent studies, a more generic approach based on inversion methods has been developed in order to determine separately the main thermo-physical properties, such as the heat capacities (solid and liquid) and/or the latent heat etc. [36,52,65]. The main idea is to combine some experimental measurements, usually the temporal evolution of the heat-flow rate, with a numerical model of the composite sample. The correct values for the intrinsic parameters of the material are retrieved by minimizing an objective function.
2. Method
2.1. Experimental Protocol
2.2. Numerical Method
- The PCM being micro-encapsulated, the thermal expansion during the phase transition can be neglected, which implies that solid and liquid densities are equal.n.b. in such a case, there is no difference between the mass and volume fractions, which are therefore both denoted by .
- The thermodynamic behavior of the PCM, i.e., its enthalpy function, obeys a binary solution model [68]:Here, the involved latent heat for the composite mortar depends on the mass fraction Y of the PCM included in this latter one:
2.3. Inversion Process
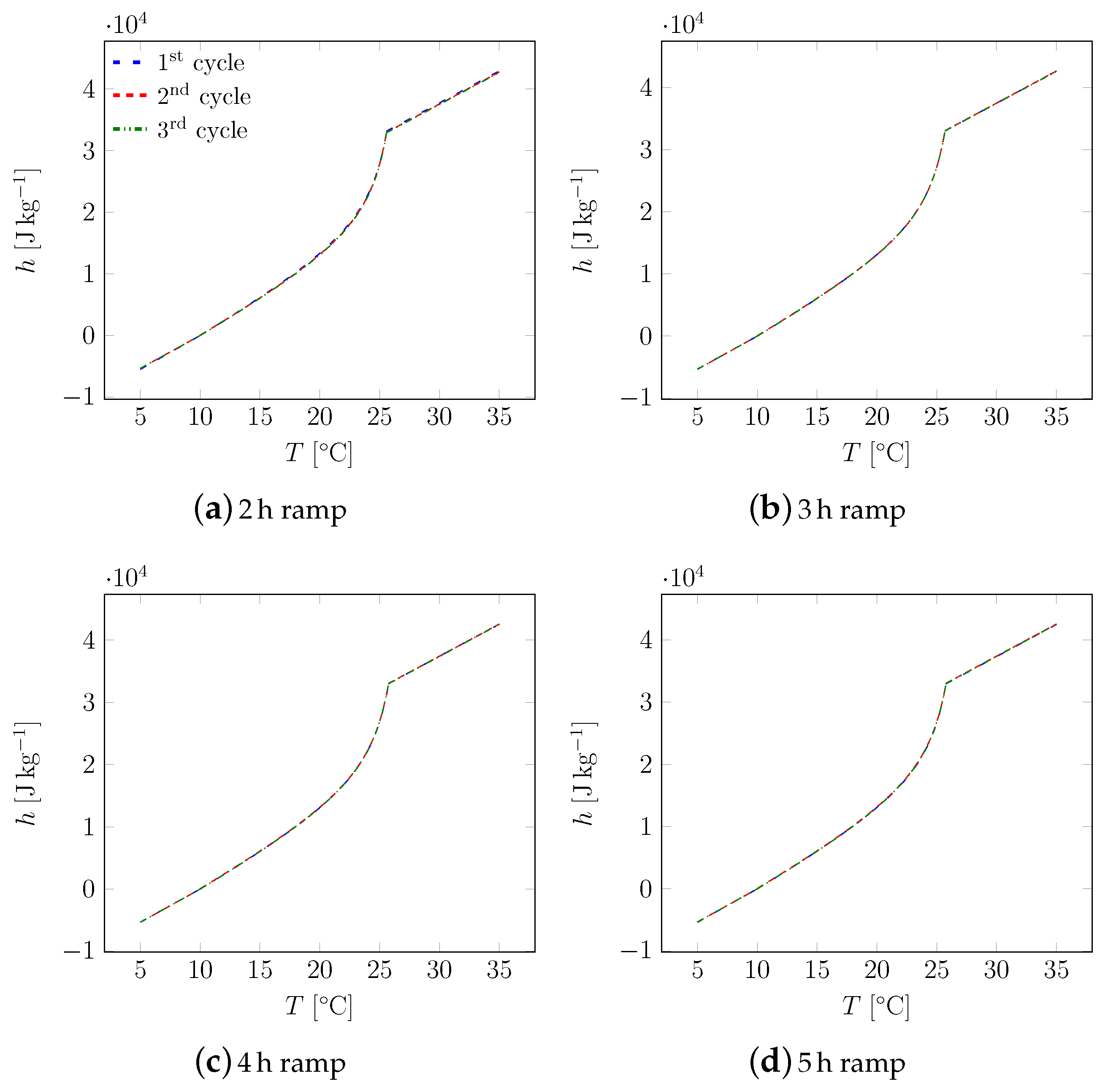
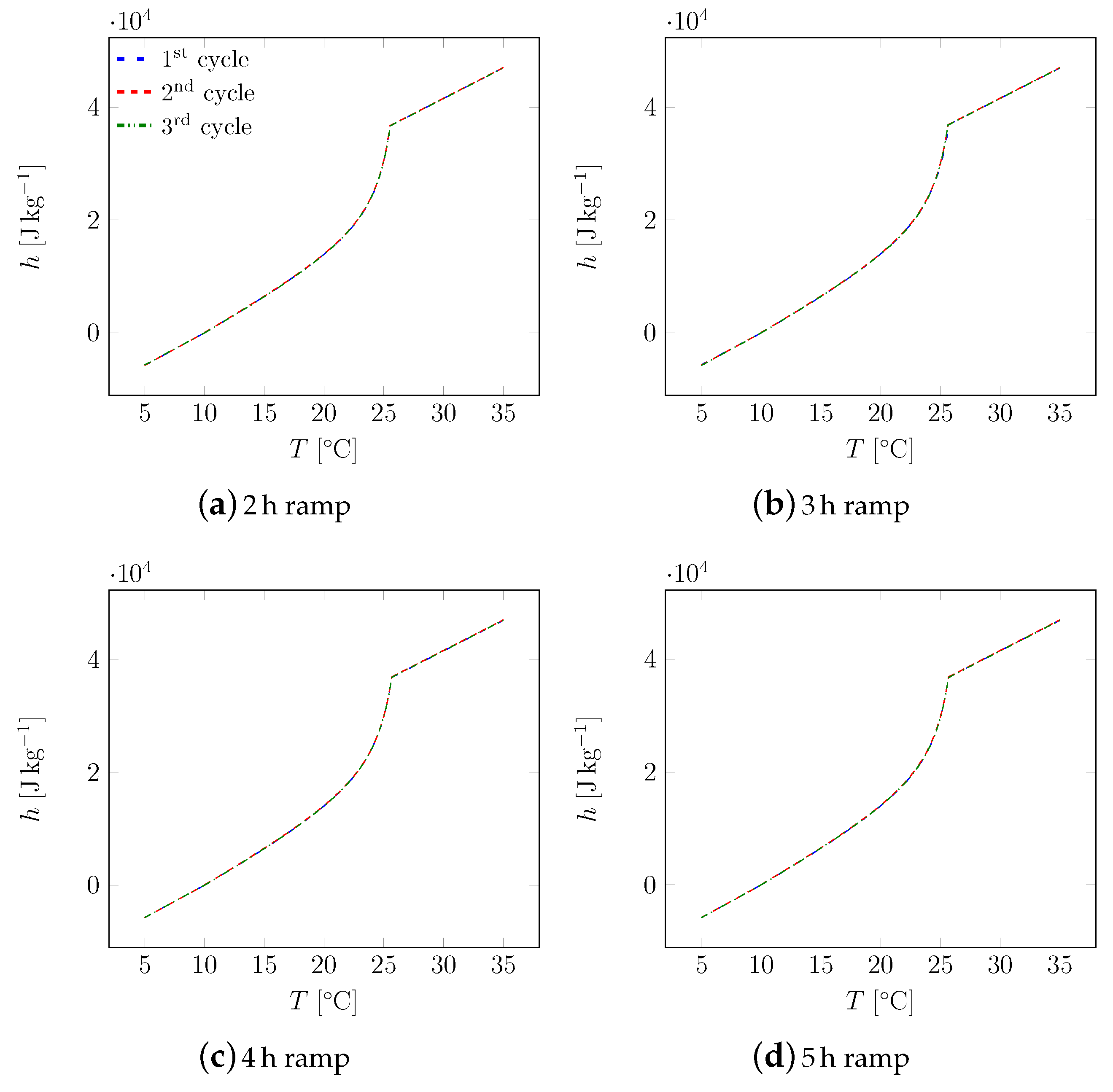
3. Results
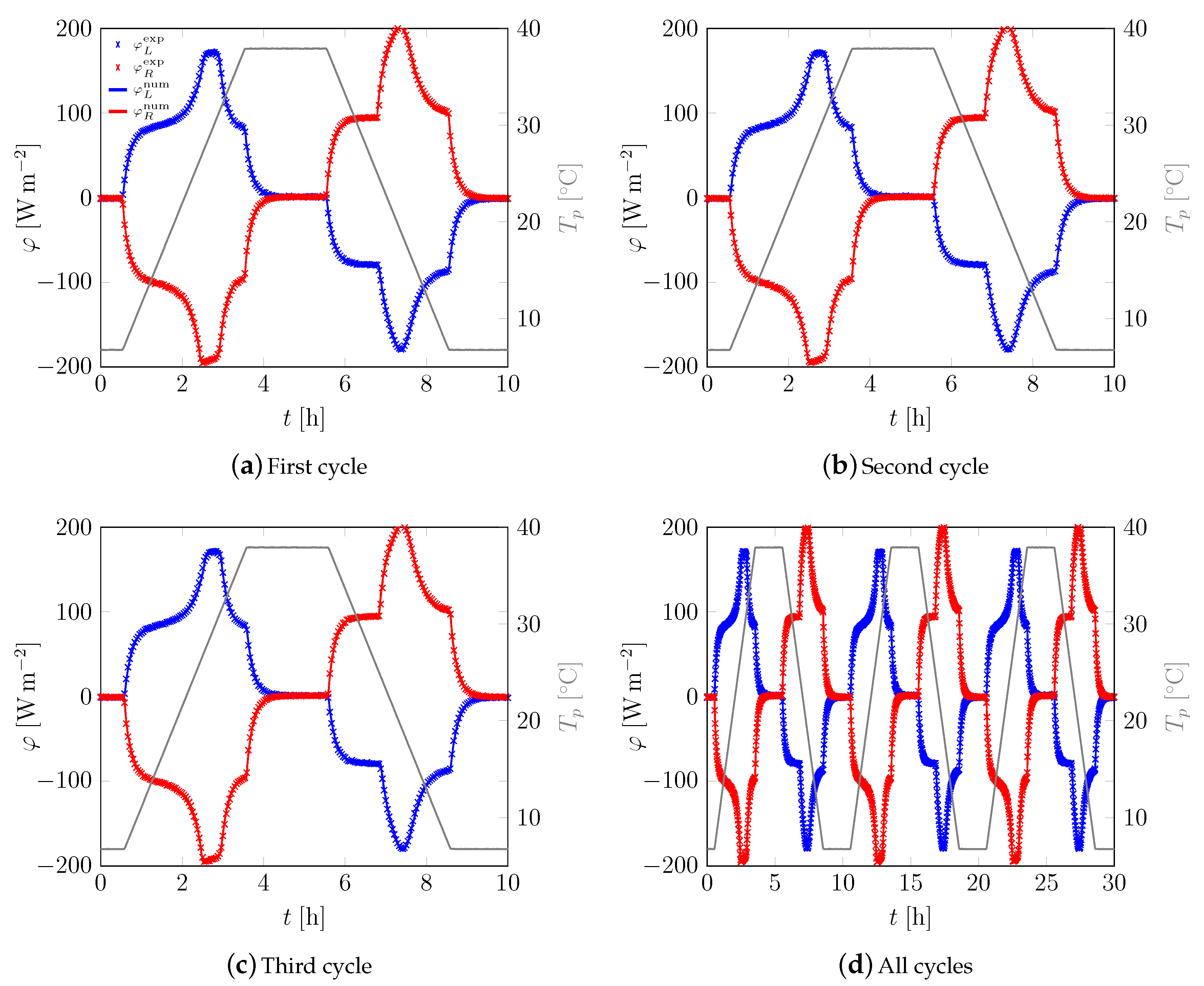
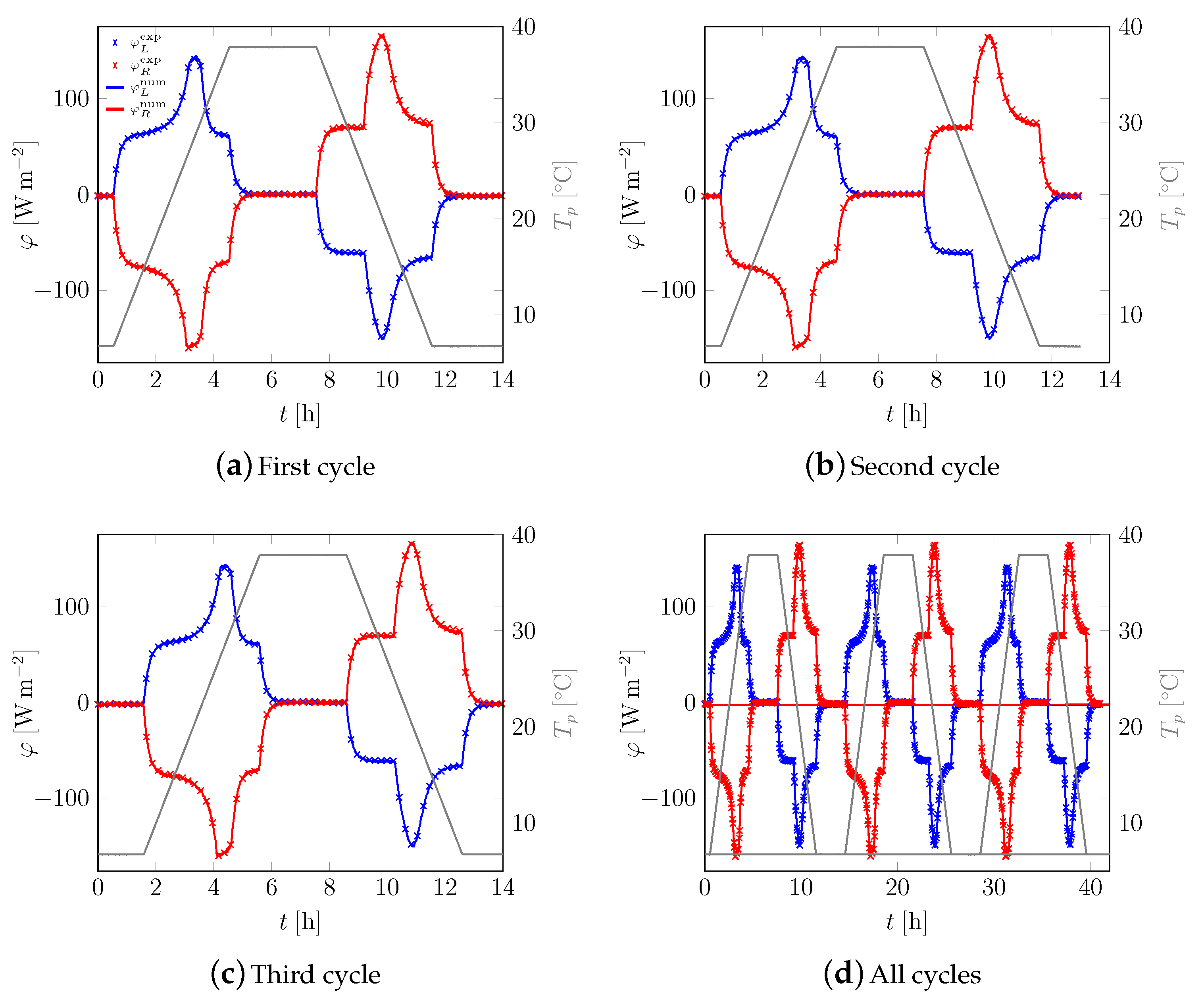
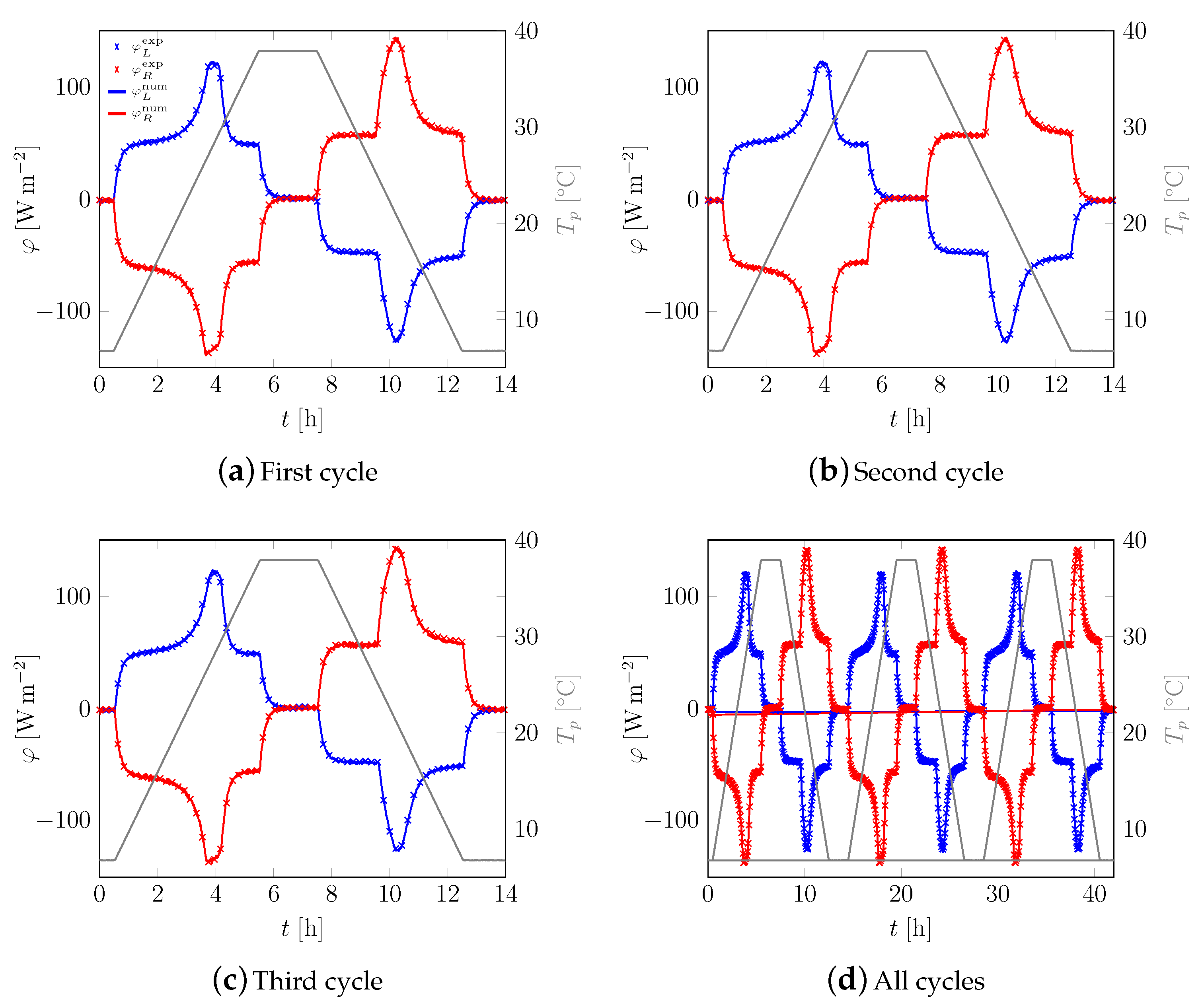
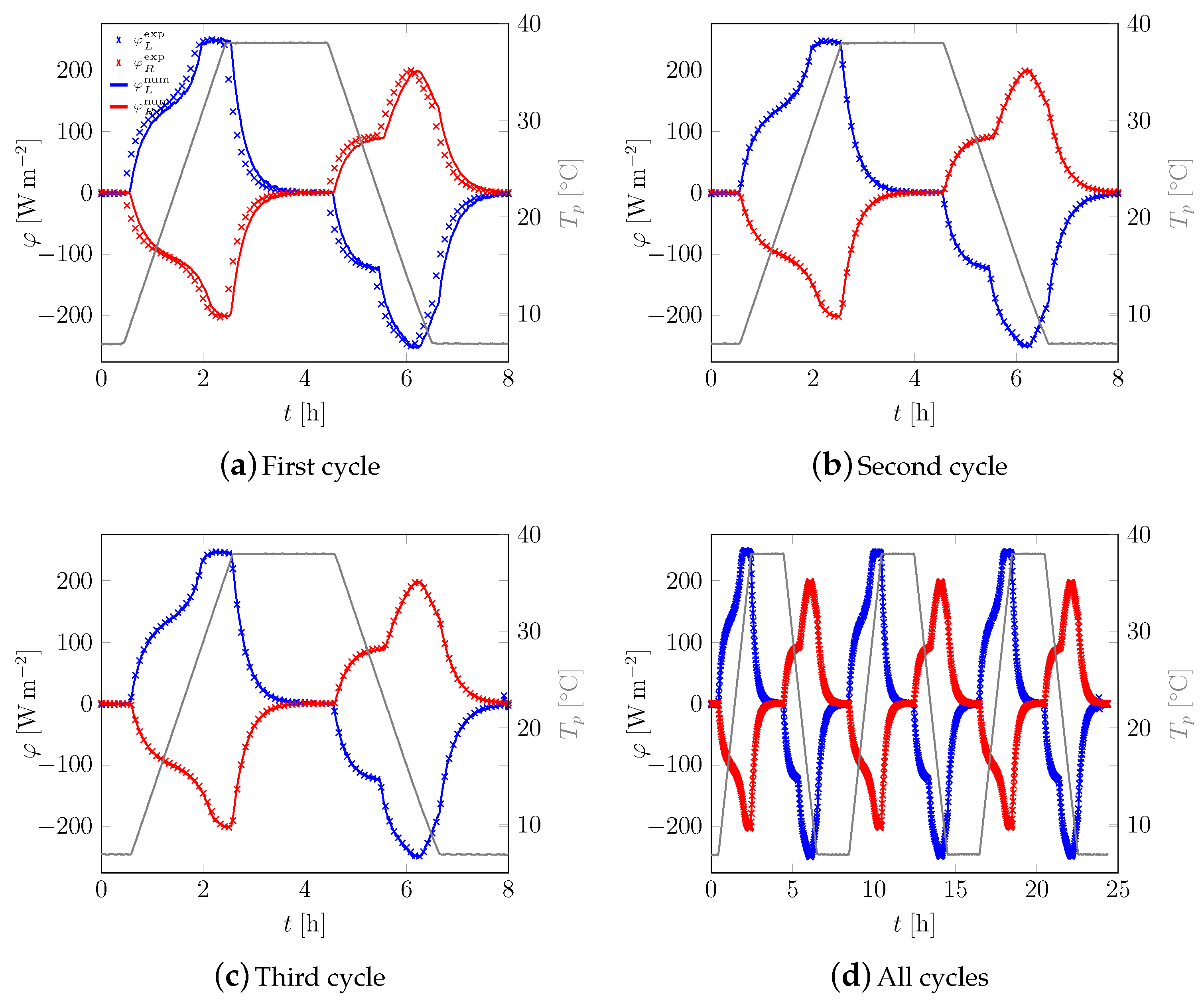
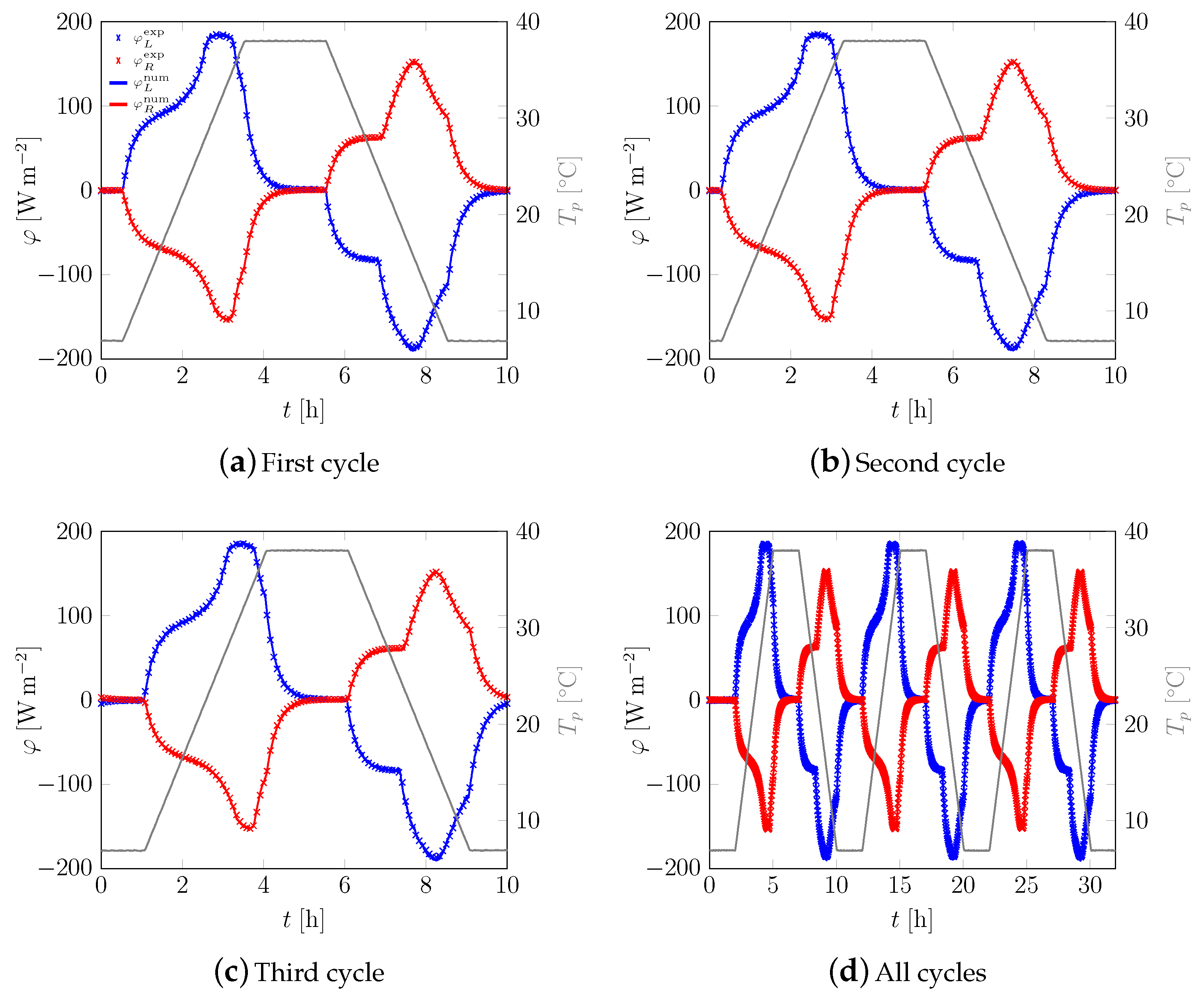
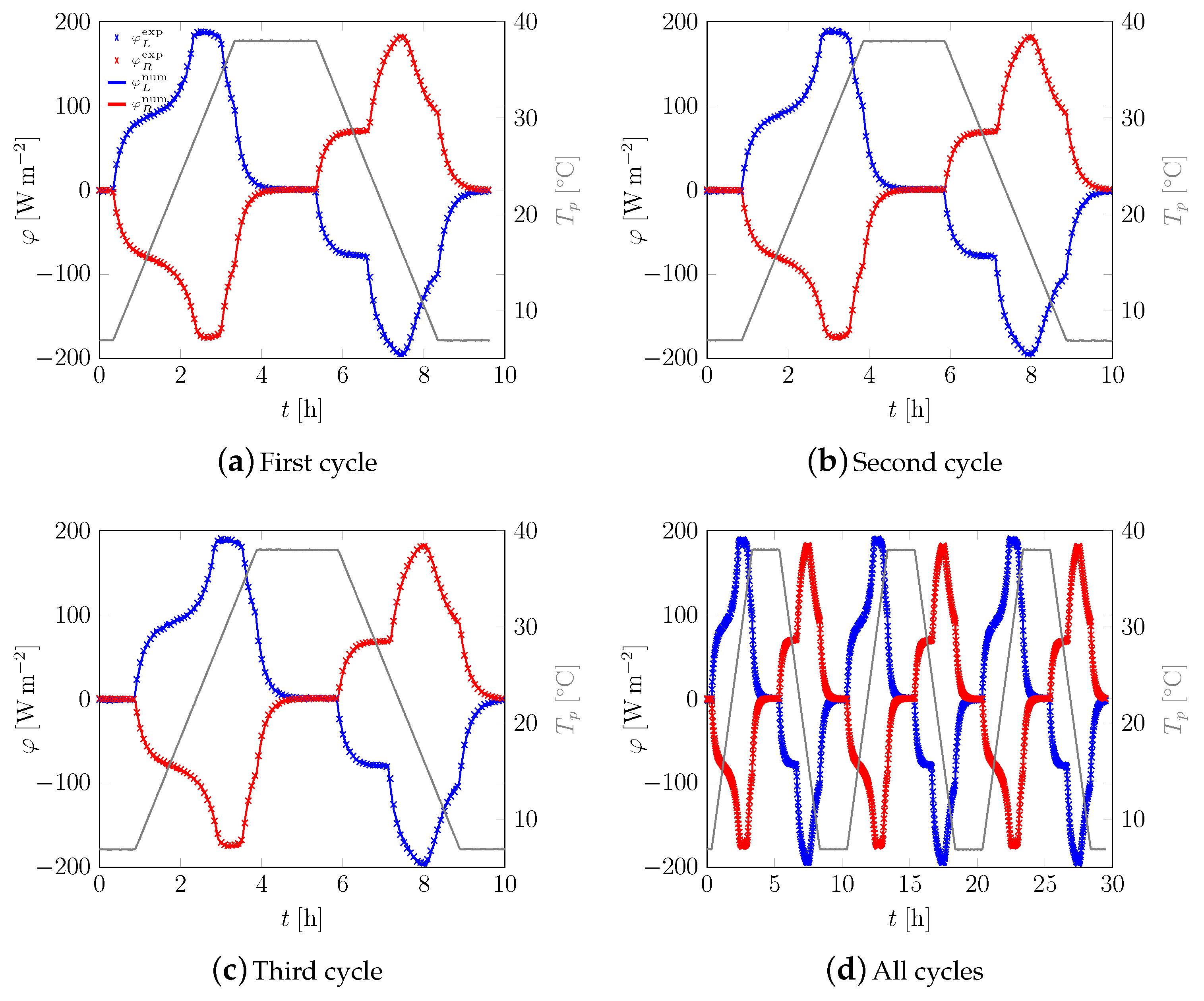
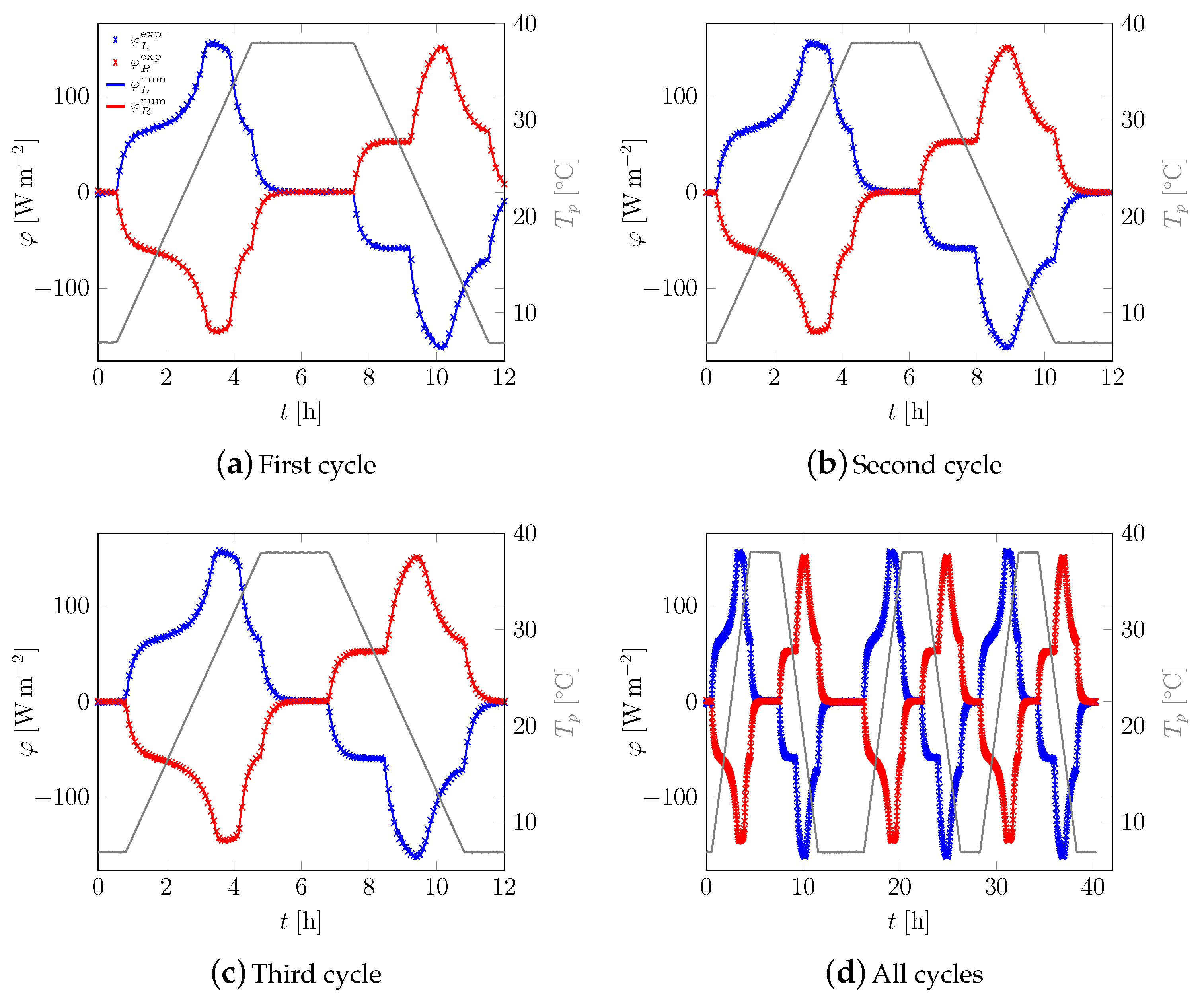
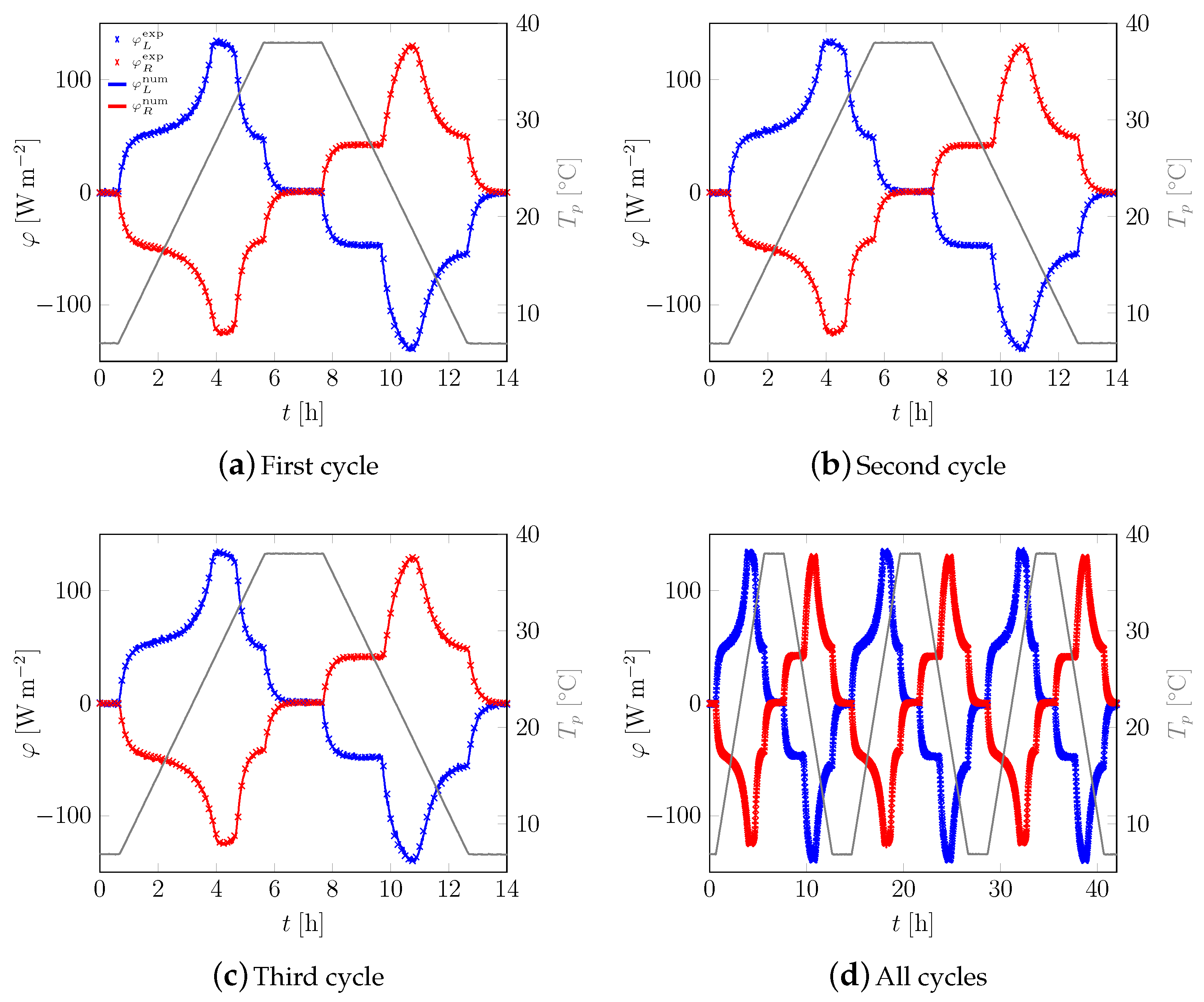
3.1. First Sample
3.2. All Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Latin symbols | |
| c | specific heat capacity, |
| D | duration, |
| e | thickness, |
| f | cost function, |
| h | specific enthalpy, |
| L | latent heat, |
| set of parameters, − | |
| R | thermal resistance, |
| T | temperature, |
| t | time, or min |
| vector of coordinates, | |
| Y | mass fraction of PCM, − |
| Greek symbols | |
| error, % | |
| heat flux, | |
| thermal conductivity, | |
| density, | |
| liquid fraction, − | |
| Subscripts and superscripts | |
| pure component (solvent phase) | |
| execution | |
| experimental | |
| L | left |
| l | liquid |
| M | melting |
| numerical | |
| p | exchange plate |
| R | right |
| s | solid |
| w | weight |
| Acronyms | |
| DSC | differential scanning calorimetry |
| GPS | generalized pattern search |
| PCM | phase change material |
Appendix A. Additional Data
| D | f | RL | RR | λs | λl | cs | cl | TM | LM | tex | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 22,577 | 1.098 · 10−2 | 4.638 · 10−3 | 0.606 | 0.6 | 1094 | 1094 | 25.57 | 27.18 | 12,322 | 0:22:39 | |
| 2 h | 22,596 | 1.085 · 10−2 | 4.39 · 10−3 | 0.594 | 0.589 | 1094 | 1094 | 25.57 | 27.17 | 12,317 | 1:05:41 |
| 23,028 | 1.084 · 10−2 | 4.39 · 10−3 | 0.594 | 0.589 | 1100 | 1094 | 25.58 | 27.17 | 12,267 | 1:12:19 | |
| 68,175 | 1.084 · 10−2 | 4.412 · 10−3 | 0.594 | 0.589 | 1094 | 1094 | 25.57 | 27.17 | 12,322 | 0:56:43 | |
| 20,403 | 1.125 · 10−2 | 4.737 · 10−3 | 0.594 | 0.589 | 1089 | 1089 | 25.57 | 27.17 | 12,478 | 2:05:37 | |
| 3 h | 19,899 | 1.154 · 10−2 | 5.028 · 10−3 | 0.611 | 0.6 | 1089 | 1089 | 25.58 | 27.19 | 12,461 | 2:06:14 |
| 19,774 | 1.139 · 10−2 | 4.918 · 10−3 | 0.606 | 0.594 | 1094 | 1094 | 25.54 | 27.09 | 12,294 | 2:05:01 | |
| 59,337 | 1.139 · 10−2 | 4.891 · 10−3 | 0.606 | 0.594 | 1089 | 1094 | 25.56 | 27.14 | 12,400 | 1:45:29 | |
| 18,095 | 1.111 · 10−2 | 5 · 10−3 | 0.6 | 0.589 | 1094 | 1094 | 25.53 | 27.06 | 12,328 | 2:27:47 | |
| 4 h | 18,642 | 1.111 · 10−2 | 4.918 · 10−3 | 0.594 | 0.583 | 1089 | 1094 | 25.54 | 27.11 | 12,422 | 0:44:17 |
| 18,230 | 1.125 · 10−2 | 5 · 10−3 | 0.6 | 0.589 | 1094 | 1094 | 25.53 | 27.07 | 12,328 | 2:50:45 | |
| 55,793 | 1.111 · 10−2 | 4.891 · 10−3 | 0.594 | 0.583 | 1089 | 1094 | 25.54 | 27.11 | 12,422 | 2:05:56 | |
| 17,821 | 1.2 · 10−2 | 5.455 · 10−3 | 0.606 | 0.594 | 1094 | 1094 | 25.53 | 27.03 | 12,300 | 4:12:57 | |
| 5 h | 17,924 | 1.2 · 10−2 | 5.455 · 10−3 | 0.606 | 0.594 | 1094 | 1094 | 25.52 | 27.01 | 12,283 | 4:31:10 |
| 17,518 | 1.216 · 10−2 | 5.625 · 10−3 | 0.617 | 0.6 | 1089 | 1094 | 25.54 | 27.08 | 12,361 | 1:20:27 | |
| 52,845 | 1.2 · 10−2 | 5.455 · 10−3 | 0.606 | 0.594 | 1094 | 1094 | 25.52 | 27 | 12,261 | 3:28:31 |
| D | f | RL | RR | λs | λl | cs | cl | TM | LM | tex | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30,313 | 1.159 · 10−2 | 3.127 · 10−2 | 0.435 | 0.442 | 1096 | 1035 | 25.59 | 27.31 | 18,135 | 01:43:24 | |
| 2 h | 23,390 | 1.224 · 10−2 | 3.14 · 10−2 | 0.431 | 0.436 | 1067 | 1037 | 25.57 | 27.28 | 18,233 | 00:50:28 |
| 21,859 | 1.24 · 10−2 | 3.127 · 10−2 | 0.431 | 0.436 | 1069 | 1036 | 25.58 | 27.28 | 18,168 | 00:59:37 | |
| 85,878 | 1.204 · 10−2 | 3.127 · 10−2 | 0.431 | 0.437 | 1074 | 1035 | 25.59 | 27.34 | 18,279 | 02:16:47 | |
| 20,785 | 1.304 · 10−2 | 3.176 · 10−2 | 0.427 | 0.437 | 1062 | 1035 | 25.7 | 27.51 | 18,352 | 06:43:38 | |
| 3 h | 20,551 | 1.321 · 10−2 | 3.189 · 10−2 | 0.427 | 0.436 | 1065 | 1031 | 25.68 | 27.43 | 18,241 | 08:27:05 |
| 21,697 | 1.33 · 10−2 | 3.189 · 10−2 | 0.428 | 0.438 | 1067 | 1030 | 25.69 | 27.46 | 18,265 | 10:36:33 | |
| 61,906 | 1.313 · 10−2 | 3.176 · 10−2 | 0.426 | 0.436 | 1066 | 1032 | 25.69 | 27.47 | 18,273 | 08:26:34 | |
| 19,051 | 1.399 · 10−2 | 3.24 · 10−2 | 0.427 | 0.443 | 1062 | 1031 | 25.76 | 27.59 | 18,343 | 13:48:26 | |
| 4h | 19,216 | 1.397 · 10−2 | 3.24 · 10−2 | 0.428 | 0.442 | 1067 | 1031 | 25.74 | 27.54 | 18,207 | 11:21:10 |
| 19,216 | 1.397 · 10−2 | 3.24 · 10−2 | 0.428 | 0.442 | 1067 | 1031 | 25.74 | 27.54 | 18,207 | 08:59:56 | |
| 57,062 | 1.399 · 10−2 | 3.24 · 10−2 | 0.427 | 0.442 | 1065 | 1031 | 25.75 | 27.57 | 18,270 | 08:19:15 | |
| 18,094 | 1.434 · 10−2 | 3.266 · 10−2 | 0.429 | 0.443 | 1073 | 1035 | 25.77 | 27.54 | 17,978 | 10:20:06 | |
| 5 h | 19,170 | 1.467 · 10−2 | 3.293 · 10−2 | 0.433 | 0.45 | 1066 | 1032 | 25.78 | 27.61 | 18,190 | 06:04:20 |
| 18,679 | 1.473 · 10−2 | 3.306 · 10−2 | 0.433 | 0.451 | 1063 | 1031 | 25.78 | 27.63 | 18,260 | 06:47:33 | |
| 56,006 | 1.465 · 10−2 | 3.293 · 10−2 | 0.433 | 0.449 | 1064 | 1032 | 25.78 | 27.63 | 18,222 | 07:19:31 |
| D | f | RL | RR | λs | λl | cs | cl | TM | LM | tex | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 17,511 | 7.893 · 10−3 | 1.364 · 10−2 | 0.384 | 0.384 | 1154 | 1088 | 25.52 | 26.91 | 20,679 | 00:54:19 | |
| 2 h | 18,899 | 7.825 · 10−3 | 1.364 · 10−2 | 0.384 | 0.384 | 1151 | 1084 | 25.52 | 26.94 | 20,778 | 04:15:25 |
| 21,183 | 7.826 · 10−3 | 1.364 · 10−2 | 0.382 | 0.382 | 1137 | 1084 | 25.54 | 27.02 | 21,027 | 3:43:08 | |
| 57,935 | 7.895 · 10−3 | 1.364 · 10−2 | 0.384 | 0.384 | 1150 | 1087 | 25.52 | 26.93 | 20,757 | 8:31:31 | |
| 21,014 | 8.333 · 10−3 | 1.429 · 10−2 | 0.384 | 0.387 | 1132 | 1081 | 25.72 | 27.37 | 21,336 | 18:28:41 | |
| 3 h | 20,915 | 8.257 · 10−3 | 1.429 · 10−2 | 0.382 | 0.387 | 1142 | 1081 | 25.63 | 27.19 | 21,094 | 41:58:24 |
| 23,850 | 8.333 · 10−3 | 1.429 · 10−2 | 0.384 | 0.387 | 1153 | 1081 | 25.62 | 27.1 | 20,848 | 10:45:50 | |
| 66,027 | 8.333 · 10−3 | 1.429 · 10−2 | 0.384 | 0.389 | 1144 | 1083 | 25.66 | 27.21 | 21,052 | 40:53:30 | |
| 28,680 | 8.571 · 10−3 | 1.452 · 10−2 | 0.382 | 0.384 | 1144 | 1081 | 25.67 | 27.26 | 21,053 | 42:29:36 | |
| 4 h | 26,641 | 8.654 · 10−3 | 1.452 · 10−2 | 0.384 | 0.387 | 1151 | 1081 | 25.67 | 27.23 | 20,983 | 108:57:29 |
| 25,323 | 8.571 · 10−3 | 1.452 · 10−2 | 0.382 | 0.387 | 1144 | 1082 | 25.67 | 27.26 | 21,067 | 32:22:59 | |
| 80,789 | 8.571 · 10−3 | 1.452 · 10−2 | 0.382 | 0.387 | 1146 | 1081 | 25.67 | 27.26 | 21,079 | 59:49:49 | |
| 28,322 | 8.824 · 10−3 | 1.475 · 10−2 | 0.384 | 0.387 | 1160 | 1086 | 25.67 | 27.16 | 20,682 | 9:59:39 | |
| 5 h | 26,548 | 8.738 · 10−3 | 1.475 · 10−2 | 0.382 | 0.387 | 1159 | 1084 | 25.67 | 27.2 | 20,763 | 44:44:31 |
| 27,889 | 8.654 · 10−3 | 1.475 · 10−2 | 0.382 | 0.387 | 1163 | 1087 | 25.67 | 27.16 | 20,587 | 13:43:08 | |
| 80,419 | 8.738 · 10−3 | 1.475 · 10−2 | 0.382 | 0.387 | 1147 | 1086 | 25.69 | 27.28 | 21,000 | 74:28:11 |



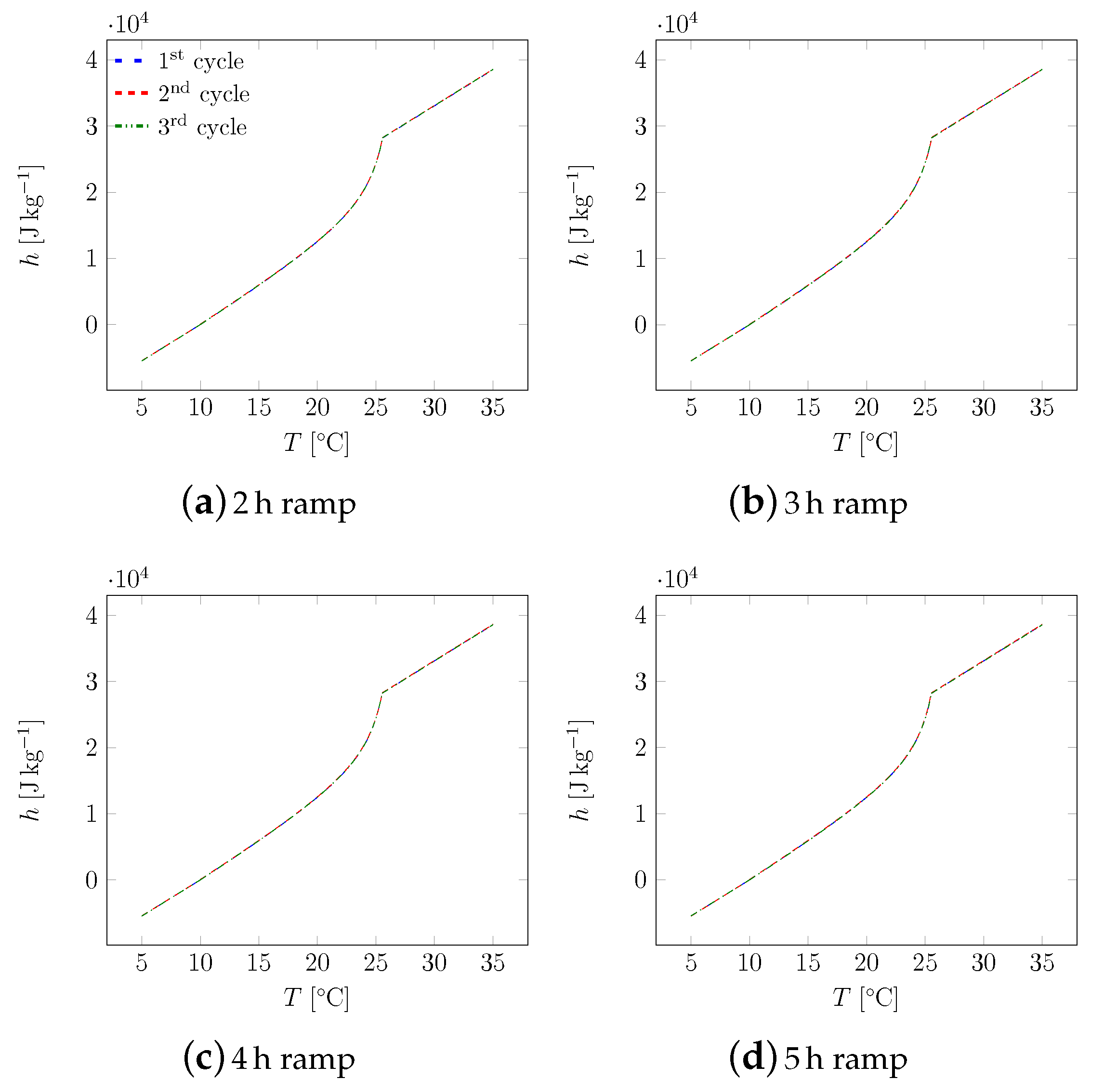
References
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sustain. Energy Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef]
- Serrano, S.; Ürge-Vorsatz, D.; Barreneche, C.; Palacios, A.; Cabeza, L.F. Heating and cooling energy trends and drivers in Europe. Energy 2017, 119, 425–434. [Google Scholar] [CrossRef]
- Zhu, N.; Ma, Z.; Wang, S. Dynamic characteristics and energy performance of buildings using phase change materials: A review. Energy Convers. Manag. 2009, 50, 3169–3181. [Google Scholar] [CrossRef]
- Tyagi, V.; Kaushik, S.; Tyagi, S.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Advanced energy storage materials for building applications and their thermal performance characterization: A review. Renew. Sustain. Energy Rev. 2016, 57, 916–928. [Google Scholar] [CrossRef]
- Kasaeian, A.; bahrami, L.; Pourfayaz, F.; Khodabandeh, E.; Yan, W.M. Experimental studies on the applications of PCMs and nano-PCMs in buildings: A critical review. Energy Build. 2017, 154, 96–112. [Google Scholar] [CrossRef]
- Cabeza, L.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Heier, J.; Bales, C.; Martin, V. Combining thermal energy storage with buildings—A review. Renew. Sustain. Energy Rev. 2015, 42, 1305–1325. [Google Scholar] [CrossRef]
- Stritih, U.; Butala, V. Energy saving in building with PCM cold storage. Int. J. Energy Res. 2007, 31, 1532–1544. [Google Scholar] [CrossRef]
- Waqas, A.; Din, Z.U. Phase change material (PCM) storage for free cooling of buildings—A review. Renew. Sustain. Energy Rev. 2013, 18, 607–625. [Google Scholar] [CrossRef]
- Kamali, S. Review of free cooling system using phase change material for building. Energy Build. 2014, 80, 131–136. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Islam, M.; Pandey, A.; Hasanuzzaman, M.; Rahim, N. Recent progresses and achievements in photovoltaic-phase change material technology: A review with special treatment on photovoltaic thermal-phase change material systems. Energy Convers. Manag. 2016, 126, 177–204. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R. Solar water heaters with phase change material thermal energy storage medium: A review. Renew. Sustain. Energy Rev. 2009, 13, 2119–2125. [Google Scholar] [CrossRef]
- Abokersh, M.H.; Osman, M.; El-Baz, O.; El-Morsi, M.; Sharaf, O. Review of the phase change material (PCM) usage for solar domestic water heating systems (SDWHS). Int. J. Energy Res. 2017, 42, 329–357. [Google Scholar] [CrossRef]
- Moreno, P.; Solé, C.; Castell, A.; Cabeza, L.F. The use of phase change materials in domestic heat pump and air-conditioning systems for short term storage: A review. Renew. Sustain. Energy Rev. 2014, 39, 1–13. [Google Scholar] [CrossRef]
- Kapsalis, V.; Karamanis, D. Solar thermal energy storage and heat pumps with phase change materials. Appl. Therm. Eng. 2016, 99, 1212–1224. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197–2209. [Google Scholar] [CrossRef]
- Baetens, R.; Petter Jelle, B.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar]
- Zhou, D.; Zhao, C.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Abuelnuor, A.A.A.; Omara, A.A.M.; Saqr, K.M.; Elhag, I.H.I. Improving indoor thermal comfort by using phase change materials: A review. Int. J. Energy Res. 2018, 42, 2084–2103. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Use of phase change materials for thermal energy storage in concrete: An overview. Constr. Build. Mater. 2013, 46, 55–62. [Google Scholar] [CrossRef]
- Rao, V.V.; Parameshwaran, R.; Ram, V.V. PCM-mortar based construction materials for energy efficient buildings: A review on research trends. Energy Build. 2018, 158, 95–122. [Google Scholar] [CrossRef]
- Hunger, M.; Entrop, A.; Mandilaras, I.; Brouwers, H.; Founti, M. The behavior of self-compacting concrete containing micro-encapsulated Phase Change Materials. Cement Concr. Compos. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Joulin, A.; Younsi, Z.; Zalewski, L.; Lassue, S.; Rousse, D.R.; Cavrot, J.P. Experimental and numerical investigation of a phase change material: Thermal-energy storage and release. Appl. Energy 2011, 88, 2454–2462. [Google Scholar]
- Meshgin, P.; Xi, Y.; Li, Y. Utilization of phase change materials and rubber particles to improve thermal and mechanical properties of mortar. Constr. Build. Mater. 2012, 28, 713–721. [Google Scholar] [CrossRef]
- Sakulich, A.; Bentz, D. Incorporation of phase change materials in cementitious systems via fine lightweight aggregate. Constr. Build. Mater. 2012, 35, 483–490. [Google Scholar] [CrossRef]
- Barreneche, C.; Navarro, M.E.; Fernández, A.I.; Cabeza, L.F. Improvement of the thermal inertia of building materials incorporating PCM. Evaluation in the macroscale. Appl. Energy 2013, 109, 428–432. [Google Scholar] [CrossRef]
- Cheng, R.; Pomianowski, M.; Wang, X.; Heiselberg, P.; Zhang, Y. A new method to determine thermophysical properties of PCM-concrete brick. Appl. Energy 2013, 112, 988–998. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Tan, J. Heat storage properties of the cement mortar incorporated with composite phase change material. Appl. Energy 2013, 103, 393–399. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, G.; Wang, S.; Fang, X.; Liu, X. Thermal energy storage cement mortar containing n-octadecane/expanded graphite composite phase change material. Renew. Energy 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Eddhahak-Ouni, A.; Drissi, S.; Colin, J.; Neji, J.; Care, S. Experimental and multi-scale analysis of the thermal properties of Portland cement concretes embedded with microencapsulated Phase Change Materials (PCMs). Appl. Therm. Eng. 2014, 64, 32–39. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Zhang, Y. Preparation technology of phase change perlite and performance research of phase change and temperature control mortar. Energy Build. 2014, 85, 506–514. [Google Scholar] [CrossRef]
- Vieira, J.; Senff, L.; Gonçalves, H.; Silva, L.; Ferreira, V.; Labrincha, J. Functionalization of mortars for controlling the indoor ambient of buildings. Energy Build. 2014, 70, 224–236. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Z.; Shao, S.; Zheng, J. Experimental and numerical study on effective thermal conductivity of novel form-stable basalt fiber composite concrete with PCMs for thermal storage. Appl. Therm. Eng. 2014, 66, 156–161. [Google Scholar] [CrossRef]
- Lecompte, T.; Bideau, P.L.; Glouannec, P.; Nortershauser, D.; Masson, S.L. Mechanical and thermo-physical behaviour of concretes and mortars containing phase change material. Energy Build. 2015, 94, 52–60. [Google Scholar] [CrossRef]
- Haurie, L.; Serrano, S.; Bosch, M.; Fernandez, A.I.; Cabeza, L.F. Single layer mortars with microencapsulated PCM: Study of physical and thermal properties, and fire behaviour. Energy Build. 2016, 111, 393–400. [Google Scholar] [CrossRef]
- Li, T.; Yuan, Y.; Zhang, N. Thermal properties of phase change cement board with capric acid/expanded perlite form-stable phase change material. Adv. Mech. Eng. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Hawes, D.; Banu, D.; Feldman, D. Latent heat storage in concrete. Sol. Energy Mater. 1989, 19, 335–348. [Google Scholar] [CrossRef]
- Hawes, D.; Banu, D.; Feldman, D. Latent heat storage in concrete. II. Sol. Energy Mater. 1990, 21, 61–80. [Google Scholar] [CrossRef]
- Hawes, D.; Feldman, D.; Banu, D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Zhou, J.; Wu, K. Development of thermal energy storage concrete. Cement Concr. Res. 2004, 34, 927–934. [Google Scholar] [CrossRef]
- Bentz, D.P.; Turpin, R. Potential applications of phase change materials in concrete technology. Cement Concr. Compos. 2007, 29, 527–532. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Jensen, R.L.; Cheng, R.; Zhang, Y. A new experimental method to determine specific heat capacity of inhomogeneous concrete material with incorporated microencapsulated-PCM. Cement Concr. Res. 2014, 55, 22–34. [Google Scholar] [CrossRef]
- Shadnia, R.; Zhang, L.; Li, P. Experimental study of geopolymer mortar with incorporated PCM. Constr. Build. Mater. 2015, 84, 95–102. [Google Scholar] [CrossRef]
- Kheradmand, M.; Azenha, M.; de Aguiar, J.L.; Krakowiak, K.J. Thermal behavior of cement based plastering mortar containing hybrid microencapsulated phase change materials. Energy Build. 2014, 84, 526–536. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Biçer, A.; Bilgin, C. Latent heat energy storage characteristics of building composites of bentonite clay and pumice sand with different organic PCMs. Int. J. Energy Res. 2014, 38, 1478–1491. [Google Scholar] [CrossRef]
- ASTM C1784. Standard Test Method for Using a Heat Flow Meter Apparatus for Measuring Thermal Storage Properties of Phase Change Materials and Products; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Shukla, N.; Kosny, J. DHFMA Method for Dynamic Thermal Property Measurement of PCM-integrated Building Materials. Curr. Sustain. Renew. Energy Rep. 2015, 2, 41–46. [Google Scholar]
- Albright, G.; Farid, M.; Al-Hallaj, S. Development of a model for compensating the influence of temperature gradients within the sample on DSC-results on phase change materials. J. Therm. Anal. Calorim. 2010, 101, 1155–1160. [Google Scholar]
- Franquet, E.; Gibout, S.; Bédécarrats, J.P.; Haillot, D.; Dumas, J.P. Inverse method for the identification of the enthalpy of phase change materials from calorimetry experiment. Thermochim. Acta 2012, 546, 61–80. [Google Scholar]
- Dumas, J.P.; Gibout, S.; Zalewski, L.; Johannes, K.; Franquet, E.; Lassue, S.; Bédécarrats, J.P.; Tittelein, P.; Kuznik, F. Interpretation of calorimetry experiments to characterise phase change materials. Int. J. Therm. Sci. 2014, 78, 48–55. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Gibout, S.; Franquet, E.; Haillot, D.; Bédécarrats, J.P.; Dumas, J.P. Challenges of the Usual Graphical Methods Used to Characterize Phase Change Materials by Differential Scanning Calorimetry. Appl. Sci. 2018, 8, 66. [Google Scholar] [CrossRef]
- Tittelein, P.; Gibout, S.; Franquet, E.; Johannes, K.; Zalewski, L.; Kuznik, F.; Dumas, J.P.; Lassue, S.; Bédécarrats, J.P.; David, D. Simulation of the thermal and energy behaviour of a composite material containing encapsulated-PCM: Influence of the thermodynamical modelling. Appl. Energy 2015, 140, 269–274. [Google Scholar] [CrossRef]
- Kuznik, F.; Johannes, K.; Franquet, E.; Zalewski, L.; Gibout, S.; Tittelein, P.; Dumas, J.P.; David, D.; Bédécarrats, J.P.; Lassue, S. Impact of the enthalpy function on the simulation of a building with phase change material wall. Energy Build. 2016, 126, 220–229. [Google Scholar] [CrossRef]
- Tittelein, P.; Gibout, S.; Franquet, E.; Zalewski, L.; Defer, D. Identification of thermal properties and thermodynamic model for a cement mortar containing PCM by using inverse method. Energy Procedia 2015, 78, 1696–1701. [Google Scholar] [CrossRef]
- Castellon, C.; Gunther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC—A study of different measurement procedures and their accuracy. Int. J. Energy Res. 2008, 32, 1258–1265. [Google Scholar]
- Günther, E.; Hiebler, S.; Mehling, H.; Redlich, R. Enthalpy of phase change materials as a function of temperature: required accuracy and suitable measurments methods. Int. J. Thermophys. 2009, 30, 1257–1269. [Google Scholar]
- Franquet, E.; Gibout, S.; Tittelein, P.; Zalewski, L.; Dumas, J.P. Experimental and theoretical analysis of a cement mortar containing microencapsulated PCM. Appl. Therm. Eng. 2014, 73, 30–38. [Google Scholar]
- Leclercq, D.; Thery, P. Apparatus for simultaneous temperature and heat-flow measurements under transient conditions. Rev. Sci. Instrum. 1983, 54, 374–380. [Google Scholar]
- Cherif, Y.; Joulin, A.; Zalewski, L.; Lassue, S. Superficial heat transfer by forced convection and radiation in a horizontal channel. Int. J. Therm. Sci. 2009, 48, 1696–1706. [Google Scholar]
- Joulin, A.; Zalewski, L.; Lassue, S.; Naji, H. Experimental investigation of thermal characteristics of a mortar with or without a micro-encapsulated phase change material. Appl. Therm. Eng. 2014, 66, 171–180. [Google Scholar] [CrossRef]
- Beckermann, C.; Viskanta, R. Natural convection solid/liquid phase change in porous media. Int. J. Heat Mass Transf. 1988, 31, 35–46. [Google Scholar]
- Beckermann, C.; Viskanta, R. Double-diffusive convection during dendritic solidification of a binary mixture. Phys. Chem. Hydrodyn. 1988, 10, 195–213. [Google Scholar]
- Ni, J.; Incropera, F. Extension of the continuum model for transport phenomena occurring during metal alloy solidification—I. The conservation equations. Int. J. Heat Mass Transf. 1995, 38, 1271–1284. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar]
- Beck, J.; Arnold, K. Parameter Estimation in Engineering and Science; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Goldberg, D.E. Genetic Algorithms in Search, Optimization and Machine Learning; Addison Wesley: New York, NY, USA, 1989. [Google Scholar]
- Gosselin, L.; Tye-Gingras, M.; Mathieu-Potvin, F. Review of utilization of genetic algorithms in heat transfer problems. Int. J. Heat Mass Transf. 2009, 52, 2169–2188. [Google Scholar]
- Wetter, M. Design optimization with GenOpt. Build. Energy Simul. User News 2000, 21, 19–28. [Google Scholar]
- Wetter, M. GenOpt®—A Generic Optimization Program. In Proceedings of the Seventh International IBPSA Conference, Rio de Janeiro, Brazil, 13–15 August 2001; Volume 1, pp. 601–608. [Google Scholar]
- Wetter, M. Generic Optimization Program User Manual Version 3.0.0; Technical Report; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2009.
- Ventolà, L.; Vendrell, M.; Giraldez, P. Newly-designed traditional lime mortar with a phase change material as an additive. Constr. Build. Mater. 2013, 47, 1210–1216. [Google Scholar] [CrossRef]
- Lee, T.; Hawes, D.; Banu, D.; Feldman, D. Control aspects of latent heat storage and recovery in concrete. Sol. Energy Mater. Sol. Cells 2000, 62, 217–237. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Sá, A.V.; Azenha, M.; de Sousa, H.; Samagaio, A. Thermal enhancement of plastering mortars with Phase Change Materials: Experimental and numerical approach. Energy Build. 2012, 49, 16–27. [Google Scholar] [CrossRef]
- Snoeck, D.; Priem, B.; Dubruel, P.; De Belie, N. Encapsulated Phase-Change Materials as additives in cementitious materials to promote thermal comfort in concrete constructions. Mater. Struct. 2016, 49, 225–239. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, F.; Cui, H.Z.; Chen, D.Z.; Ouyang, X.; Xu, S.Z.; Wang, J.X.; Huang, Y.T.; Zuo, J.D.; Tang, J.N. A novel phase-change cement composite for thermal energy storage: Fabrication, thermal and mechanical properties. Appl. Energy 2016, 170, 130–139. [Google Scholar] [CrossRef]
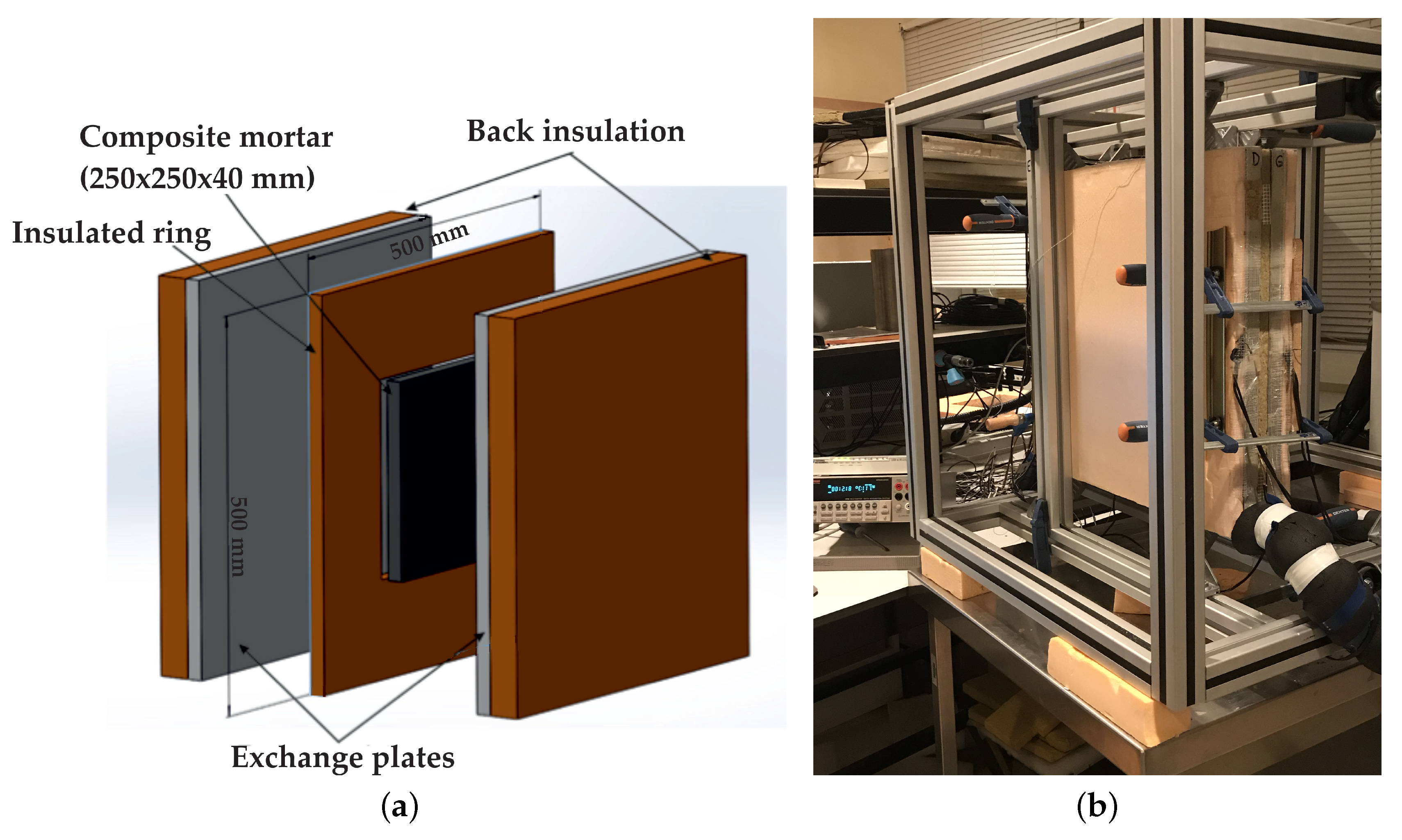
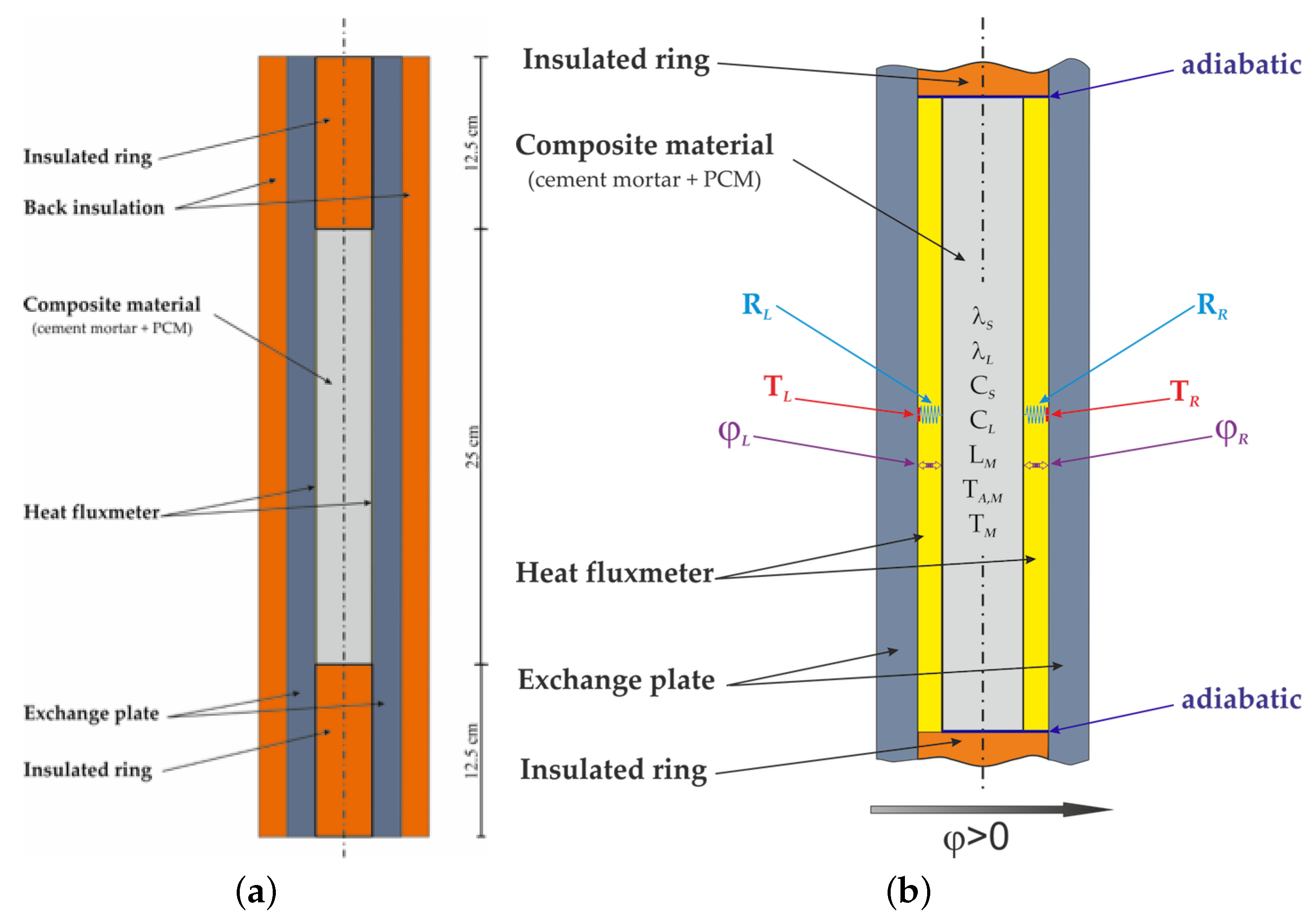
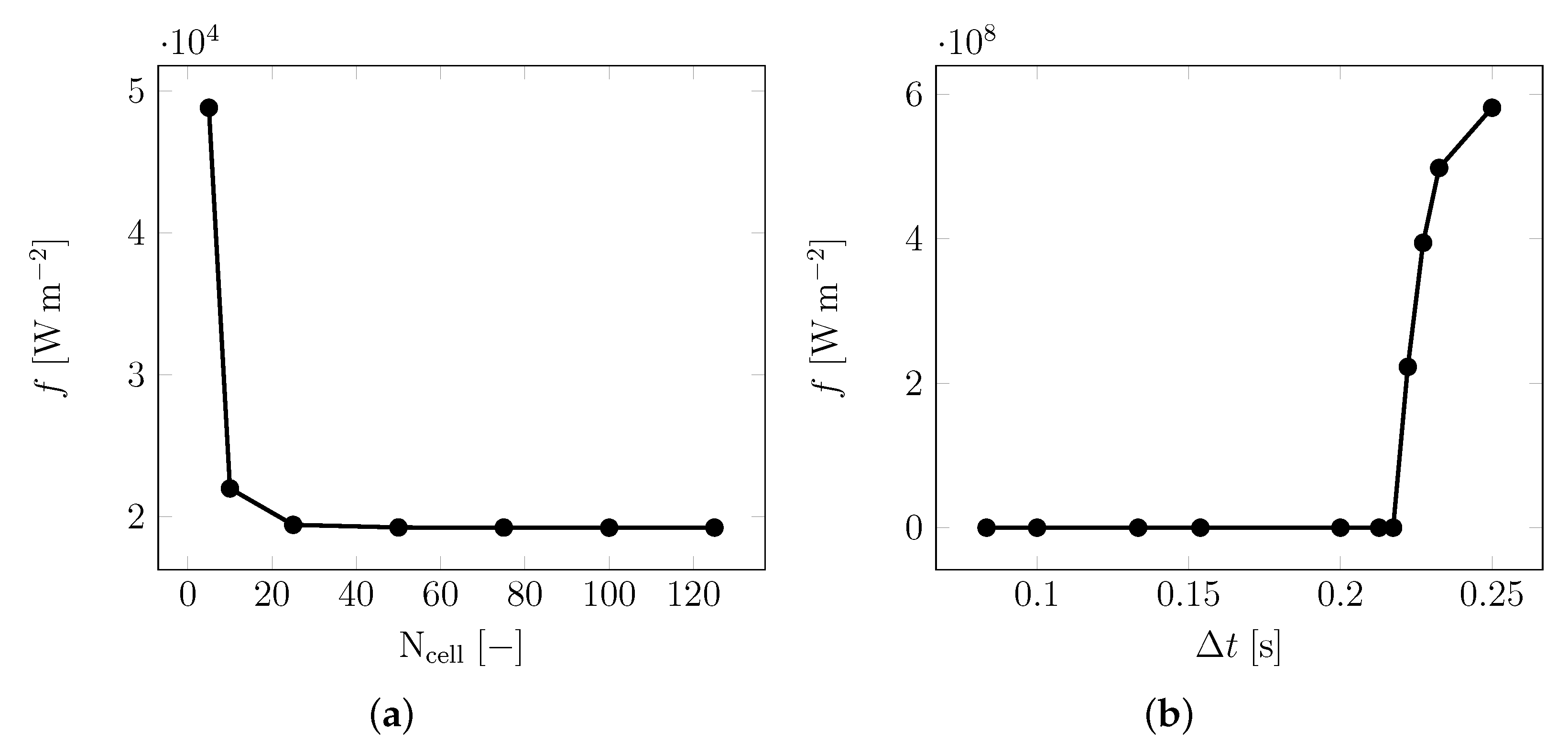
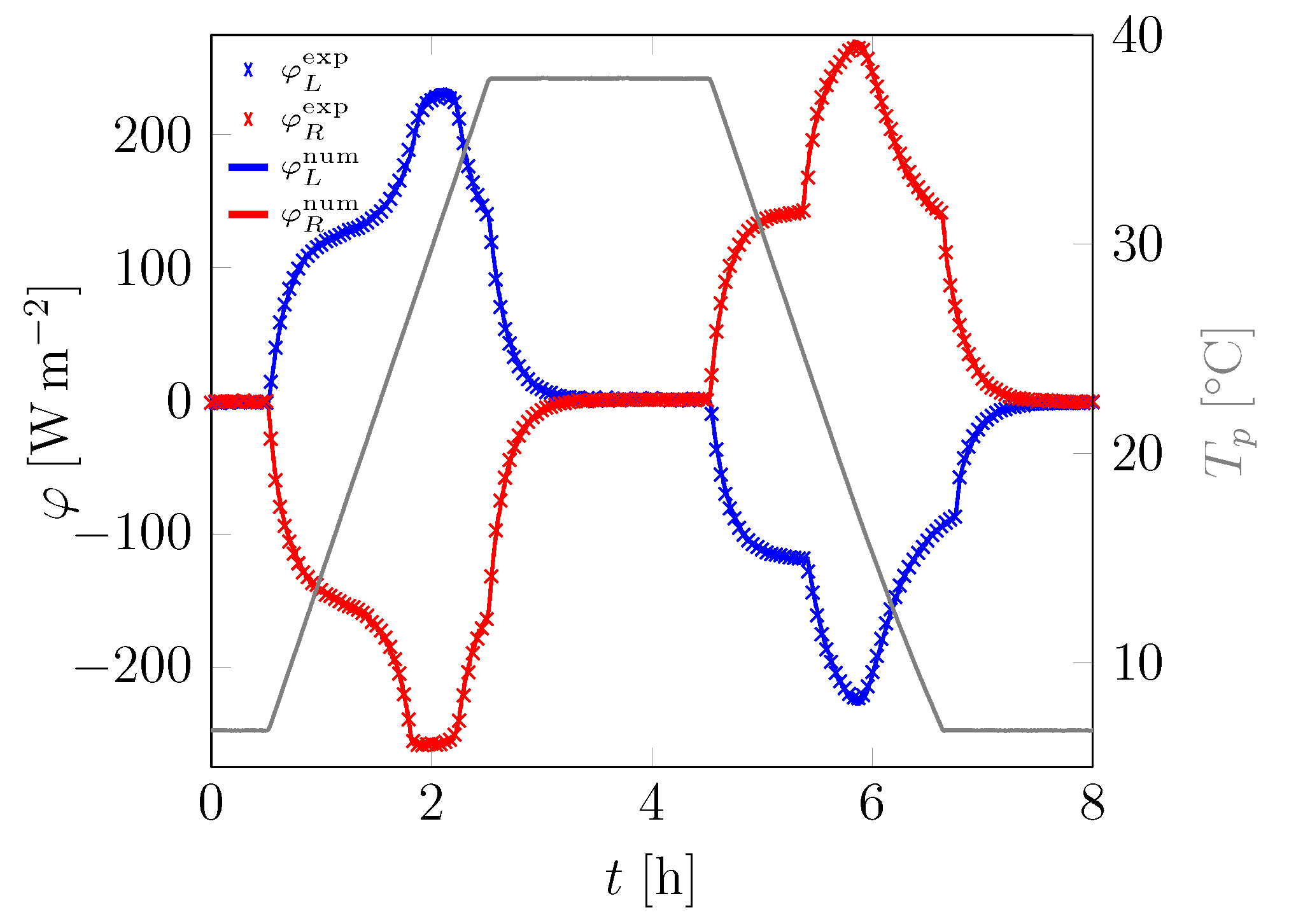
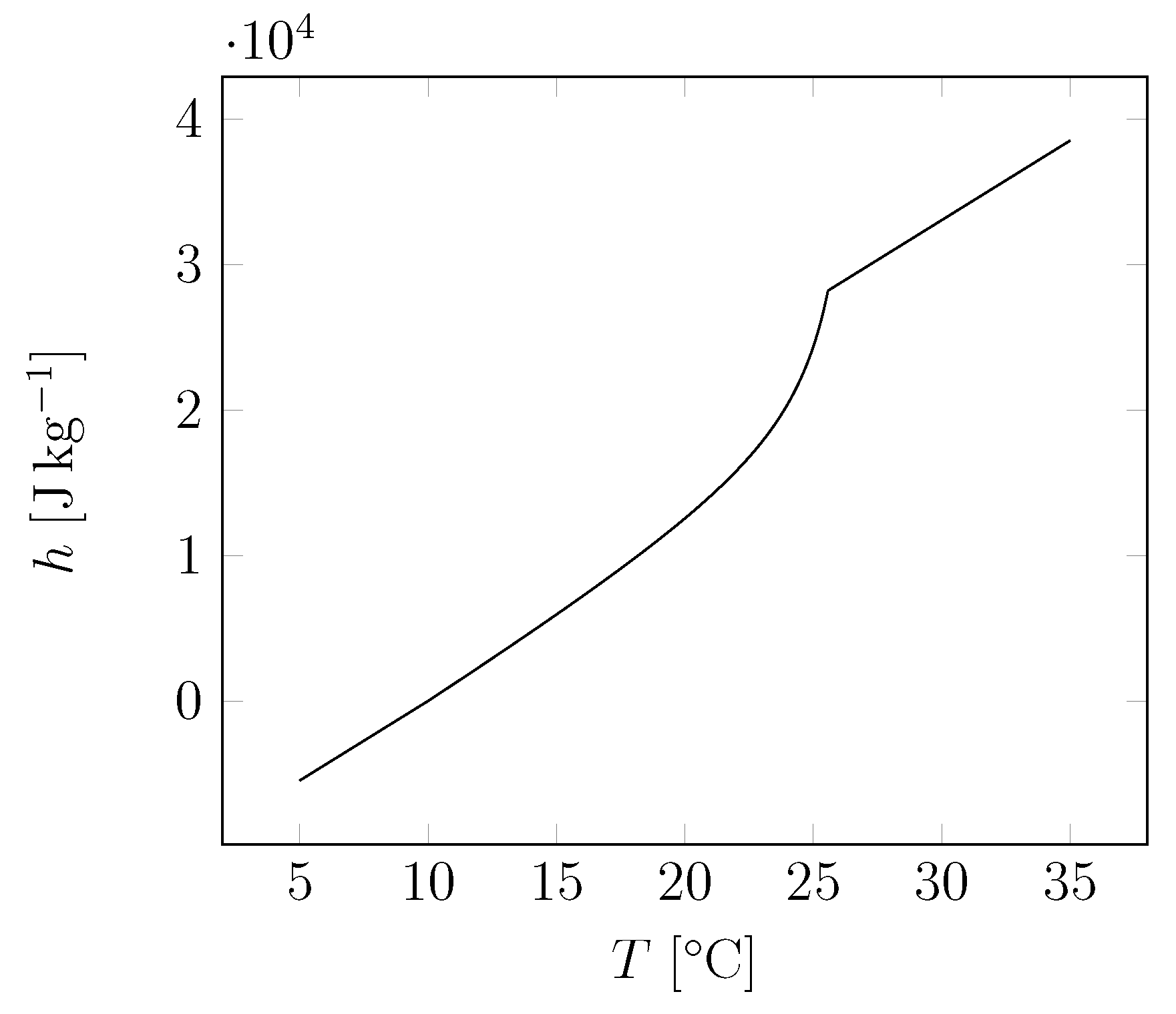

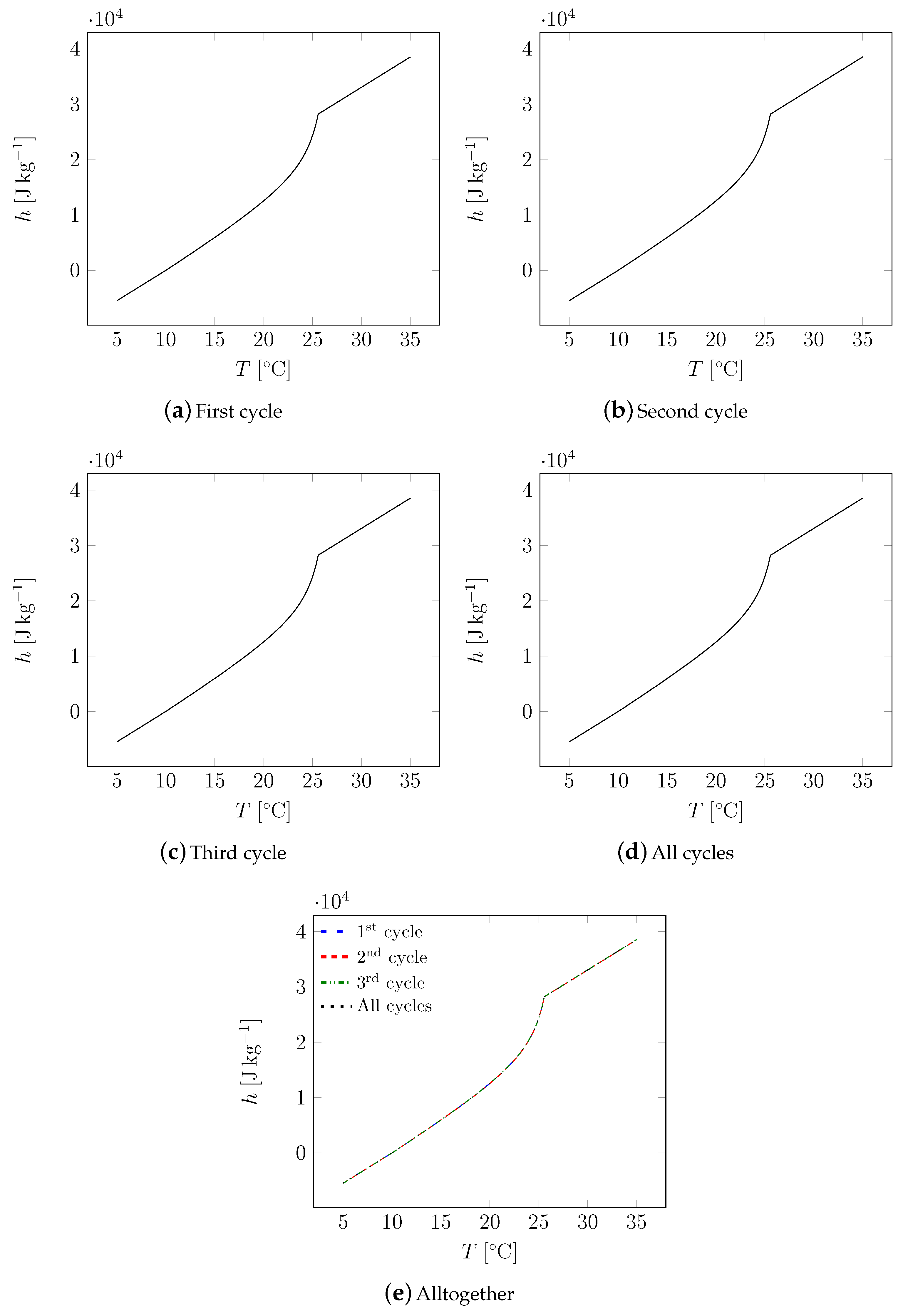
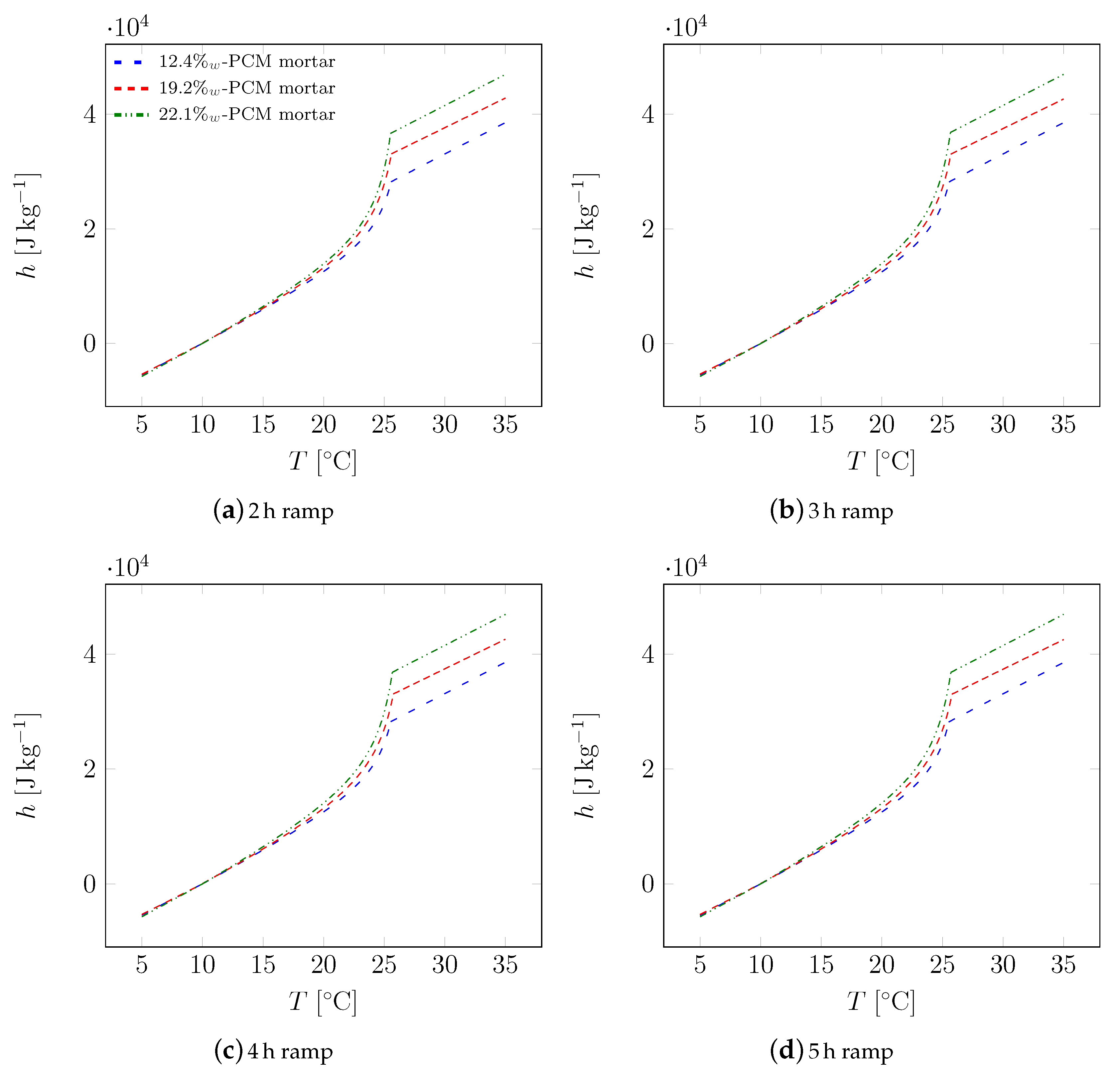
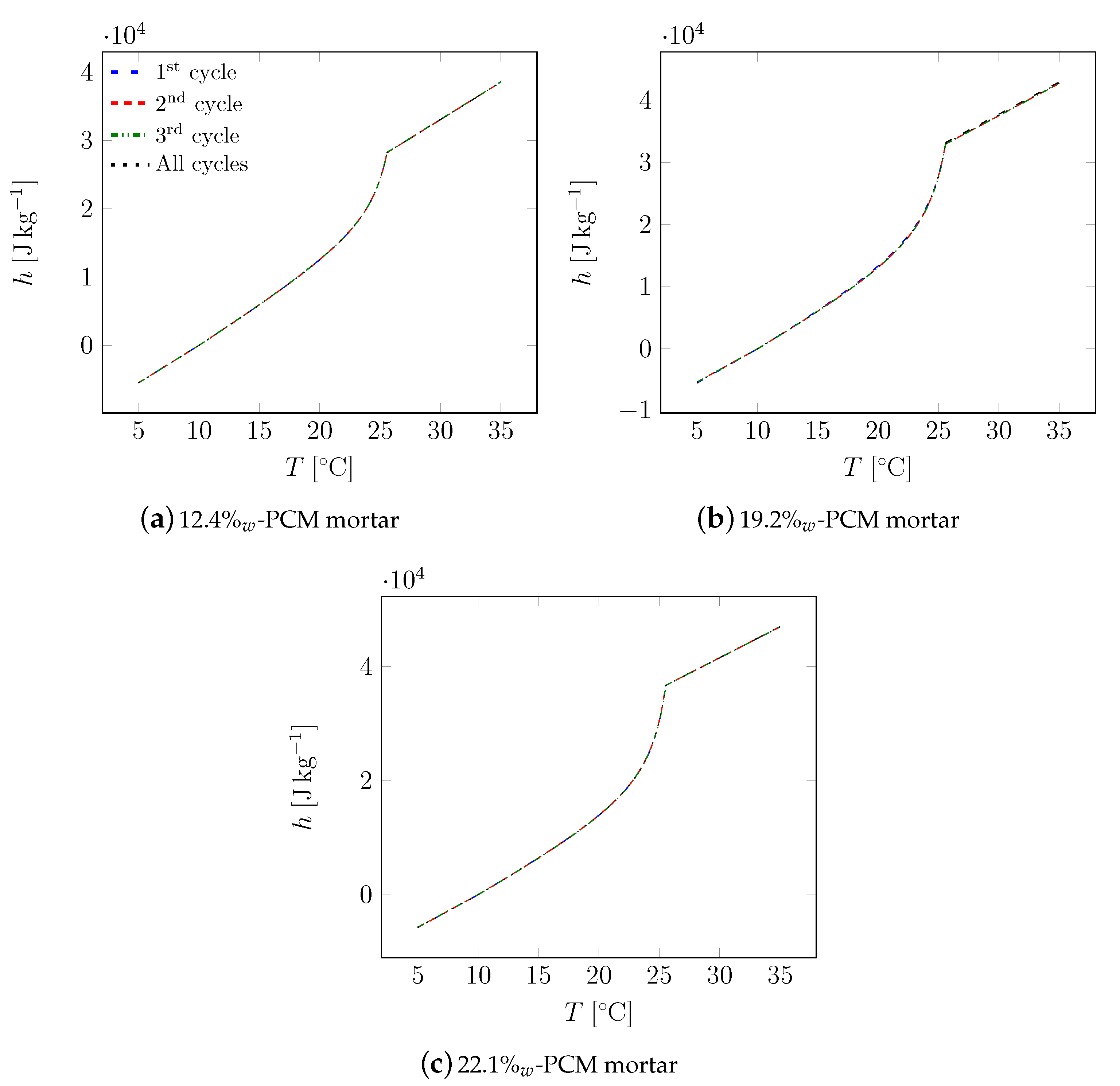
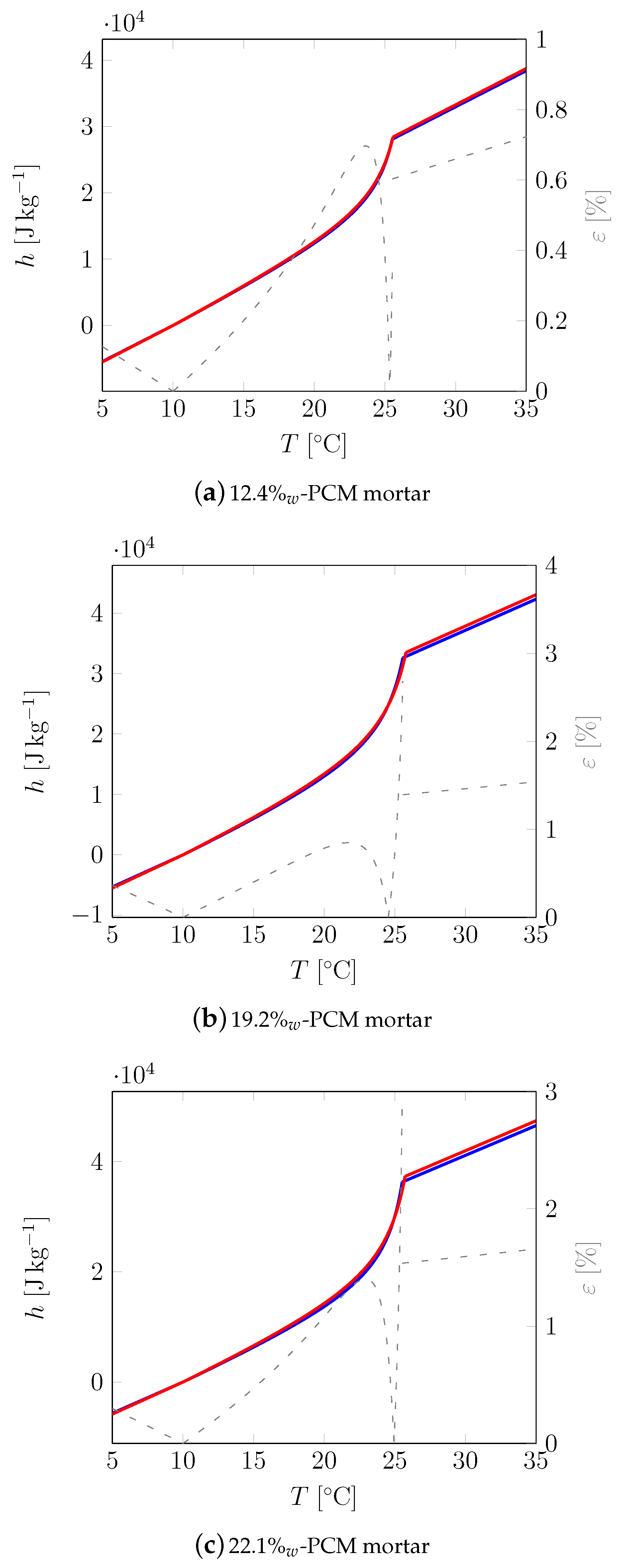
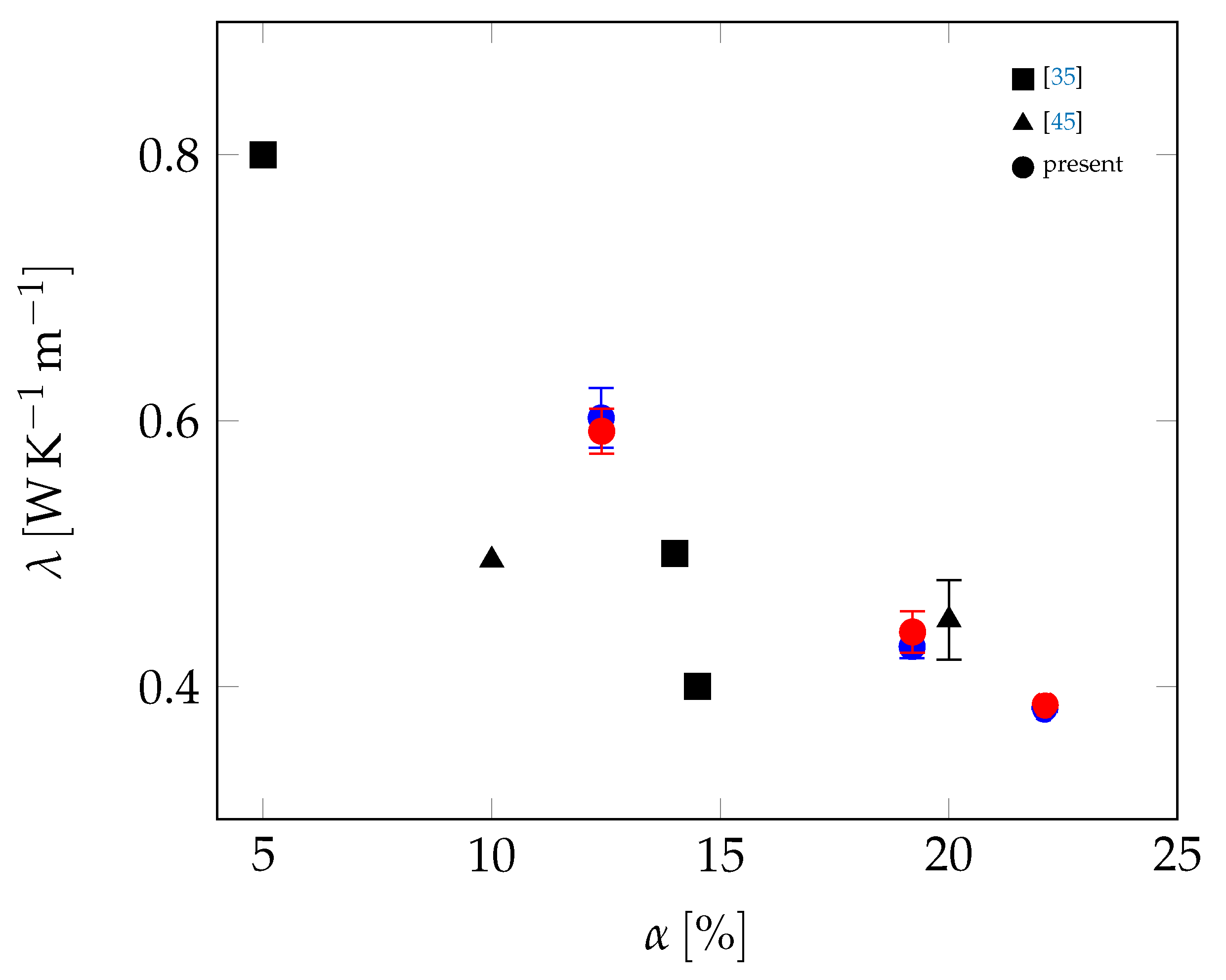
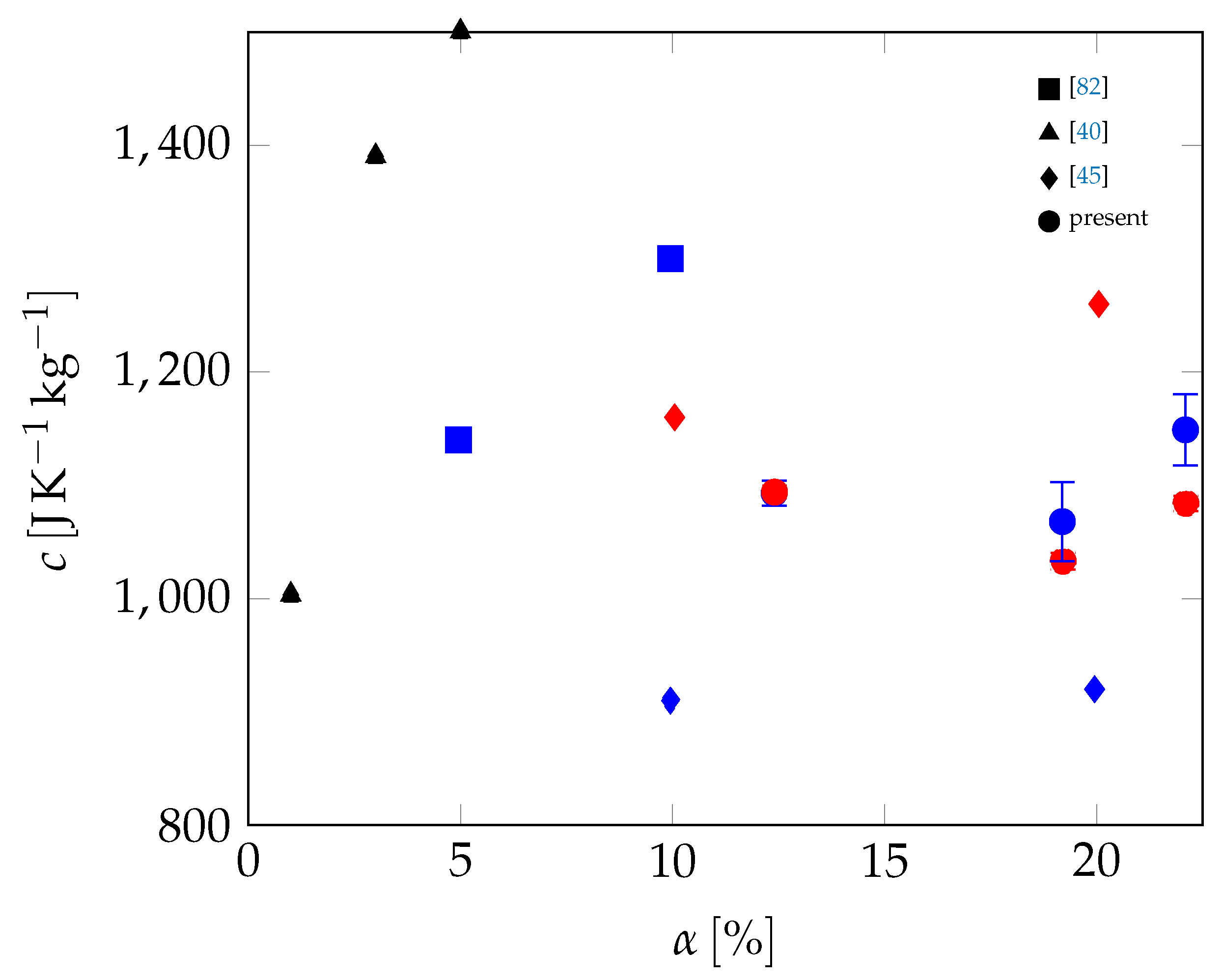
| Material | Sand | Cement | Water | PCM |
|---|---|---|---|---|
| 1600 | 3060 | 1000 | 300 |
| Mortar | (kg m) | |
|---|---|---|
| 12.4 | 0.039 | 1412 |
| 19.2 | 0.0388 | 1257 |
| 22.1 | 0.0385 | 1231 |
| Mortar | Stat. | RL (W−1K m2) | RR (W−1K m2) | λs (W K−1 m−1) | λl (W K−1 m−1) | cs (J K−1 kg−1) | cl (J K−1 kg−1) | TM (°C) | (°C) | LM (J kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Min | 1.216 · 10−2 | 5.625 · 10−3 | 0.594 | 0.583 | 1089 | 1089 | 25.52 | 27 | 12,26 | |
| 12.4%w | Max | 1.084 · 10−2 | 4.39 · 10−3 | 0.617 | 0.6 | 1100 | 1094 | 25.58 | 27.19 | 12,478 |
| Avg. | 1.135 · 10−2 | 4.921 · 10−3 | 0.602 | 0.592 | 1093 | 1094 | 25.55 | 27.11 | 12,348 | |
| ε | 12.16% | 28.12% | 3.74% | 2.86% | 1.02% | 0.51% | 0.22% | 0.7% | 1.77% | |
| Min | 1.473 · 10−2 | 3.306 · 10−2 | 0.426 | 0.436 | 1062 | 1030 | 25.57 | 27.28 | 17,978 | |
| 19.2%w | Max | 1.159 · 10−2 | 3.127 · 10−2 | 0.435 | 0.451 | 1096 | 1037 | 25.78 | 27.63 | 18,352 |
| Avg. | 1.338 · 10−2 | 3.21 · 10−2 | 0.43 | 0.441 | 1068 | 1033 | 25.7 | 27.48 | 18,227 | |
| ε | 27.09% | 5.71% | 2.03% | 3.54% | 3.26% | 0.72% | 0.85% | 1.3% | 2.08% | |
| Min | 8.824 · 10−3 | 1.475 · 10−2 | 0.382 | 0.382 | 1132 | 1081 | 25.52 | 26.91 | 20,587 | |
| 22.1%w | Max | 7.826 · 10−3 | 1.364 · 10−2 | 0.384 | 0.389 | 1163 | 1088 | 25.72 | 27.37 | 21,336 |
| Avg. | 8.362 · 10−3 | 1.429 · 10−2 | 0.383 | 0.386 | 1149 | 1084 | 25.63 | 27.15 | 20,924 | |
| ε | 12.75% | 8.2% | 0.58% | 1.74% | 2.75% | 0.62% | 0.78% | 1.69% | 3.64% |
| Fatty Acids | Alcohols | Ethers | Paraffins | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Micronal | Mikrathermic | Devan | ||||||||||||||||||||||
| Reference | Hexadecanoic Acid (Butyl Palmitate) | Octadecanoic Acid (Butyl Stearate) | Decanoic Acid (Capric Acid) | Dodecanoic Acid (Lauric Acid) | Tetradecanoic Acid (Myristic Acid) | Dodecanol | Tetradecanol | PEG400 | PEG600 | PEG1000 | DS5001 | DS5001 X | DS 5008 X | DS 5039 X | DS5040X | D18 | D24 | D28 | Microtek MPCM28 | MC18 | MC24 | MC28 | Octadecan | Unicere 55 |
| [47] | ✓ | ✓ | ✓ | |||||||||||||||||||||
| [48] | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||
| [49] | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||
| [83] | ✓ | ✓ | ||||||||||||||||||||||
| [51] | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||
| [84] | ✓ | |||||||||||||||||||||||
| [31] | ✓ | |||||||||||||||||||||||
| [85] | ✓ | |||||||||||||||||||||||
| [33] | ✓ | |||||||||||||||||||||||
| [34] | ✓ | ✓ | ||||||||||||||||||||||
| [35] | ✓ | |||||||||||||||||||||||
| [37] | ✓ | |||||||||||||||||||||||
| [82] | ✓ | |||||||||||||||||||||||
| [39] | ✓ | |||||||||||||||||||||||
| [40] | ✓ | |||||||||||||||||||||||
| [41] | ✓(eutectic mixture) | |||||||||||||||||||||||
| [54] | ✓ | ✓ | ✓ | |||||||||||||||||||||
| [52] | ✓ | |||||||||||||||||||||||
| [42] | ✓ | |||||||||||||||||||||||
| [44] | ✓ | |||||||||||||||||||||||
| [53] | ✓ | |||||||||||||||||||||||
| [45] | ✓ | |||||||||||||||||||||||
| [86] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| [87] | ✓ | |||||||||||||||||||||||
| [46] | ✓ | |||||||||||||||||||||||
| 12.4 | 19.2 | 22.1 | |
|---|---|---|---|
| Avg. (J kg) | 99,696 | 94,930 | 94,792 |
| 0.7% | 4.11% | 4.25% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewski, L.; Franquet, E.; Gibout, S.; Tittelein, P.; Defer, D. Efficient Characterization of Macroscopic Composite Cement Mortars with Various Contents of Phase Change Material. Appl. Sci. 2019, 9, 1104. https://doi.org/10.3390/app9061104
Zalewski L, Franquet E, Gibout S, Tittelein P, Defer D. Efficient Characterization of Macroscopic Composite Cement Mortars with Various Contents of Phase Change Material. Applied Sciences. 2019; 9(6):1104. https://doi.org/10.3390/app9061104
Chicago/Turabian StyleZalewski, Laurent, Erwin Franquet, Stéphane Gibout, Pierre Tittelein, and Didier Defer. 2019. "Efficient Characterization of Macroscopic Composite Cement Mortars with Various Contents of Phase Change Material" Applied Sciences 9, no. 6: 1104. https://doi.org/10.3390/app9061104
APA StyleZalewski, L., Franquet, E., Gibout, S., Tittelein, P., & Defer, D. (2019). Efficient Characterization of Macroscopic Composite Cement Mortars with Various Contents of Phase Change Material. Applied Sciences, 9(6), 1104. https://doi.org/10.3390/app9061104







