Addition of Salt Ions before Spraying Improves Heat- and Cold-Induced Gel Properties of Soy Protein Isolate (SPI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soy Protein Isolate Spray Drying
2.3. Preparation of Heat- and Cold-Induced Gels
2.4. Determination of Gel Texture
2.5. Determination of Gel Whiteness
2.6. Measurement of Water Holding Capacity
2.7. Determination of the Molecular Forces in the Gels
2.8. Determination of the Gel Microstructure
2.9. Statistical Analysis
3. Results and Discussion
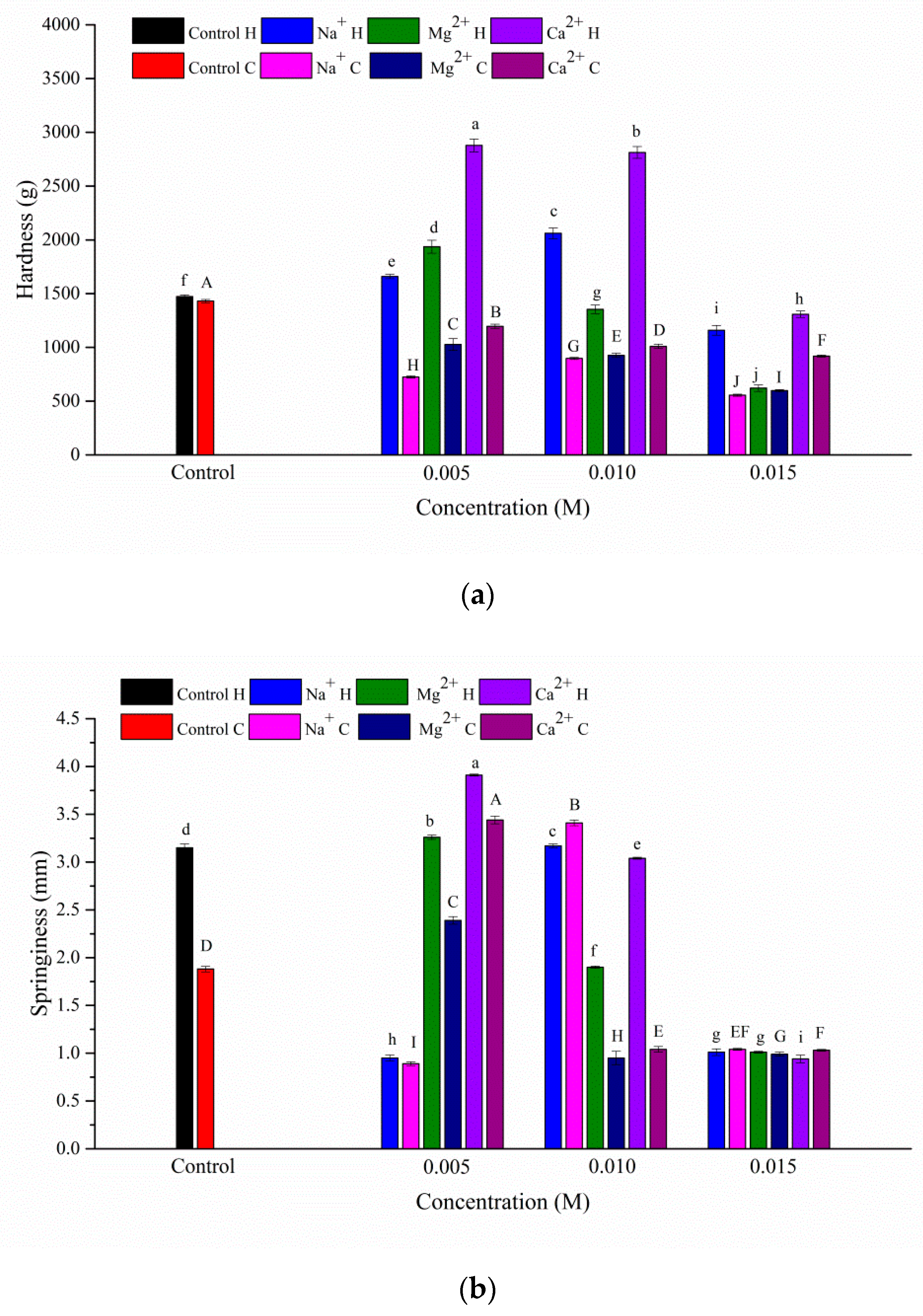
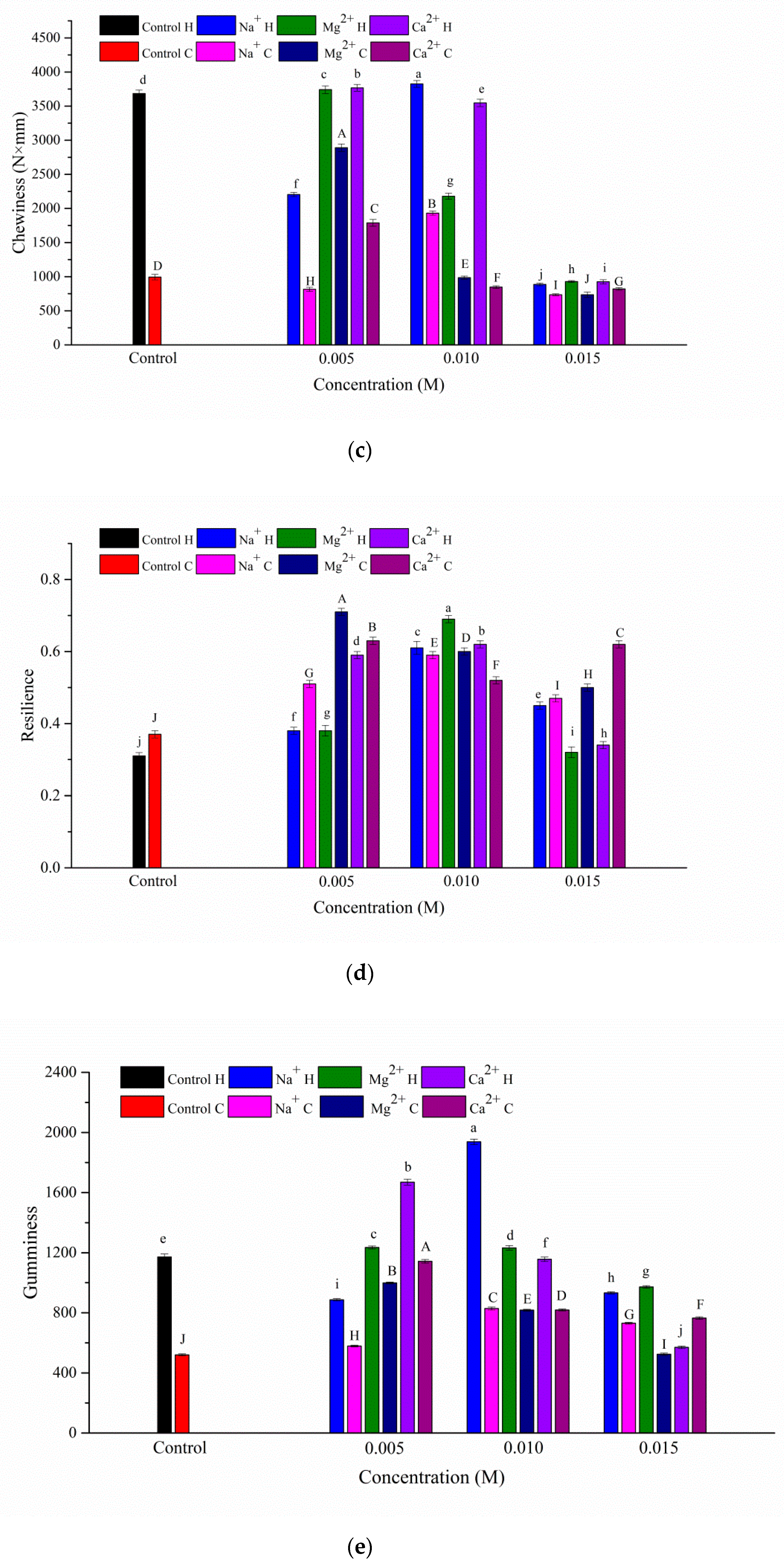
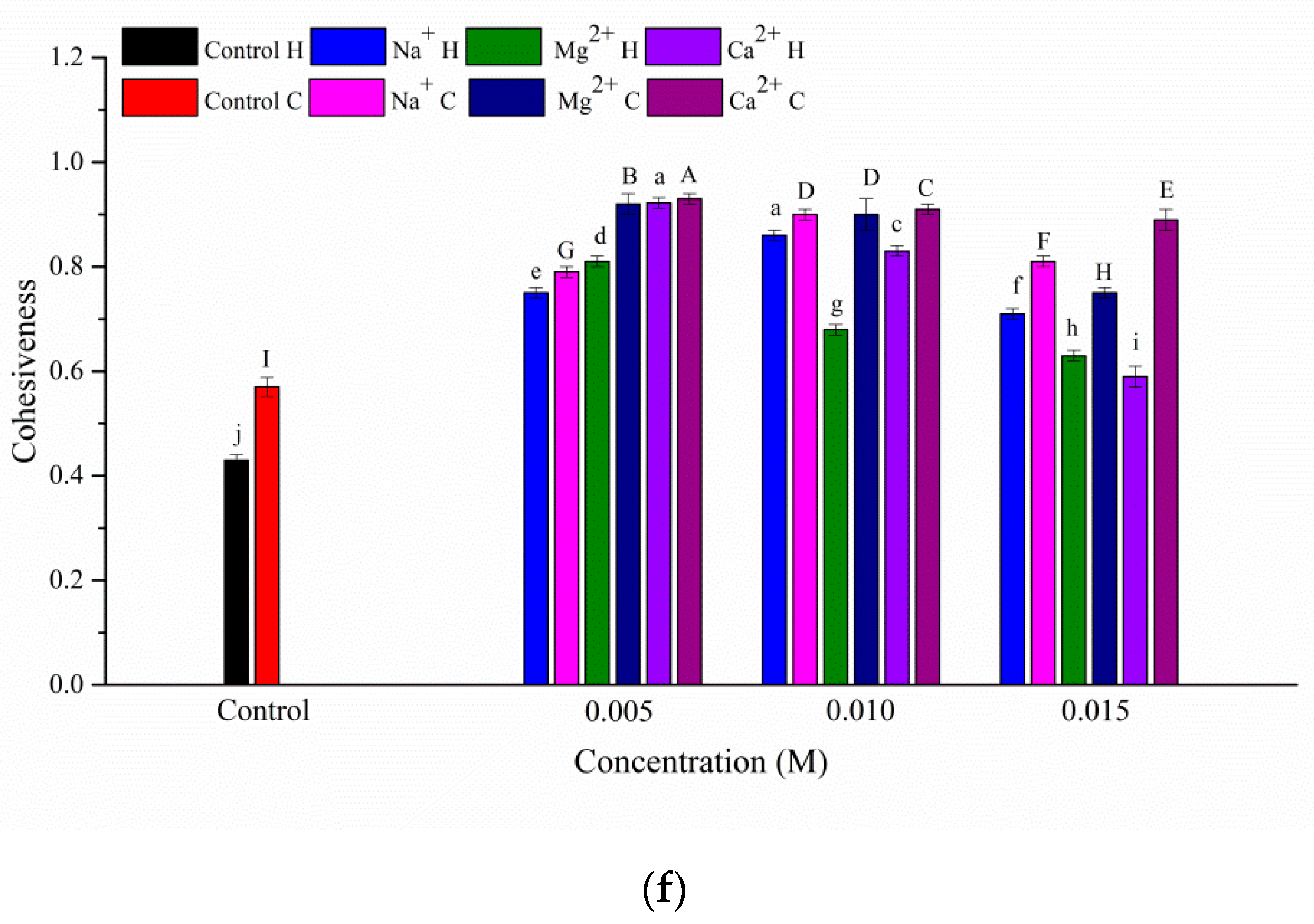
3.1. Texture Properties of the Gels
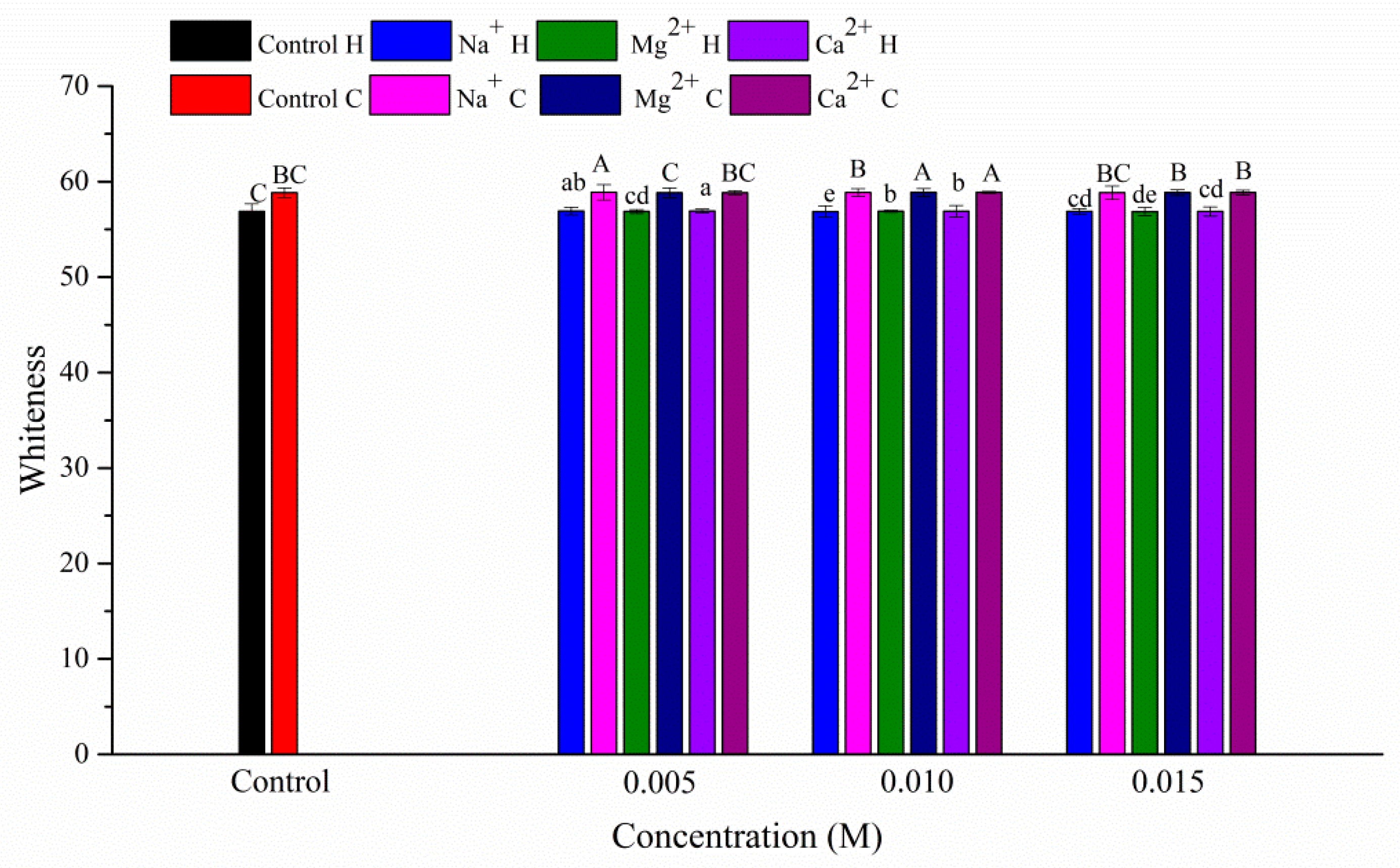
3.2. Whiteness of the Gels
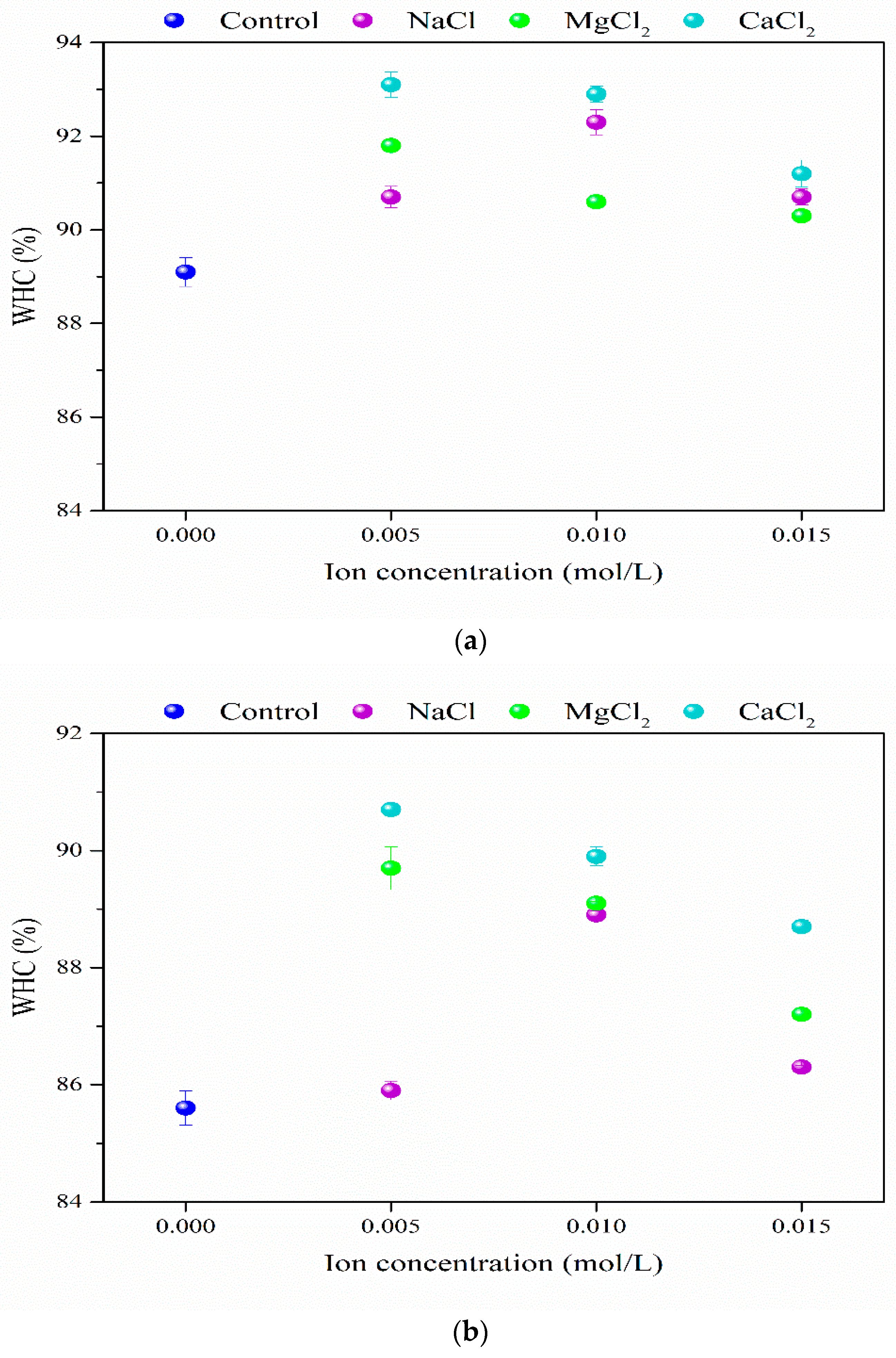
3.3. WHC
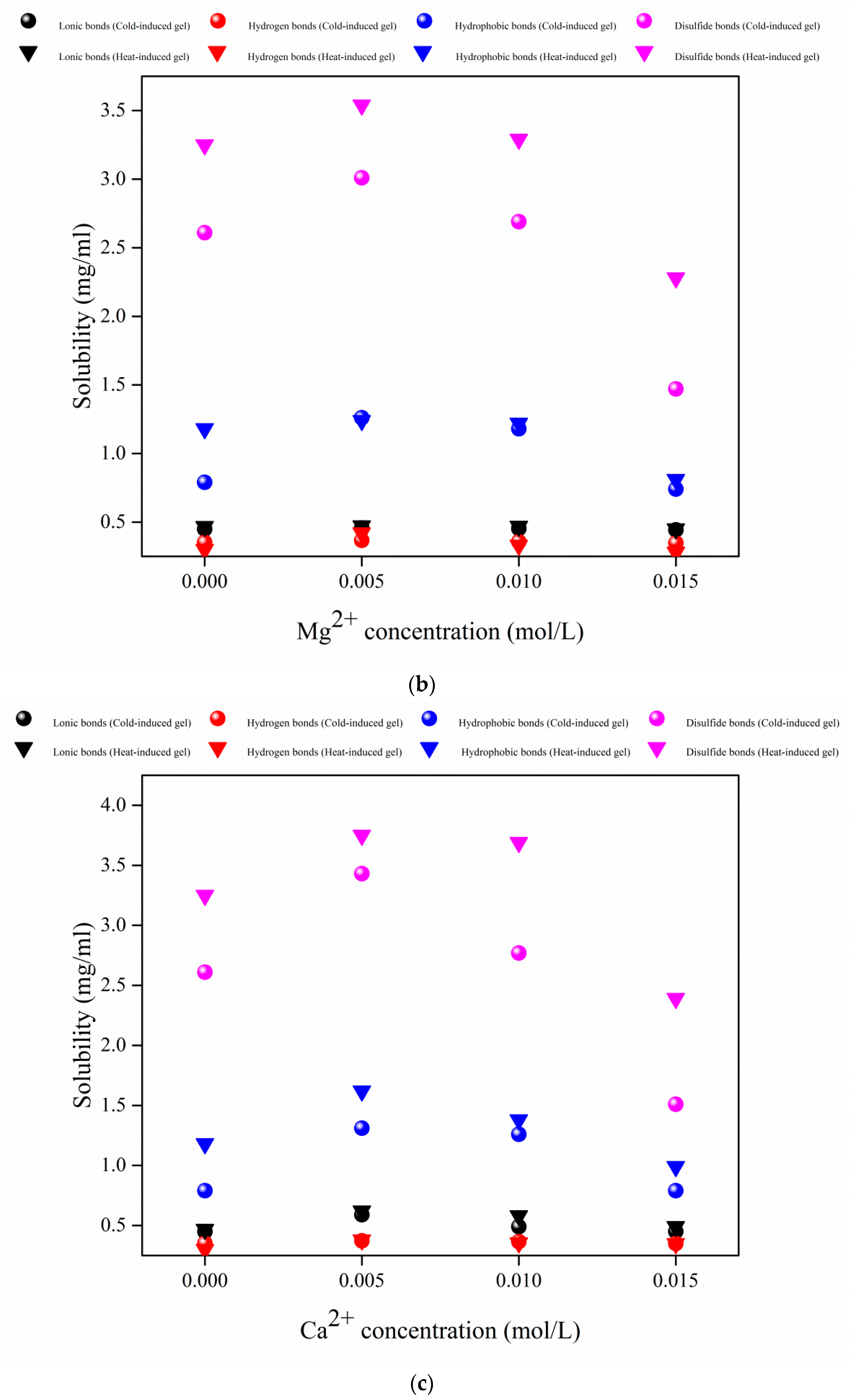
3.4. Chemical Interaction Forces Analysis
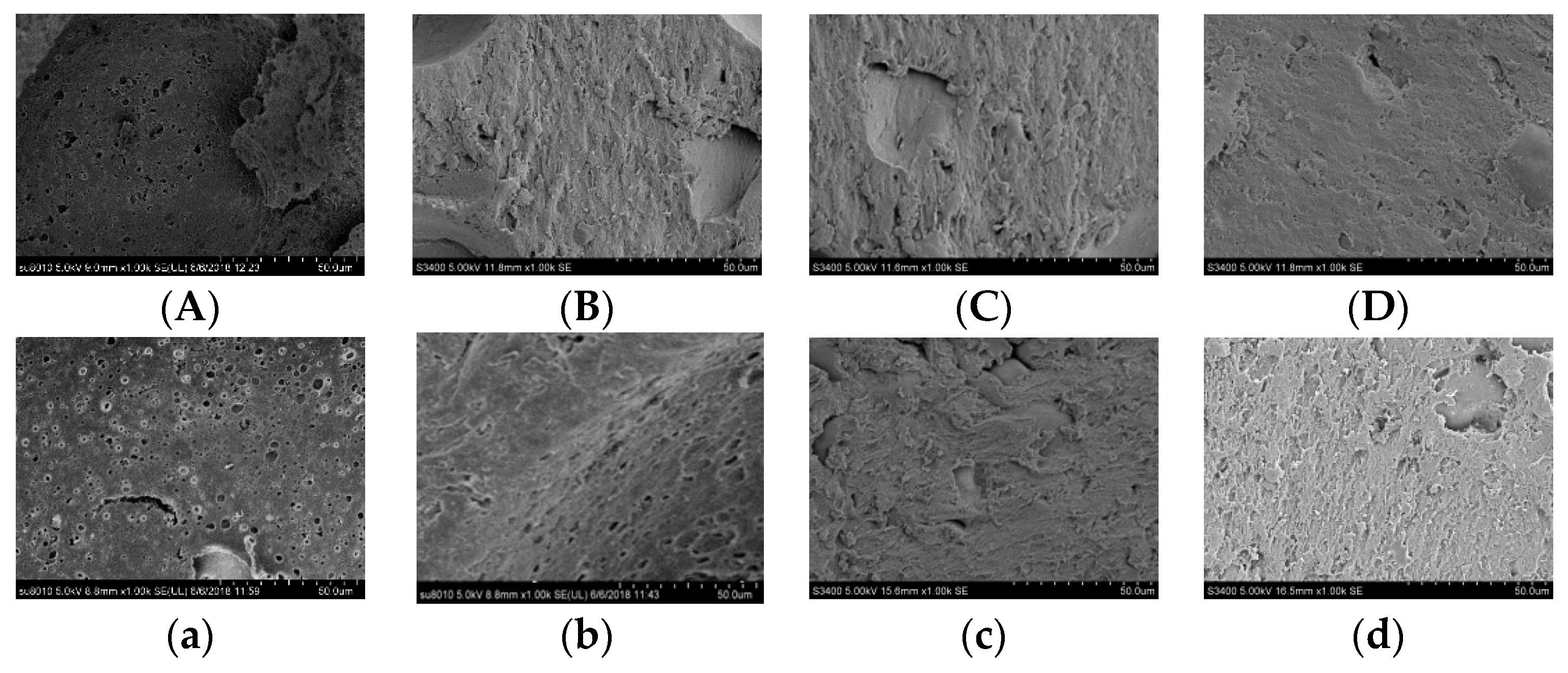
3.5. Microstructure Analysis of the Gels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niu, H.L.; Li, Y.; Han, J.C.; Liu, Q.; Kong, B.H. Gelation and rheological properties of myofibrillar proteins influenced by the addition of soybean protein isolates subjected to an acidic pH treatment combined with a mild heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Kudre, T.G.; Benjakul, S. Combining effect of microbial transglutaminase and bambara groundnut protein isolate on gel properties of surimi from sardine (Sardinella albella). Food Biophys. 2013, 8, 240–249. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liang, J.; Jiang, L.Z.; Li, Y.; Wang, J.; Zhang, H.; Sui, X.N. Effect of the interaction between myofibrillar protein and heat-induced soy protein isolates on gel properties. CyTA J. Food 2015, 13, 527–534. [Google Scholar] [CrossRef]
- Qin, X.; Chen, S.S.; Li, X.J.; Luo, S.Z.; Zhong, X.Y.; Jiang, S.T.; Zhao, Y.Y.; Zheng, Z.H. Gelation properties of transglutaminase-induced soy protein isolate and wheat gluten mixture with ultrahigh pressure pretreatment. Food Bioprocess Technol. 2017, 10, 866–874. [Google Scholar] [CrossRef]
- Niu, H.L.; Xia, X.F.; Wang, CH.; Kong, B.H.; Liu, Q. Thermal stability and gel quality of myofibrillar protein as affected by soy protein isolates subjected to an acidic pH and mild heating. Food Chem. 2018, 242, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Chen, X.; McClements, D.J.; Zou, L.Q.; Liu, W. pH-, ion- and temperature-dependent emulsion gels: Fabricated by addition of whey protein to gliadin-nanoparticle coated lipid droplets. Food Hydrocoll. 2018, 77, 870–878. [Google Scholar] [CrossRef]
- Schuldt, S.; Raak, N.; Jaros, D.; Rohm, H. Acid-induced formation of soy protein gels in the presence of NaCl. LWT Food Sci. Technol. 2014, 57, 634–639. [Google Scholar] [CrossRef]
- Kim, J.H.J.; Varankovich, N.V.; Nickerson, M.T. The effect of pH on the gelling behaviour of canola and soy protein isolates. Food Res. Int. 2016, 81, 31–38. [Google Scholar] [CrossRef]
- Luciana, D.G.; Pablo, R.S.; Adriana, N.M. Encapsulation of fish oil in soybean protein particles by emulsification and spray drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S. Physicochemical and functional properties of lentil protein isolates prepared by different drying methods. Food Chem. 2011, 129, 1513–1522. [Google Scholar] [CrossRef]
- Wang, W.J.; Shen, M.Y.; Liu, S.; Jiang, L.; Song, Q.Q.; Xie, J.H. Gel properties and interactions of Mesona blumes polysaccharide-soy protein isolates mixed gel: The effect of salt addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zeng, M.M.; Qin, F.; Adhikarib, B.; He, Z.Y.; Chen, J. Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018, 242, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Zhang, H.; Gao, L.; Wang, L.; Hai, F.; Qian, H.F. Influence of pH and ionic strength on heat-induced formation and rheological properties of cottonseed protein gels. Food Bioprod. Process. 2015, 96, 27–34. [Google Scholar] [CrossRef]
- Chen, N.N.; Chassenieux, C.; Taco, T. Kinetics of NaCl induced gelation of soy protein aggregates: Effects of temperature, aggregate size, and protein concentration. Food Hydrocoll. 2018, 77, 66–74. [Google Scholar] [CrossRef]
- Vilela, J.A.P.; Cavallieri, Â.L.F.; Cunha, R.L.D. The influence of gelation rate on the physical properties/structure of salt-induced gels of soy protein isolateegellan gum. Food Hydrocoll. 2011, 25, 1710–1718. [Google Scholar] [CrossRef]
- Yu, N.N.; Xu, Y.; Jiang, Q.X.; Xia, W. Molecular forces involved in heat-induced freshwater surimi gel: Effects of various bond disrupting agents on the gel properties and protein conformation changes. Food Hydrocoll. 2017, 69, 193–201. [Google Scholar] [CrossRef]
- Sochaya, C.; Soottawat, B. Impact of microbial transglutaminase on gelling properties of indian mackerel fish protein isolates. Food Chem. 2017, 136, 929–937. [Google Scholar] [CrossRef]
- Valdez-Hurtadoa, S.; López-Bermúdezb, L.S.; Higuera-Barrazab, O.A.; Del Toro-Sanchezb, C.L.; Ruiz-Cruzc, S.; Suárez-Jiménezb, M.G.; Marquez-Riosb, E. Effect of ultrasonication time on the functional properties of giant squid (Dosidicus gigas) mantle protein concentrate. Food Biosci. 2019, 27, 1–5. [Google Scholar] [CrossRef]
- Wang, K.; Arntfield, S.D. Probing the molecular forces involved in binding of selected volatile flavour compounds to salt-extracted pea proteins. Food Chem. 2016, 211, 235–242. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Boil. Chem. 1949, 177, 751–766. [Google Scholar]
- Lin, L.H.; Shen, M.Y.; Liu, S.C.; Tang, W.; Wang, Z.J.; Xie, M.Y.; Xie, J. An acidic heteropolysaccharide from Mesona chinensis: Rheological properties, gelling behavior and texture characteristics. Int. J. Biol. Macromol. 2018, 107, 1591–1598. [Google Scholar] [CrossRef]
- Haga, S.; Ohashi, T. Heat induced gelation of a mixture of myosin B and soybean protein. Agric. Boil. Chem. 1984, 48, 1001–1007. [Google Scholar] [CrossRef]
- Pan, T.; Guo, H.Y.; Li, Y.; Song, J.H.; Ren, F.Z. The effects of calcium chloride on the gel properties of porcine myosin-κ-carrageenan mixtures. Food Hydrocoll. 2017, 63, 467–477. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Mahrouqi, A.I. Instrumental texture profile analysis of gelatin gel extracted from grouper skin and commercial (bovine and porcine) gelatin gels. Int. J. Food Sci. Nutr. 2009, 60, 229–242. [Google Scholar] [CrossRef]
- Alfonso, T.; Jose, G.M.; Juan, A.S.; Isabel, G. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Mulvihill, D.M.; Kinsella, J.E. Gelation of β-lactoglobulin: Effects of sodium chloride and calcium chloride on the rheological and structural properties of gels. J. Food Sci. 1988, 53, 231–236. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Yang, H.; Liu, R.; Rong, J.; Zhao, S.; Xiong, S. Effects of CaCl2 on chemical interactions and gel properties of surimi gels from two species of carps. Eur. Food Res. Technol. 2011, 233, 569–576. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces study of heat induced myofibrillar protein gel as affected by partial substitution of NaCl with KCl, MgCl2 and CaCl2. CyTA J. Food 2016, 14, 239–247. [Google Scholar] [CrossRef]
- Phanngam, K.S.B.; Thummanoon, P.; Angel, B.E.; Sittipong, N. Impact of divalent salts and bovine gelatin on gel properties of phosphorylated gelatin from the skin of unicorn leatherjacket. LWT Food Sci. Technol. 2014, 55, 477–482. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Ganasen, P.; Benjakul, S. Physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cations. LWT Food Sci. Technol. 2010, 43, 77–85. [Google Scholar] [CrossRef]
- Benjakul, S.; Oungbho, K.; Visessanguan, W.; Thiansilakul, Y.; Roytrakul, S. Characteristics of gelatin from the skins of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. Food Chem. 2009, 116, 445–451. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- Soottawat, B.; Wonnop, V.; Yuwathida, K. The effect of whitening agents on the gel-forming ability and whiteness of surimi. Int. J. Food Sci. Technol. 2004, 39, 773–781. [Google Scholar] [CrossRef]
- Balange, A.K.; Benjakul, S. Effect of oxidised phenolic compounds on the gel property of mackerel (Rastrelliger kanagurta) surimi. LWT Food Sci. Technol. 2009, 6, 1059–1064. [Google Scholar] [CrossRef]
- Kuan, Y.H.; Nafchi, A.M.; Huda, N.; Ariffin, F.; Karim, A.A. Effects of sugars on the gelation kinetics and texture of duck feet gelatin. Food Hydrocoll. 2016, 58, 267–275. [Google Scholar] [CrossRef]
- Hermansson, A.-M. Water-and fatholding. In Functional Properties of Food Macromolecules; Mitchell, J.R., Ledward, D.A., Eds.; Elsevier Applied Science Publishers: London, UK, 1986; pp. 273–314. [Google Scholar]
- Sun, J.A.; Li, X.; Xu, X.L.; Zhou, G.H. Influence of various levels of flaxseed gum addition on the water-holding capacities of heat-induced porcine myofibrillar protein. J. Food Sci. 2011, 76, C472–C478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.S.; Li, W.W.; Qin, F.; Chen, J. Effects of oligosaccharides and soy soluble polysaccharide on the rheological and textural properties of calcium sulfate-induced soy protein gels. Food Bioprocess Technol. 2017, 10, 556–567. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Doi, E. Rheological characteristics and gelation mechanism of tofu (soybean curd). J. Agric. Food Chem. 1995, 43, 1808–1812. [Google Scholar] [CrossRef]
- Shan, H.; Lu, S.W.; Jiang, L.Z.; Wang, L.K.; Liao, H.; Zhang, R.Y.; Dai, C.J.; Yao, X.M.; Zhang, Y.L.; Su, P.; et al. Gelation property of alcohol-extracted soy protein isolate and effects of various reagents on the firmness of heat-induced gels. Int. J. Food Prop. 2014, 18, 627–637. [Google Scholar] [CrossRef]
- Lin, W.L.; Zeng, Q.X.; Zhu, Z.W.; Song, G.S. Relation between protein characteristics and tpa texture characteristics of crisp grass carp (Ctenopharyngodon idellus c. et v) and grass carp (Ctenopharyngodon idellus). J. Text. Stud. 2012, 43, 1–11. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, R.; Zhao, J.; Chen, Q.; Kong, B. Structural and gel textural properties of soy protein isolate when subjected to extreme acid pH-shifting and mild heating processes. J. Agric. Food Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- O’kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J. Agric. Food Chem. 2004, 52, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Zheng, W. Silver carp (Hypophthalmichthys molitrix) surimi acid-induced gel extract characteristics: A comparison with heat-induced gel. Int. J. Food Prop. 2015, 18, 821–832. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the size and content of protein aggregates on the rheological and structural properties of soy protein isolate emulsion gels induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, Z.; Yin, L.; Cheng, Y.; Li, L. Effect of preheating temperature and calcium ions on the properties of cold-set soybean protein gel. Food Res. Int. 2010, 43, 1673–1683. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cavallieri, Â.L.; Da Cunha, R.L. Cold-set whey protein gels induced by calcium or sodium salt addition. Int. J. Food Sci. Technol. 2010, 45, 348–357. [Google Scholar] [CrossRef]
- Moritaka, H.; Fukuba, H.; Kumeno, K.; Nakahama, N.; Nishinari, K. Effect of monovalent and divalent cations of the rheological properties of gellan gels. Food Hydrocoll. 1991, 4, 495–507. [Google Scholar] [CrossRef]


| Buffer Series | NaCl | Tris-HCl, pH 8.0 | SDS | Urea | β-ME |
|---|---|---|---|---|---|
| S1 | 0.6 mol/L | ||||
| S2 | 20 mmol/L | ||||
| S3 | 20 mmol/L | 1% | |||
| S4 | 20 mmol/L | 1% | 8 mol/L | ||
| S5 | 20 mmol/L | 1% | 8 mol/L | 2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Teng, F.; Wang, N.; Zhang, X.-N.; Regenstein, J.M.; Liu, J.-S.; Li, Y.; Wang, Z.-J. Addition of Salt Ions before Spraying Improves Heat- and Cold-Induced Gel Properties of Soy Protein Isolate (SPI). Appl. Sci. 2019, 9, 1076. https://doi.org/10.3390/app9061076
Zheng L, Teng F, Wang N, Zhang X-N, Regenstein JM, Liu J-S, Li Y, Wang Z-J. Addition of Salt Ions before Spraying Improves Heat- and Cold-Induced Gel Properties of Soy Protein Isolate (SPI). Applied Sciences. 2019; 9(6):1076. https://doi.org/10.3390/app9061076
Chicago/Turabian StyleZheng, Li, Fei Teng, Na Wang, Xue-Na Zhang, Joe M. Regenstein, Ji-Shan Liu, Yang Li, and Zhong-Jiang Wang. 2019. "Addition of Salt Ions before Spraying Improves Heat- and Cold-Induced Gel Properties of Soy Protein Isolate (SPI)" Applied Sciences 9, no. 6: 1076. https://doi.org/10.3390/app9061076
APA StyleZheng, L., Teng, F., Wang, N., Zhang, X.-N., Regenstein, J. M., Liu, J.-S., Li, Y., & Wang, Z.-J. (2019). Addition of Salt Ions before Spraying Improves Heat- and Cold-Induced Gel Properties of Soy Protein Isolate (SPI). Applied Sciences, 9(6), 1076. https://doi.org/10.3390/app9061076





