Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Materials and Preparation
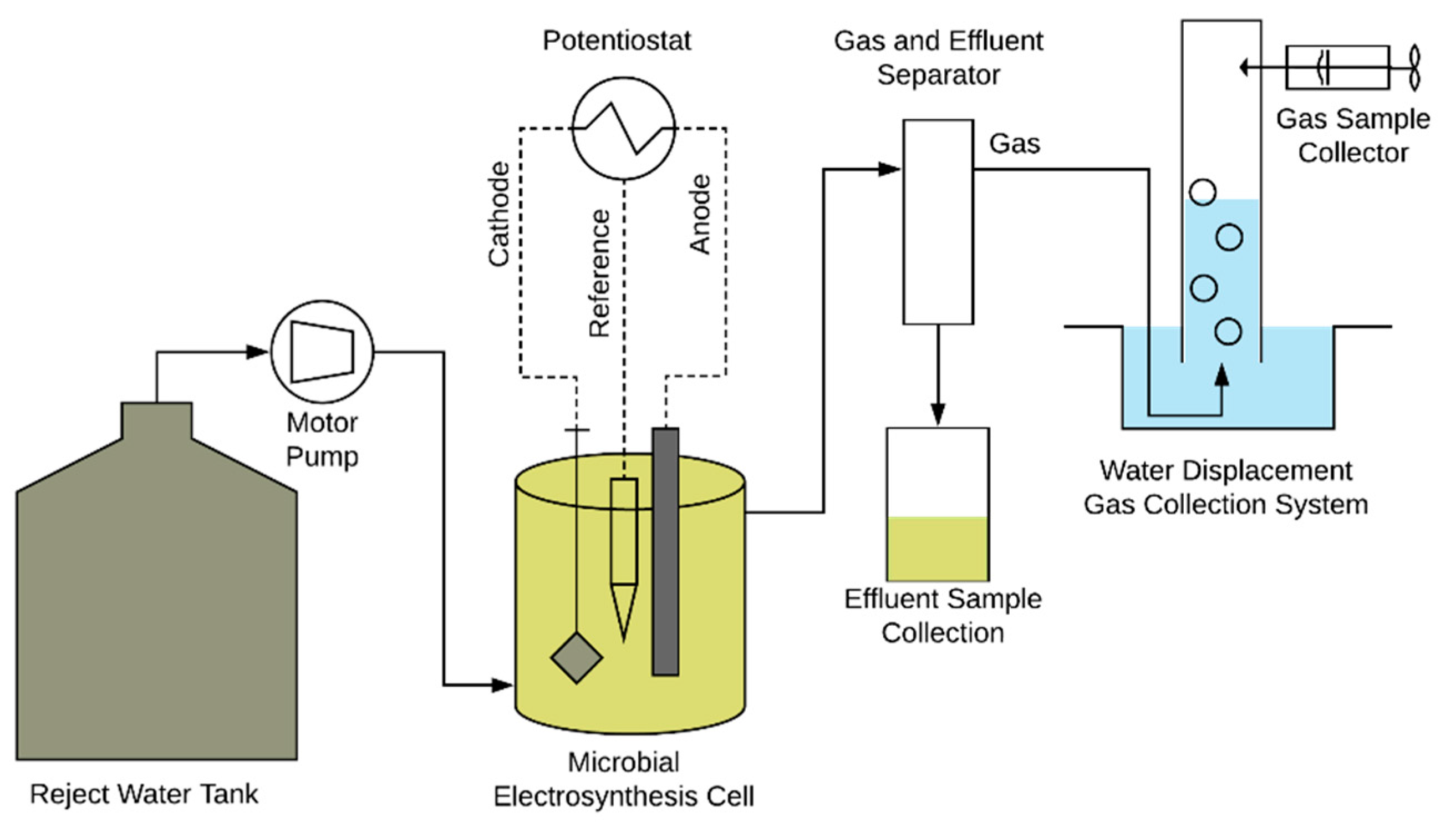
2.2. Reactor Setup and Operation
2.2.1. Biofilm Growth
2.2.2. Biomethane Production Experiment
2.2.3. Optimisation of Cathode Potential
2.2.4. Optimisation of pH
2.2.5. Verification of Optimised MES Performance
2.3. Analytical Methods and Calculations
3. Results and Discussion
3.1. Biofilm Growth
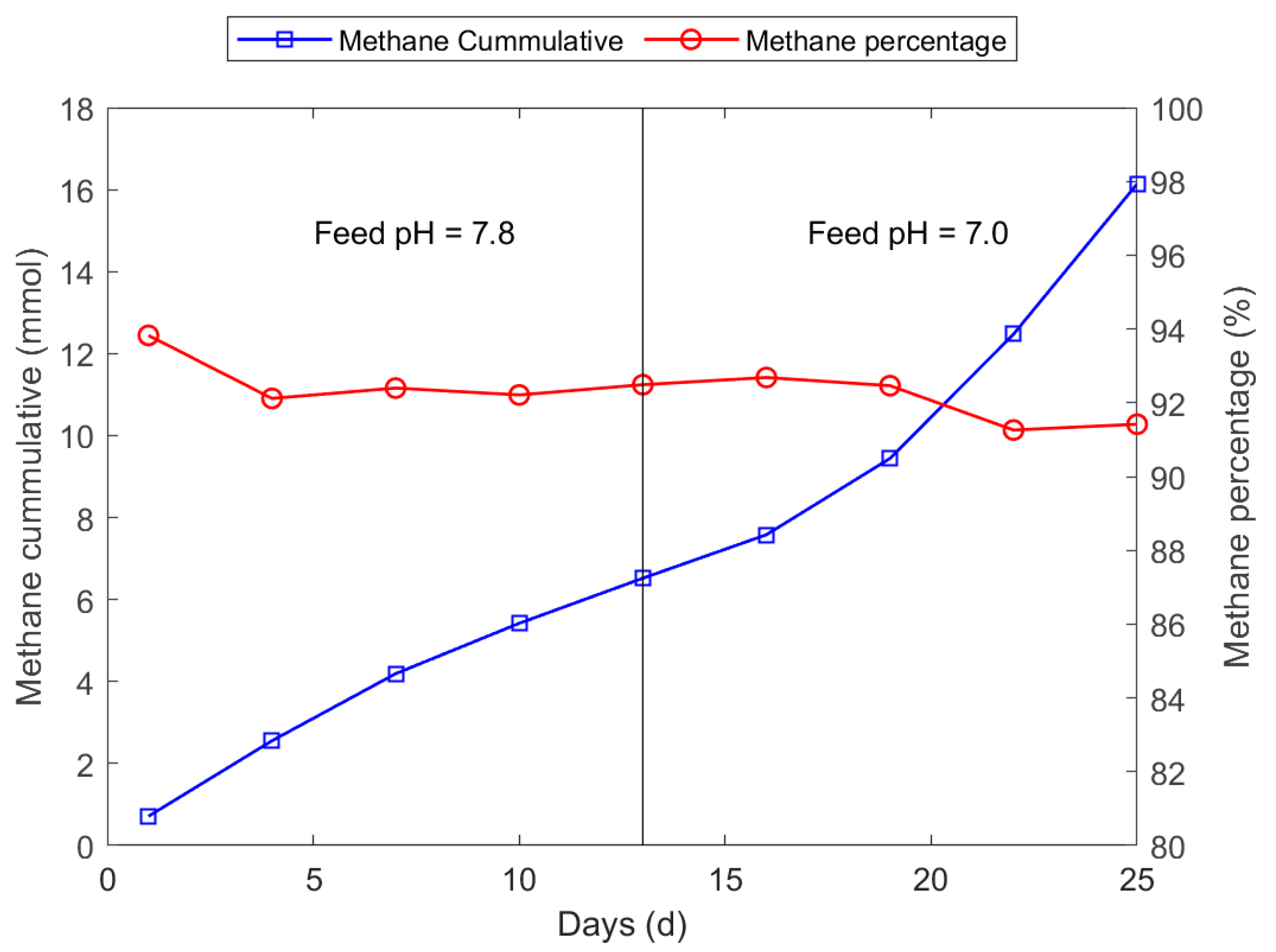
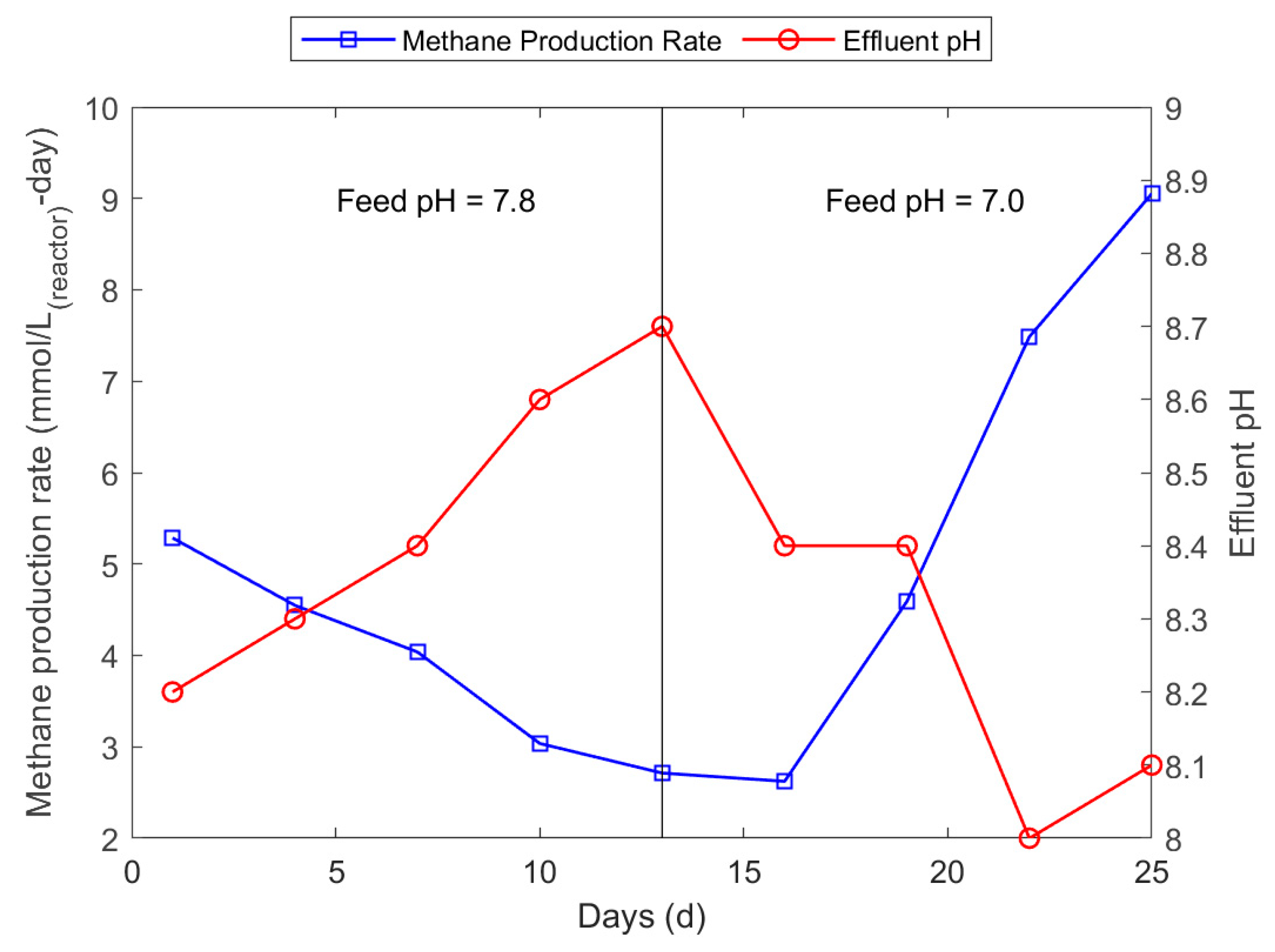
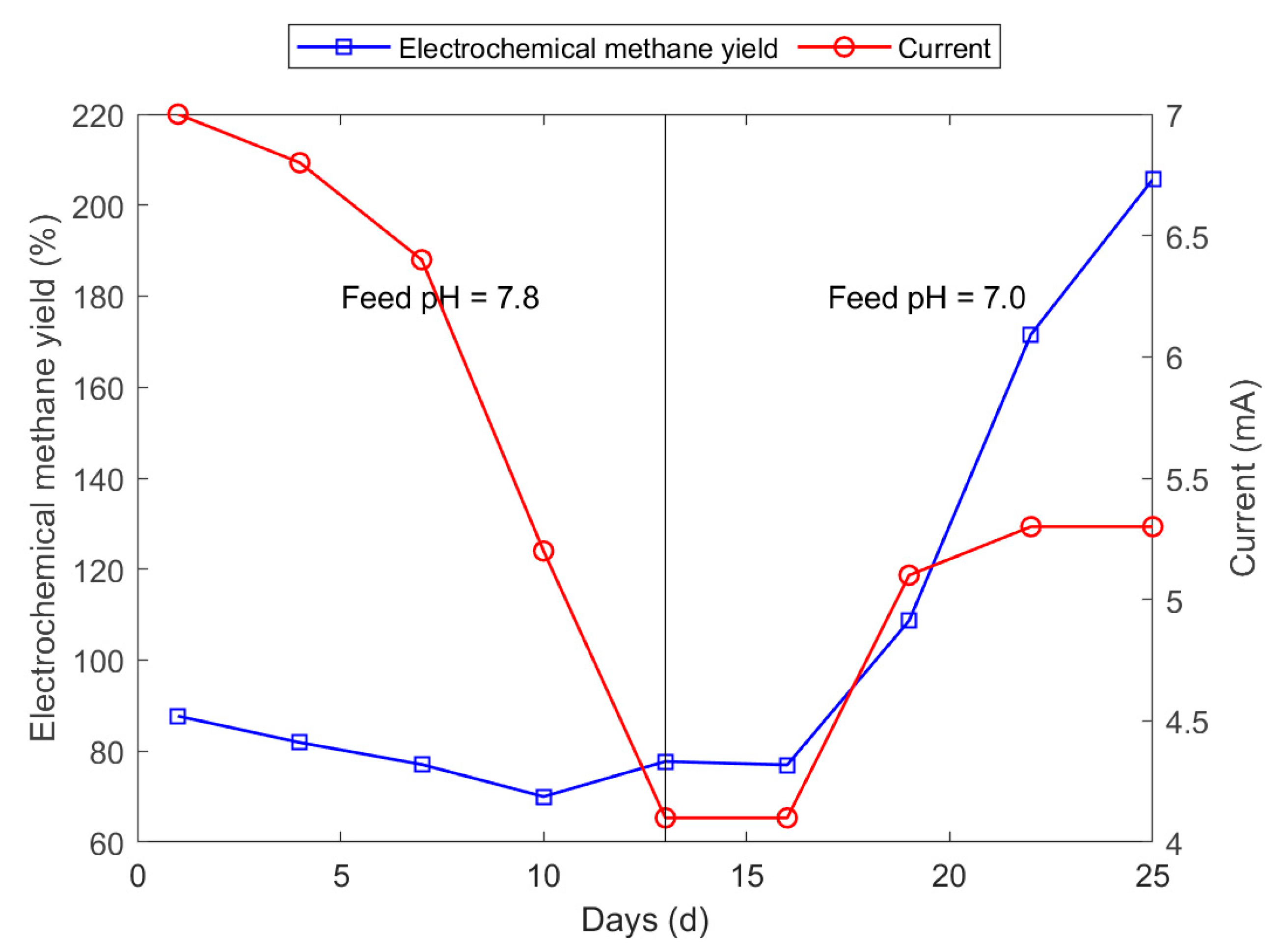
3.2. Biomethane Production Experiment
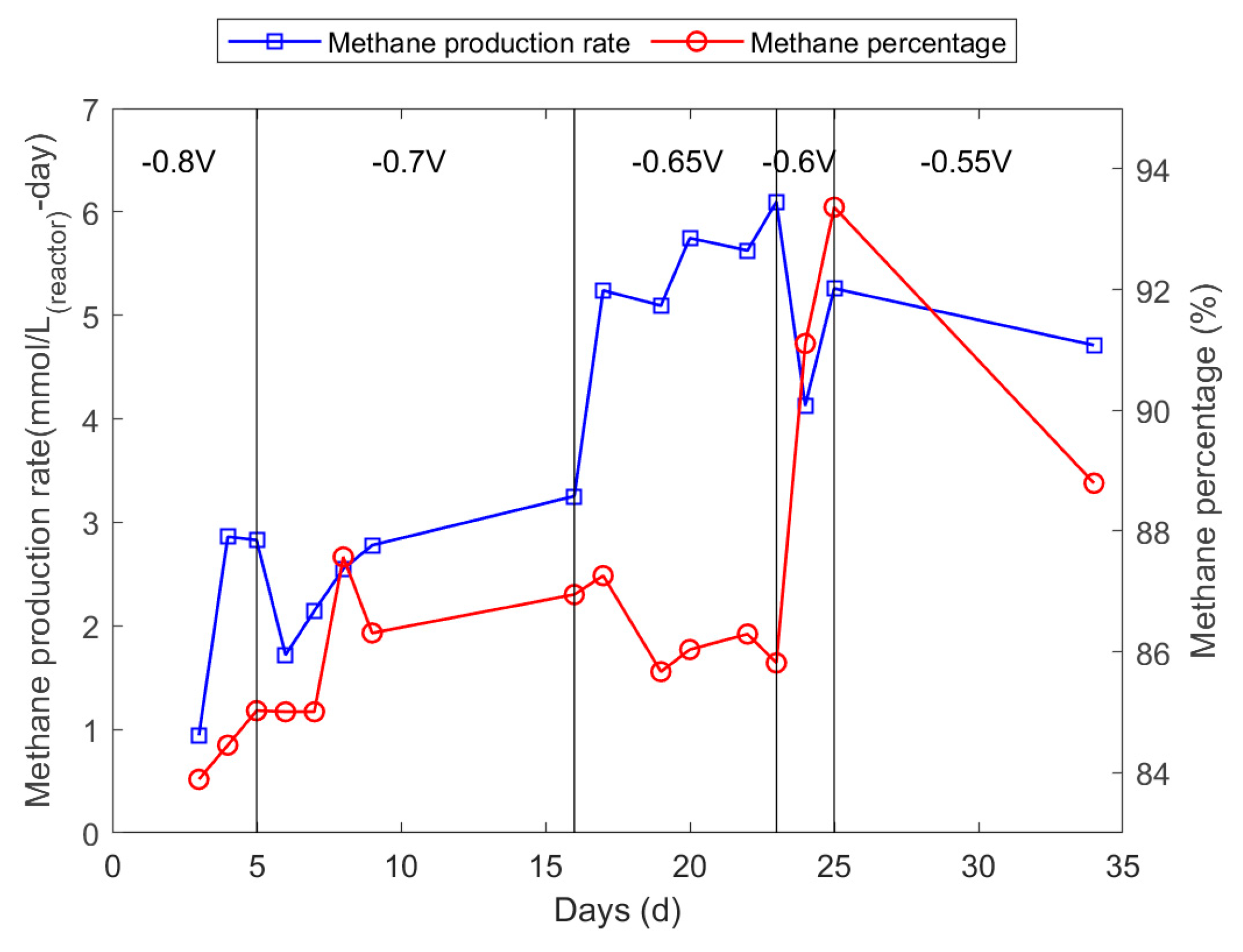
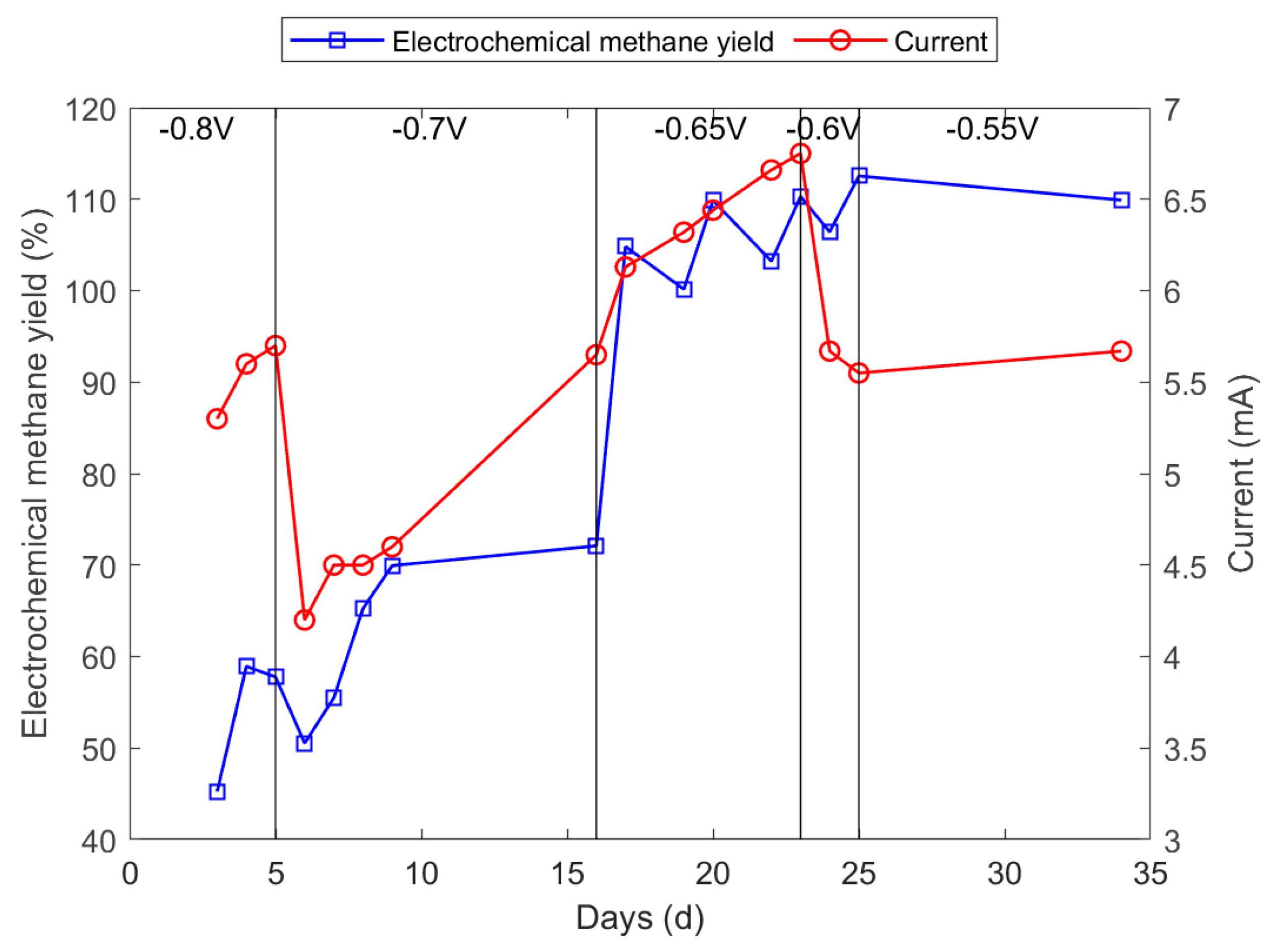
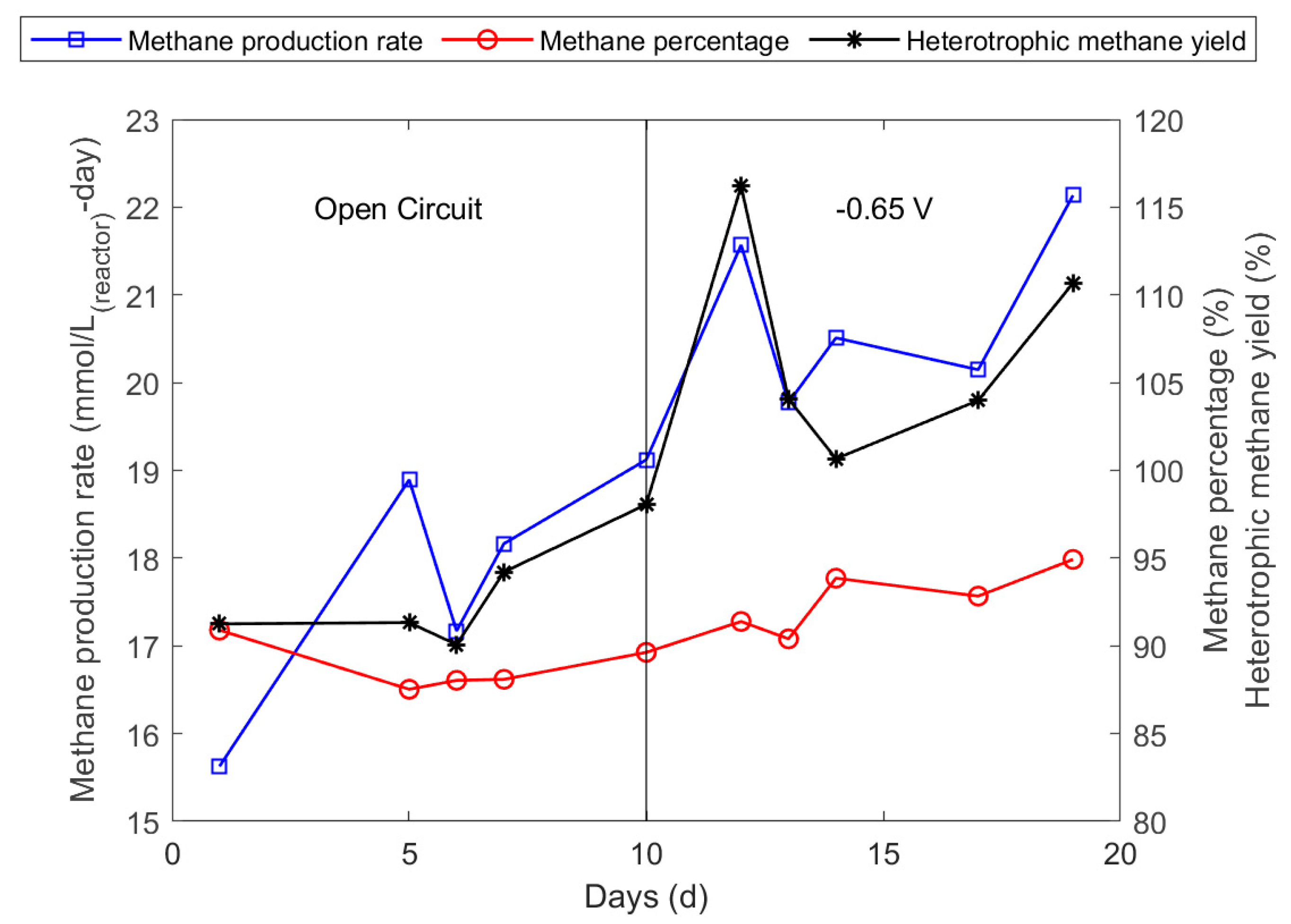
3.3. Optimisation of Cathode Potential
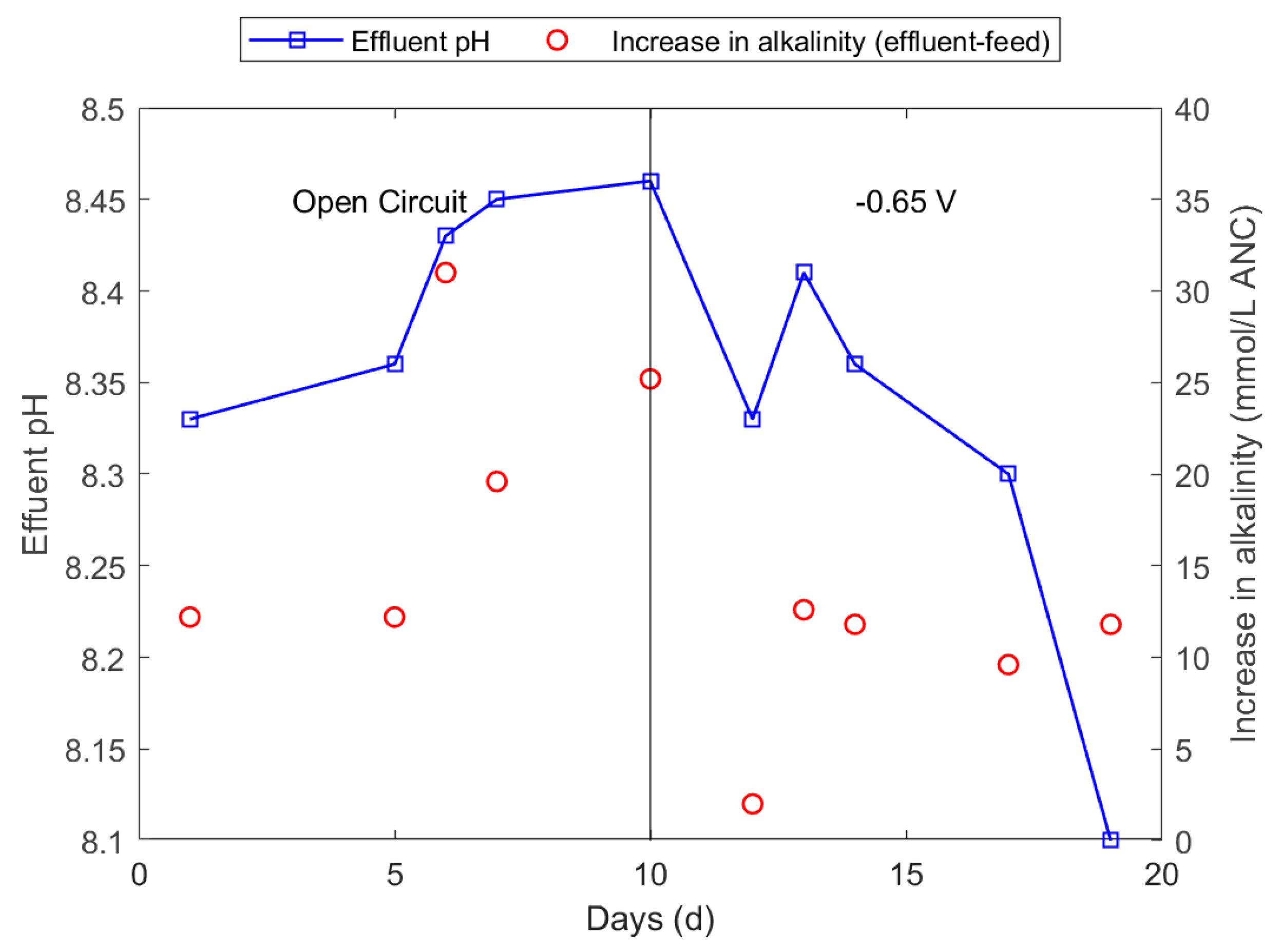
3.4. Optimisation of pH
3.5. Verification of Optimised MES Performance
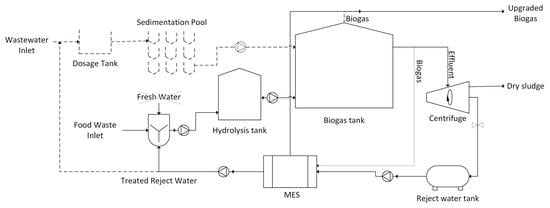
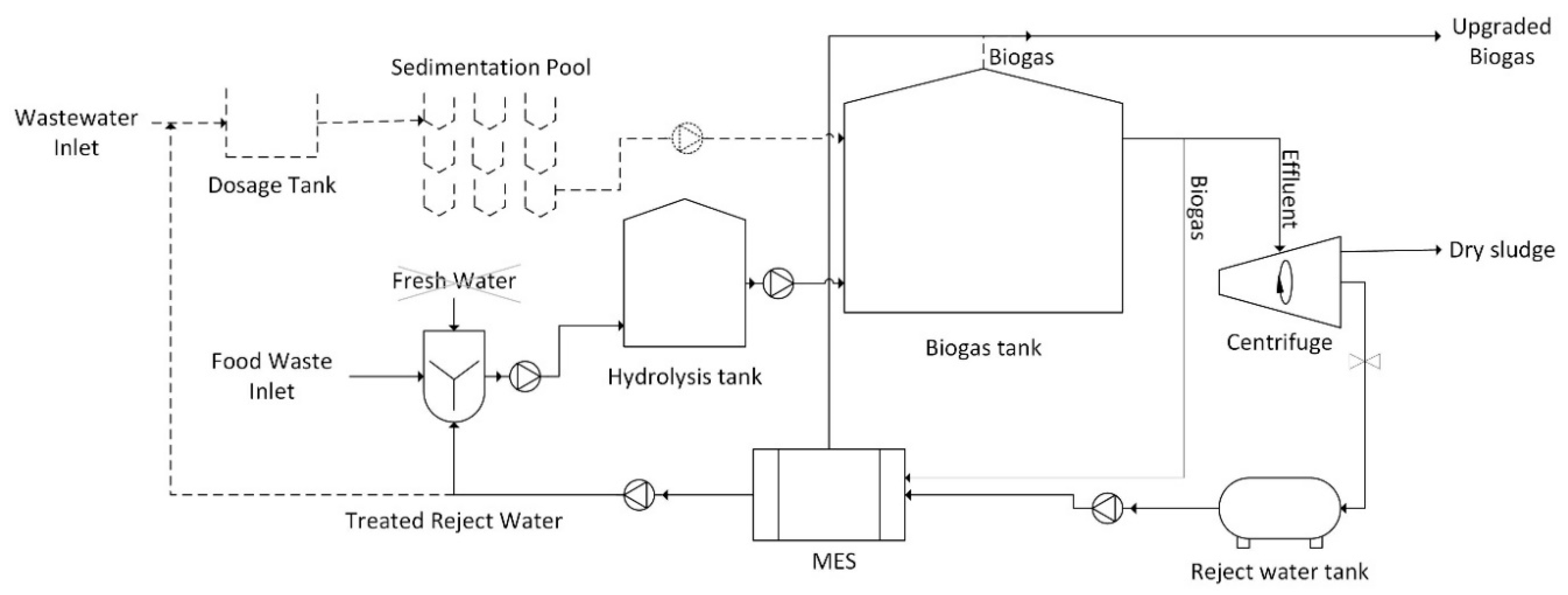
3.6. Proposed AD-MES Integrated System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelabhotla, A.B.T.; Dinamarca, C. Electrochemically mediated CO2 reduction for bio-methane production: A review. Rev. Environ. Sci. Bio/Technol. 2018, 17, 531–551. [Google Scholar] [CrossRef]
- Sánchez, O.G.; Birdja, Y.Y.; Bulut, M.; Vaes, J.; Breugelmans, T.; Pant, D. Recent advances in industrial CO2 electroreduction. Curr. Opin. Green Sustain. Chem. 2019, 16, 47–56. [Google Scholar] [CrossRef]
- Angenent, L.T.; Usack, J.G.; Xu, J.; Hafenbradl, D.; Posmanik, R.; Tester, J.W. Integrating electrochemical, biological, physical, and thermochemical process units to expand the applicability of anaerobic digestion. Bioresour. Technol. 2018, 247, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.R. Microbial Catalysis of Methane from Carbon Dioxide. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2012; pp. 1–107. [Google Scholar]
- Jiang, Y.; Su, M.; Zhang, Y.; Zhan, G.; Tao, Y.; Li, D. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrog. Energy 2013, 38, 3497–3502. [Google Scholar] [CrossRef]
- Siegert, M.; Li, X.F.; Yates, M.D.; Logan, B.E. The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. Front. Microbiol. 2015, 5, 778. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, F.; Liu, J.; Zhang, X.; Huang, X.; Logan, B.E. Methane production in microbial reverse-electrodialysis methanogenesis cells (MRMCs) using thermolytic solutions. Environ. Sci. Technol. 2014, 48, 8911–8918. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Kuramochi, Y.; Fukushima, N.; Maeda, H.; Sato, K.; Kobayashi, H. Bioelectrochemical analyses of the development of a thermophilic biocathode catalyzing electromethanogenesis. Environ. Sci. Technol. 2015, 49, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; San-Martín, M.I.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Siegert, M.; Yates, M.D.; Call, D.F.; Zhu, X.; Spormann, A.; Logan, B.E. Comparison of nonprecious metal cathode materials for methane production by electromethanogenesis. ACS Sustain. Chem. Eng. 2014, 2, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, Z.; Yang, C.; Zhou, A.; Guo, Z.; Liang, B.; Varrone, C.; Wang, A.J. Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol. Biofuels 2016, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cai, W.; Guo, Z.; Wang, L.; Yang, C.; Varrone, C.; Wang, A. Microbial electrolysis contribution to anaerobic digestion of waste activated sludge, leading to accelerated methane production. Renew. Energy 2016, 91, 334–339. [Google Scholar] [CrossRef]
- Villano, M.; Monaco, G.; Aulenta, F.; Majone, M. Electrochemically assisted methane production in a biofilm reactor. J. Power Sources 2011, 196, 9467–9472. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Holmes, D.E. Bioelectrochemical removal of carbon dioxide (CO2): An innovative method for biogas upgrading. Bioresour. Technol. 2014, 173, 392–398. [Google Scholar] [CrossRef]
- Dykstra, C.M.; Pavlostathis, S.G. Methanogenic Biocathode Microbial Community Development and the Role of Bacteria. Environ. Sci. Technol. 2017, 51, 5306–5316. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Yin, Q.; Zhu, X.; Zhan, G.; Bo, T.; Yang, Y.; Tao, Y.; He, X.; Li, D.; Yan, Z. Enhanced methane production in an anaerobic digestion and microbial electrolysis cell coupled system with co-cultivation of Geobacter and Methanosarcina. J. Environ. Sci. (China) 2016, 42, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Chen, S.; Quan, X.; Yu, Q. Bioelectrochemical enhancement of anaerobic methanogenesis for high organic load rate wastewater treatment in a up-flow anaerobic sludge blanket (UASB) reactor. Sci. Rep. 2014, 4, 6658–6665. [Google Scholar] [CrossRef]
- Xu, H.; Gong, S.; Sun, Y.; Ma, H.; Zheng, M.; Wang, K. High-rate hydrogenotrophic methanogenesis for biogas upgrading: The role of anaerobic granules. Environ. Technol. 2015, 36, 529–537. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-situ biogas upgrading with pulse H2additions: The relevance of methanogen adaption and inorganic carbon level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D.M. Bioresource Technology An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Gregory, K.B.; Bond, D.R.; Lovley, D.R. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef]
- Nelabhotla, A.B.T.; Dinamarca, C. Optimisation of Electrochemical Treatment of Artificial Wastewater using Cyclic Voltammetry. Int. J. Environ. Sci. Dev. 2018, 9, 218–221. [Google Scholar] [CrossRef]
- Huong Le, T.X.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon N. Y. 2017, 122, 564–591. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X. Biogas production from anaerobic co-digestion of food waste with dairy manure in a two-phase digestion system. Appl. Biochem. Biotechnol. 2010, 160, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E. Effect of reseeding and pH control on the performance of a two-stage mesophilic anaerobic digester operating on acid cheese whey. Can. Agric. Eng. 2000, 42, 173–183. [Google Scholar]
- Dodds, W.K.; Whiles, M.R. Chapter 13—Carbon. In Aquatic Ecology, 2nd ed.; Academic Press: London, UK, 2010; pp. 323–343. ISBN 978-0-12-374724-2. [Google Scholar]
- Dębowski, M.; Szwaja, S.; Zieliński, M.; Kisielewska, M.; Stańczyk-Mazanek, E. The Influence of Anaerobic Digestion Effluents (ADEs) Used as the Nutrient Sources for Chlorella sp. Cultivation on Fermentative Biogas Production. Waste Biomass Valorization 2017, 8, 1153–1161. [Google Scholar] [CrossRef]
- Hashemi, S.; Niazi, S.; Dinamarca, C.; Janka, E.; Heggholmen, A.; Wang, S.; Bakke, R. Biological reject water treatment for enhanced carbon recovery. In Proceedings of the 2nd International Conference Bioresource Technology for Bioenergy Bioproducts and Environmental Sustainability, Sitges, Spain, 16–19 September 2018. [Google Scholar]
| Parameter | Result |
|---|---|
| Biogas production | 550 mL (22.5 mmol) |
| Methane | 500 mL (20.5 mmol) |
| Carbon dioxide | 34 mL (1.4 mmol) |
| COD Consumption | 1644.3 mg (52%) |
| Voltage | −0.8 V vs. SHE |
| Methane Concentration | 90.9% |
| Mass Balance Efficiency | 80.5% |
| Electrochemical Methane Yield | 467.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelabhotla, A.B.T.; Dinamarca, C. Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Appl. Sci. 2019, 9, 1056. https://doi.org/10.3390/app9061056
Nelabhotla ABT, Dinamarca C. Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Applied Sciences. 2019; 9(6):1056. https://doi.org/10.3390/app9061056
Chicago/Turabian StyleNelabhotla, Anirudh Bhanu Teja, and Carlos Dinamarca. 2019. "Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes" Applied Sciences 9, no. 6: 1056. https://doi.org/10.3390/app9061056
APA StyleNelabhotla, A. B. T., & Dinamarca, C. (2019). Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Applied Sciences, 9(6), 1056. https://doi.org/10.3390/app9061056