Eosinophilic Gastroenteritis with Appendix Involvement: Role of Intestinal Ultrasound
Abstract
1. Introduction
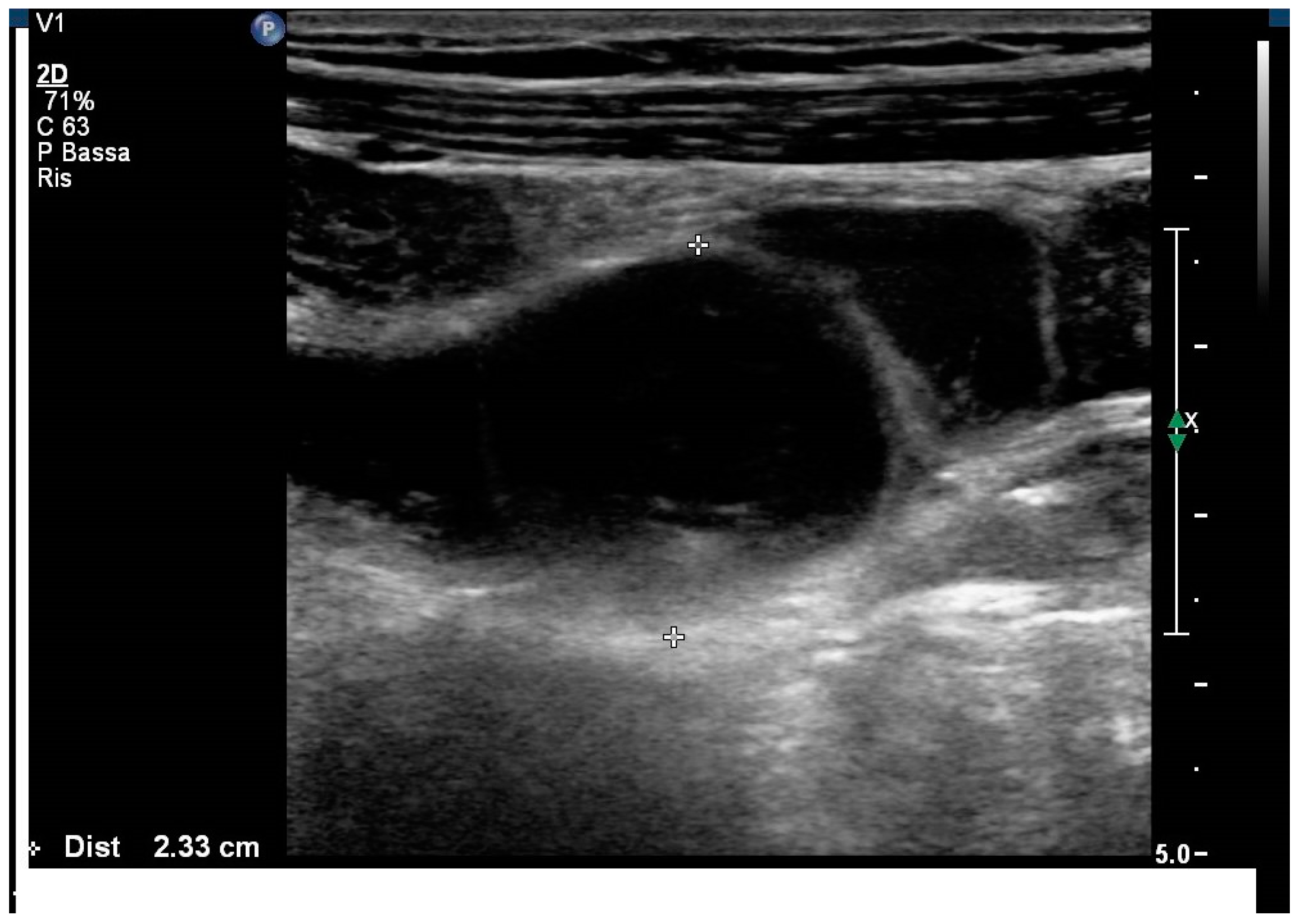
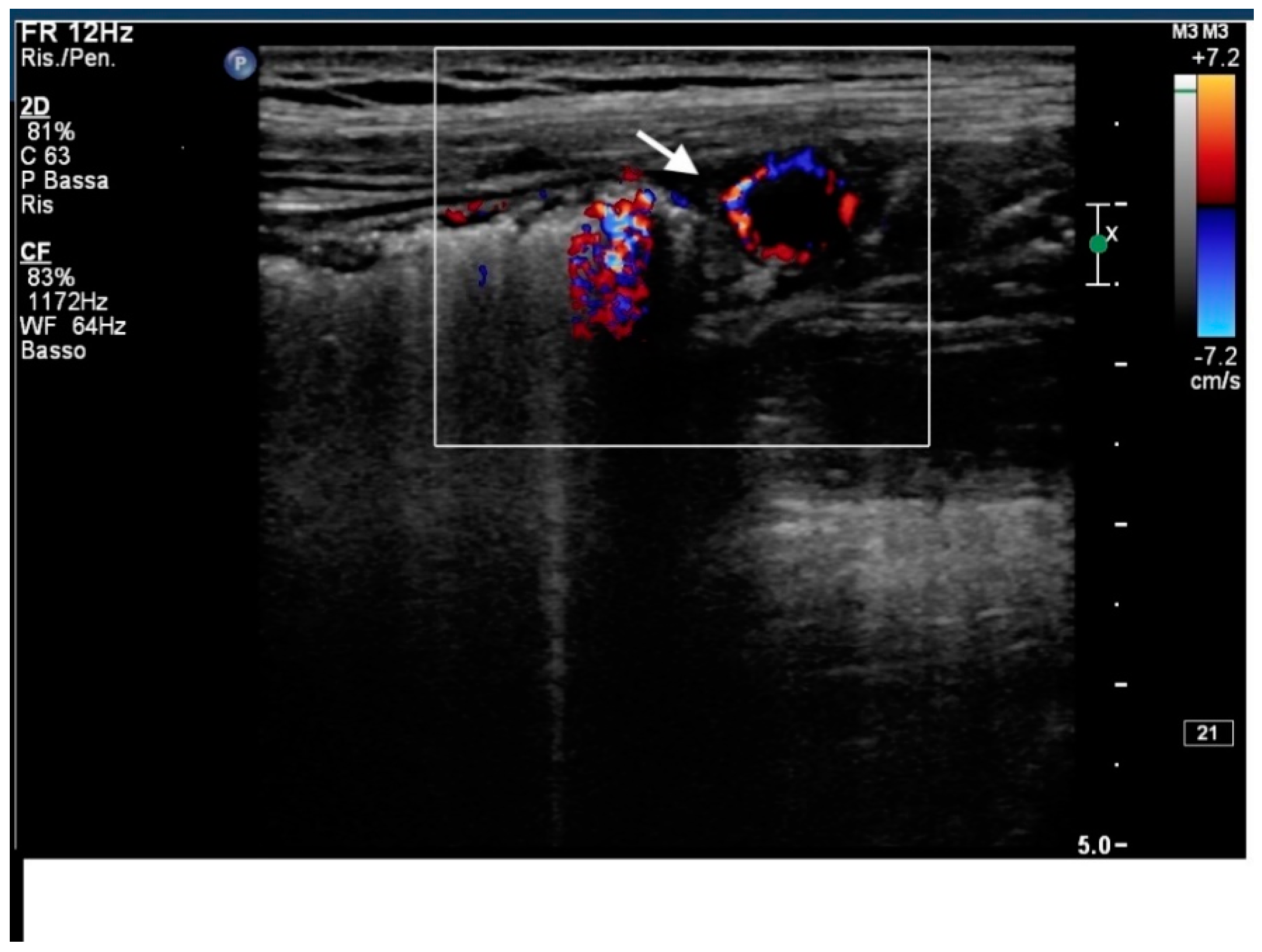
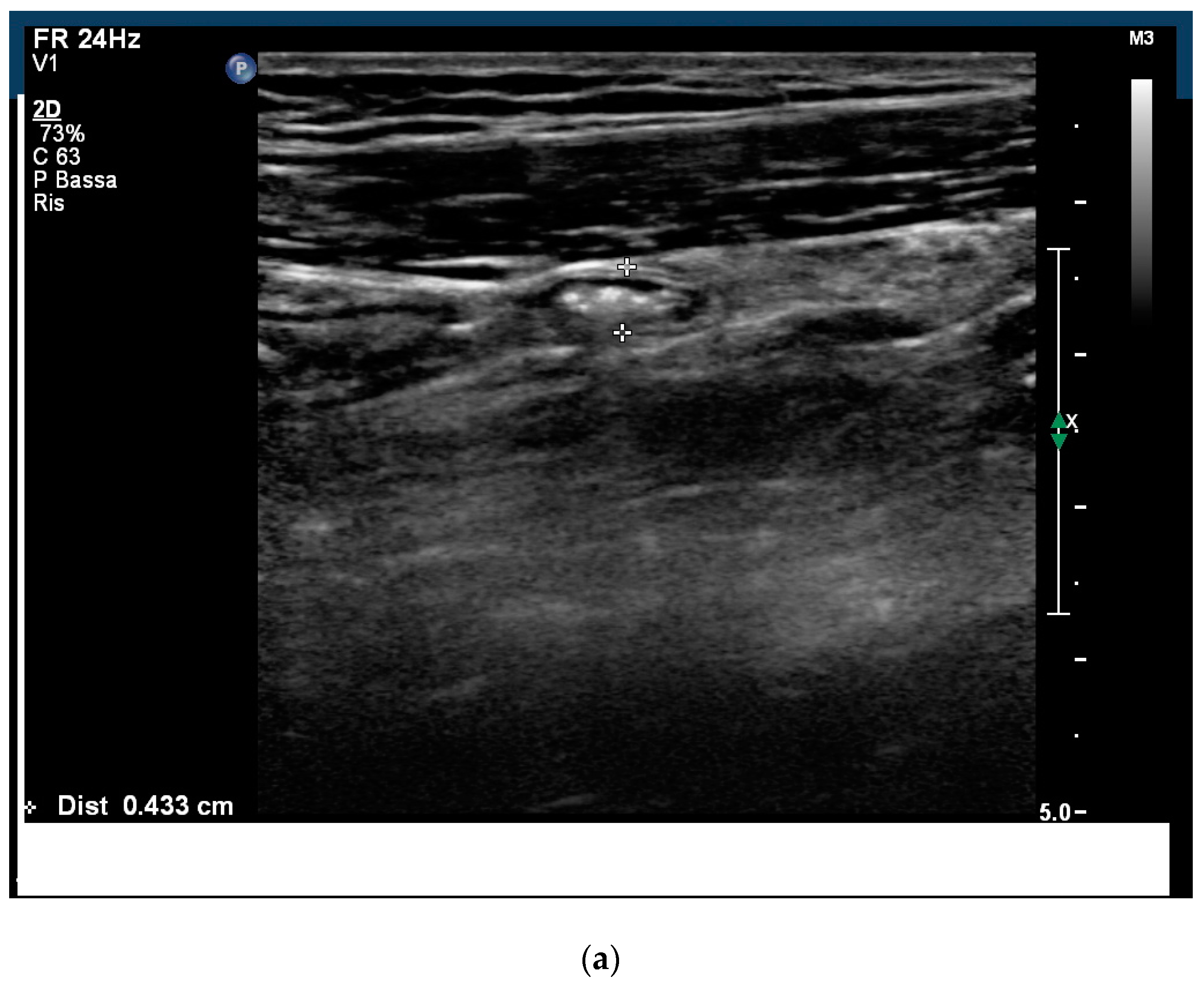
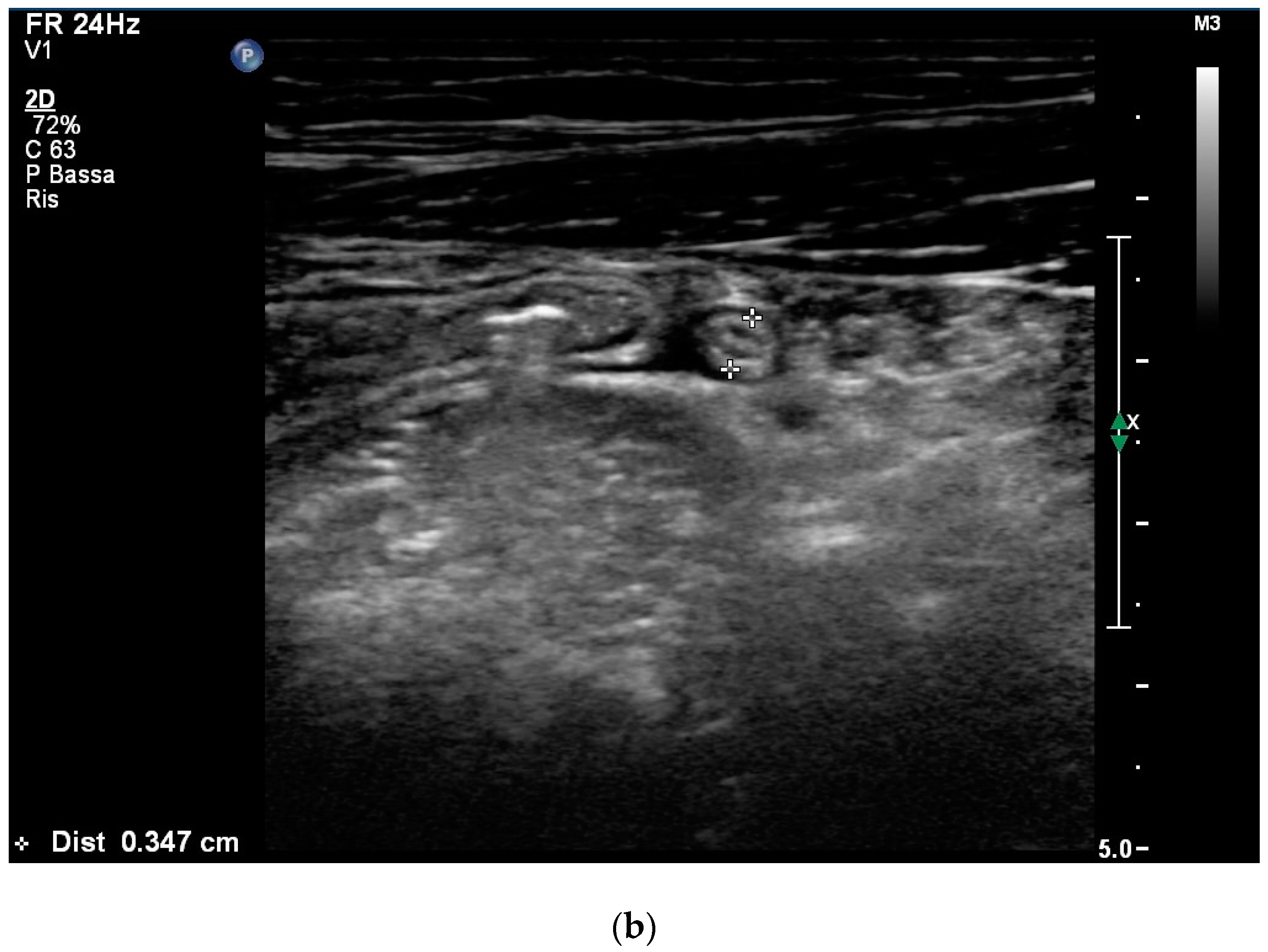
2. Case Report
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kaijser, R. Allergic diseases of the gut from the point of view of the surgeon. Arch. Klin. Chir. 1937, 188, 36–64. [Google Scholar]
- Zhang, M.M.; Li, Y.Q. Eosinophilic gastroenteritis: A state-of-the-art review. Gastroenterol. Hepatol. 2017, 32, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.C.; Hargrove, R.L.; Sleisenger, M.H.; Jeffries, G. Eosinophilic gastroenteritis. Medicine 1970, 49, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Woosley, J.T.; Dellon, E.S. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig. Liver Dis. 2015, 47, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Salloum, L.; Tshibaka, C.; Moser, R. Eosinophilic gastroenteritis mimicking acute appendicitis. Am. Surg. 2000, 66, 990–992. [Google Scholar] [PubMed]
- Croese, J.; Prociv, P.; Maguire, E.J.; Crawford, A.P. Eosinophilic enteritis presenting as surgical emergencies: A report of six cases. Med. J. Aust. 1990, 153, 415–417. [Google Scholar] [PubMed]
- Ingle, S.B.; Hinge, C.R. Eosinophilic gastroenteritis: An usual type of gastroenteritis. World J. Gastroenterol. 2013, 19, 5061–5066. [Google Scholar] [CrossRef]
- Mallin, M.; Craven, P.; Steenblik, J.; Forbes, B.; Boehm, K.; Youngquist, S. Diagnosis of appendicitis by bedside ultrasound in the ED. Am. J. Emerg. Med. 2015, 33, 430–432. [Google Scholar] [CrossRef]
- Jung, Y.; Rothenberg, M.E. Roles and Regulation of Gastrointestinal Eosinophils in Immunity and Disease. J. Immunol. 2014, 193, 999–1005. [Google Scholar] [CrossRef]
- Walker, M.M.; Potter, M.; Talley, N.J. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol. Hepatol. 2018, 3, 271–280. [Google Scholar] [CrossRef]
- Mansoor, E.; Saleh, M.A.; Cooper, G.S. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin. Gastroenterol. Hepatol. 2017, 15, 1733–1741. [Google Scholar] [CrossRef]
- Spergel, J.M.; Book, W.M.; Mays, E.; Song, L.; Shah, S.S.; Talley, N.J.; Bonis, P.A. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 300–306. [Google Scholar] [CrossRef]
- Kim, N.I.; Jo, Y.J.; Song, M.H.; Kim, S.H.; Kim, T.H.; Park, Y.S.; Eom, W.Y.; Kim, S.W. Clinical features of eosinophilic gastroenteritis. Korean J. Gastroenterol. 2004, 44, 217–223. [Google Scholar]
- Pineton de Chambrun, G.; Gonzalez, F.; Canva, J.Y.; Gonzalez, S.; Houssin, L.; Desreumaux, P.; Cortot, A.; Colombel, J.F. Natural history of eosinophilic gastroenteritis. Clin. Gastroenterol. 2008, 22, 455–479. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Eosinophilic gastrointestinal disorders (EGID). J. Allergy Clin. Immunol. 2004, 113, 11–28. [Google Scholar] [CrossRef]
- Chang, J.Y.; Choung, R.S.; Lee, R.M.; Locke, G.R., III; Schleck, C.D.; Zinsmeister, A.R.; Smyrk, T.C.; Talley, N.J. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin. Gastroenterol. Hepatol. 2010, 8, 669–675. [Google Scholar] [CrossRef]
- Ko, H.M.; Morotti, R.A.; Yershov, O.; Chehade, M. Eosinophilic Gastritis in Children: Clinicopathological Correlation, Disease Course and Response to Therapy. Am. J. Gastroenterol. 2014, 109, 1277–1285. [Google Scholar] [CrossRef]
- Phaw, N.A.; Tsai, H.H. Eosinophilic gastroenteritis: A challenge to diagnose and treat. BMJ Case Rep. 2016. [Google Scholar] [CrossRef]
- Anuradha, C.; Mittal, R.; Yacob, M.; Manipadam, M.T.; Kurian, S.; Eapen, A. Eosinophilic disorders of the gastrointestinal tract: Imaging features. Diagn. Interv. Radiol. 2012, 18, 183–188. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Harima, Y. A Case of Eosinophilic Gastroenteritis Forming a Rigid Chamber Mimicking Giant Duodenal Ulcer on Computed Tomography Imaging. Am. J. Case Rep. 2016, 17, 259–263. [Google Scholar] [CrossRef]
- Brandon, J.L.; Schroeder, S.; Furuta, G.T.; Capocelli, K.; Masterson, J.C.; Fenton, L.Z. CT imaging features of eosinophilic colitis in children. Pediatr. Radiol. 2013, 43, 697–702. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Huh, J.S.; Je, B.K.; Hong, K.D.; Lee, J.H. Eosinophilic Gastrointestinal Disorder Presenting as Intractable Vomiting and Ascites in a Young Girl. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 198–203. [Google Scholar] [CrossRef]
- Alsulaiman, R.M. Eosinophilic ascites: A case report and literature review. J. Family Community Med. 2015, 22, 183–185. [Google Scholar] [CrossRef]
- Bleibel, F.; Fragoza, K.; Faller, G.T. Acute eosinophilic ascites in a middle-aged man. Case Rep. Gastrointest. Med. 2012, 2012, 896523. [Google Scholar] [CrossRef]
- Dellon, E.S.; Jones, P.D.; Martin, N.B.; Kelly, M.; Kim, S.C.; Freeman, K.L.; Dellon, E.P.; Ferris, M.E.; Shaheen, N.J. Health-care transition from pediatric to adult-focused gastroenterology in patients with eosinophilic esophagitis. Dis. Esophagus 2013, 26, 7–13. [Google Scholar] [CrossRef]
- Collins, M.H. Histopathology associated with eosinophilic gastrointestinal diseases. Immunol. Allergy Clin. N. Am. 2009, 29, 109–117. [Google Scholar] [CrossRef]
- Turner, K.O.; Sinkre, R.A.; Neumann, W.L.; Genta, R.M. Primary colonic eosinophilia and eosinophilic colitis in adults. Am. J. Surg. Pathol. 2017, 41, 225–233. [Google Scholar] [CrossRef]
- Yantiss, R.K. Eosinophils in the GI tract: How many is too many and what do they mean? Mod. Pathol. 2015, 28, S7–S21. [Google Scholar] [CrossRef]
- Gupta, N.; Aggawal, A.; Gupta, R.; Sule, S.; Wolf, D.C. The management of eosinophilic gastroenteritis. Scand. J. Gastroenterol. 2015, 50, 1309–1314. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Serrano-Montalban, B.; Arias, A.; Redondo, O.; Tenias, J.M. Efficacy of dietary treatment for inducing disease remission in eosinophilic gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 56–64. [Google Scholar]
- Lee, C.M.; Changchien, C.S.; Chen, P.C.; Lin, D.Y.; Sheen, I.S.; Wang, C.S.; Tai, D.I.; Sheen-Chen, S.M.; Chen, W.J.; Wu, C.S. Eosinophilic gastroenteritis: 10 years experience. Am. J. Gastroenterol. 1993, 88, 70–74. [Google Scholar] [PubMed]
- Verheijden, N.A.; Ennecker-Jans, S.A. A rare cause of abdominal pain: Eosinophilic gastroenteritis. Neth J. Med. 2010, 68, 367–369. [Google Scholar] [PubMed]
- Alfadda, A.; Storr, M.A.; Shaffer, E.A. Eosinophilic colitis: Epidemiology, clinical features, and current management. Ther. Adv. Gastroenterol. 2010, 4, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, R.A.; Prindiville, T.P.; Pecha, R.E.; Ruebner, B.H. Unusual presentations of eosinophilic gastroenteritis: Case series and review of literature. World J. Gastroenterol. 2009, 15, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Elsing, C.; Placke, J.; Gross-Weege, W. Budesonide for the treatment of obstructive eosinophilic jejunitis. Z. Gastroenterol. 2007, 45, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Seelen, J.L.; You, P.H.; De Vries, A.C.; Puylaert, J.B.C.M. Eosinophilic enteritis presenting as acute abdomen: US features of two cases. Gastrointest. Radiol. 1992, 17, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Alnaser, S.; Aljebreen, A.M. Endoscopic Ultrasound and Hisopathologic Correlates in Eosinophilic Gastroenteritis. Saudi J. Gastroenterol. 2007, 13, 91–94. [Google Scholar] [PubMed]
- Savino, A.; Salvatore, R.; Cafarotti, A.; De Sanctis, S.; Angelucci, D.; Mohn, A.; Chiarelli, F.; Pelliccia, P. Role of ultrasonography in the diagnosis and follow-up of pediatric eosinophilic gastroenteritis: A case report and review of the literature. Ultraschall Med. 2011, 32 (Suppl. 2), E57–E62. [Google Scholar] [CrossRef] [PubMed]
- Buljevac, M.; Urek, M.C.; Stoos-Veić, T. Sonography in diagnosis and follow-up of serosal eosinophilic gastroenteritis treated with corticosteroid. J. Clin. Ultrasound 2005, 33, 43–46. [Google Scholar] [CrossRef]
- Shin, W.G.; Park, C.H.; Lee, Y.S.; Kim, K.O.; Yoo, K.S.; Kim, J.H.; Park, C.K. Eosinophilic enteritis presenting as intussusception in adult. Korean J. Intern. Med. 2007, 22, 13–17. [Google Scholar] [CrossRef]
- Mostbeck, G.; Adam, E.J.; Nielsen, M.B.; Claudon, M.; Clevert, D.; Nicolau, C.; Nyhsen, C.; Owens, C.M. How to diagnose acute appendicitis: Ultrasound first. Insights Imaging 2016, 7, 255–263. [Google Scholar] [CrossRef]
- Yilmaz, M.; Akbulut, S.; Kutluturk, K.; Sahin, N.; Arabaci, E.; Ara, C.; Yilmaz, S. Unusual histopathological findings in appendectomy specimens from patients with suspected acute appendicitis. World J. Gastroenterol. 2013, 19, 4015–4022. [Google Scholar] [CrossRef]
- Alemayehu, H.; Snyder, C.L.; St Peter, S.D.; Ostlie, D.J. Incidence and outcomes of unexpected pathology findings after appendectomy. J. Pediatr. Surg. 2014, 49, 1390–1393. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertè, R.; Soru, P.; Vecchi, M.; Fraquelli, M. Eosinophilic Gastroenteritis with Appendix Involvement: Role of Intestinal Ultrasound. Appl. Sci. 2019, 9, 1037. https://doi.org/10.3390/app9061037
Bertè R, Soru P, Vecchi M, Fraquelli M. Eosinophilic Gastroenteritis with Appendix Involvement: Role of Intestinal Ultrasound. Applied Sciences. 2019; 9(6):1037. https://doi.org/10.3390/app9061037
Chicago/Turabian StyleBertè, Roberto, Pietro Soru, Maurizio Vecchi, and Mirella Fraquelli. 2019. "Eosinophilic Gastroenteritis with Appendix Involvement: Role of Intestinal Ultrasound" Applied Sciences 9, no. 6: 1037. https://doi.org/10.3390/app9061037
APA StyleBertè, R., Soru, P., Vecchi, M., & Fraquelli, M. (2019). Eosinophilic Gastroenteritis with Appendix Involvement: Role of Intestinal Ultrasound. Applied Sciences, 9(6), 1037. https://doi.org/10.3390/app9061037




