Detection of Additives with the Help of Discrete Geometrical Invariants
Abstract
Featured Application
Abstract
1. Introduction and Formulation of the Problem
- (1)
- Determination of some output parameters that are related to monotone concentration of the electroactive components in conditions of strongly fluctuating currents on the C(“active”) and Pt (“inertial”) electrodes, for construction of the desired calibration curves.
- (2)
- Comparison of the obtained characteristics for the C- and Pt-electrodes and derivation of the calibration/monotone curves with respect to the given d-tryptophan concentration.
- (a)
- Its generality (universality) implies the potential to compare any couple of random sets assigned by their coordinates (Y1k, Y2k) in a 2D plane.
- (b)
- All parameters are expressed in terms of the integer moments and their inter-correlations.
- (c)
- The number of parameters is finite, and they have clear geometrical interpretation.
- (d)
- The proposed method oriented for analysis of discrete sets is free from any unjustified supposition and treatment errors.
- (e)
- The proposed approach can compare random sequences having different sampling volumes in the frame of the same set of statistical parameters.
2. Materials and Methods
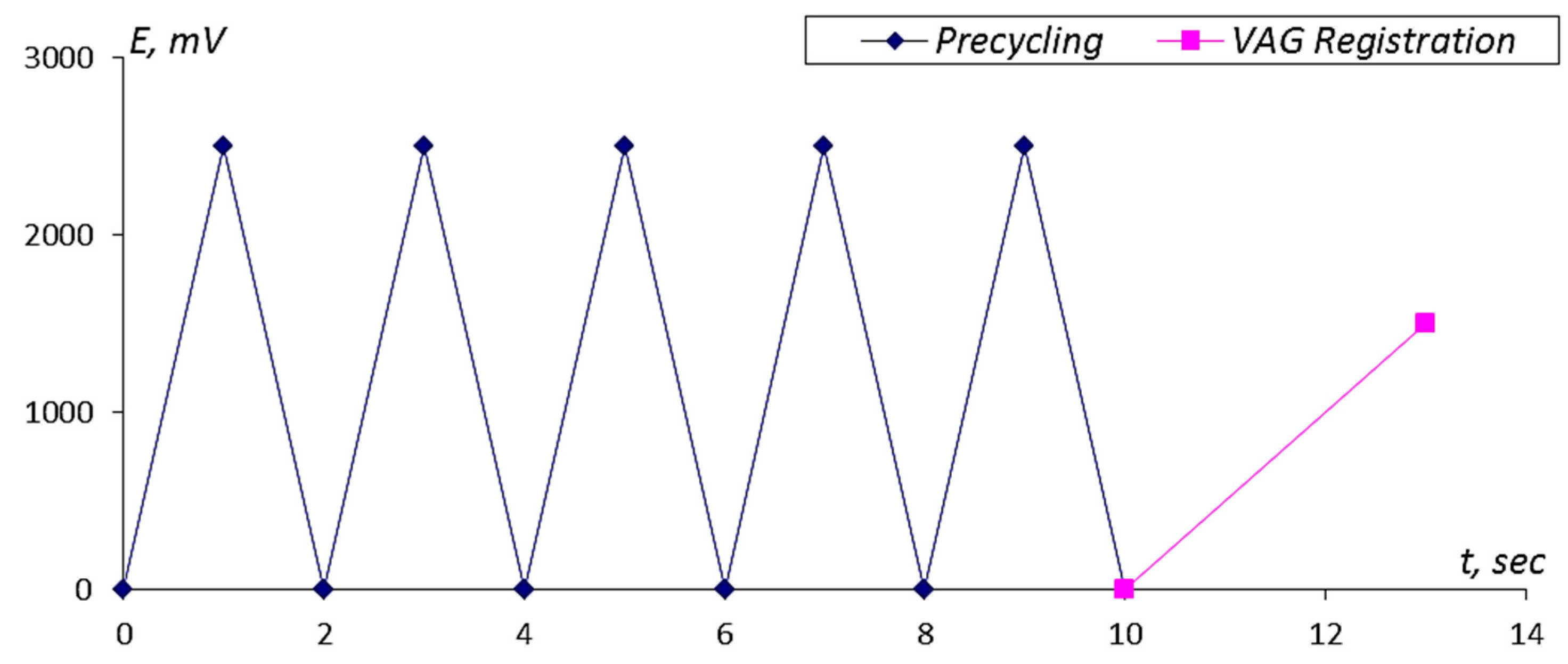
- (1)
- Electrochemical regeneration (precycling)—five successive cycles with a potential scan rate of 2.5 V/s in the range of potentials from 0–2.5 V.
- (2)
- Registration of the VAG curve in the analyzed solution at a potential scan rate of 0.5 V/s in the range of potentials from 0–1.5 V.
3. Description of the Treatment Procedure
- (a)
- Which curve is more sensitive to the presence of a Pt- or C-electrode?
- (b)
- How many monotone curves can be derived from the parameters that form the invariants of the second/fourth orders?
- (c)
- Which space is more sensitive to the presence of d-tryptophan in the solute: the data space or the measurement space?
- We should take into account the behavior of parameter σB,C(c), which incorporates the influence of the higher moments. This dependence can be important for other applications.
- The behavior of the value of the invariant I4(c) (derived from Equation (A17))) is also important and should be taken into account, as well.
4. Results and Discussions
- (1)
- The C- and Pt-electrodes at the repeated functioning cycles exhibit the aging phenomenon that leads, in turn, to the temporal signal drift. This drift distorts the measured VAGs. This effect is expressed clearly in the analysis of the curves associated with the measurement space. For a quantitative description, as well as the control and elimination of this negative phenomenon, we use the DGI method that frees the system from the imposed model and errors related to the treatment procedure.
- (2)
- The integral curves in the measurement space because of the DGI application demonstrate the sensitivity of both electrodes to the amino-acid concentration variations (see the monotone curves obtained for the data space).
- (3)
- The aging effect confirms our previous conclusions; after achieving the activated state, the electrode behavior at the given potential sweep depends on the number of cycles to a lesser degree. This effect is expressed clearly for the more inertial Pt-electrodes. For C-electrodes, we presumably observe the varied behavior covering all 1000 measurements.
- (4)
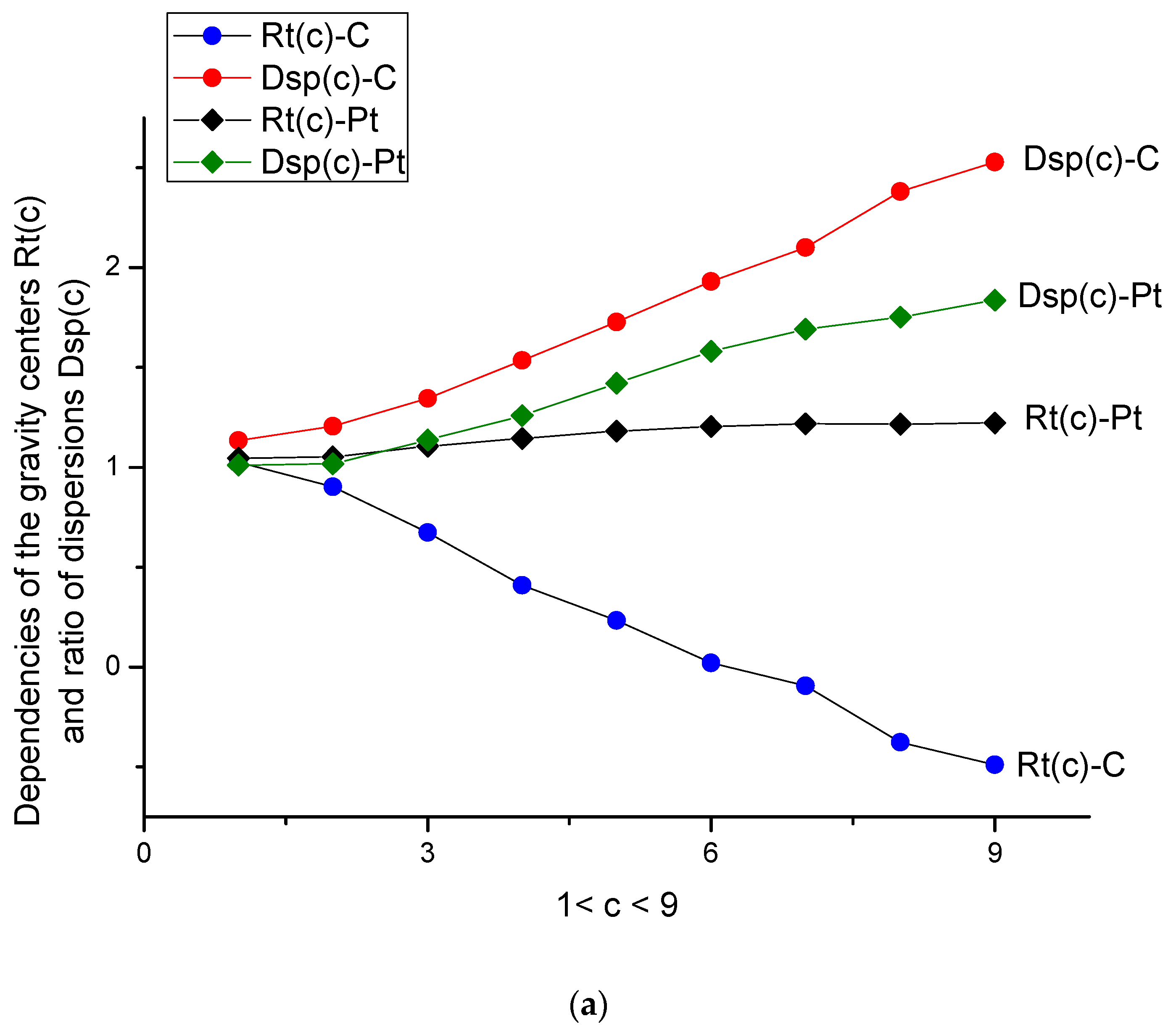
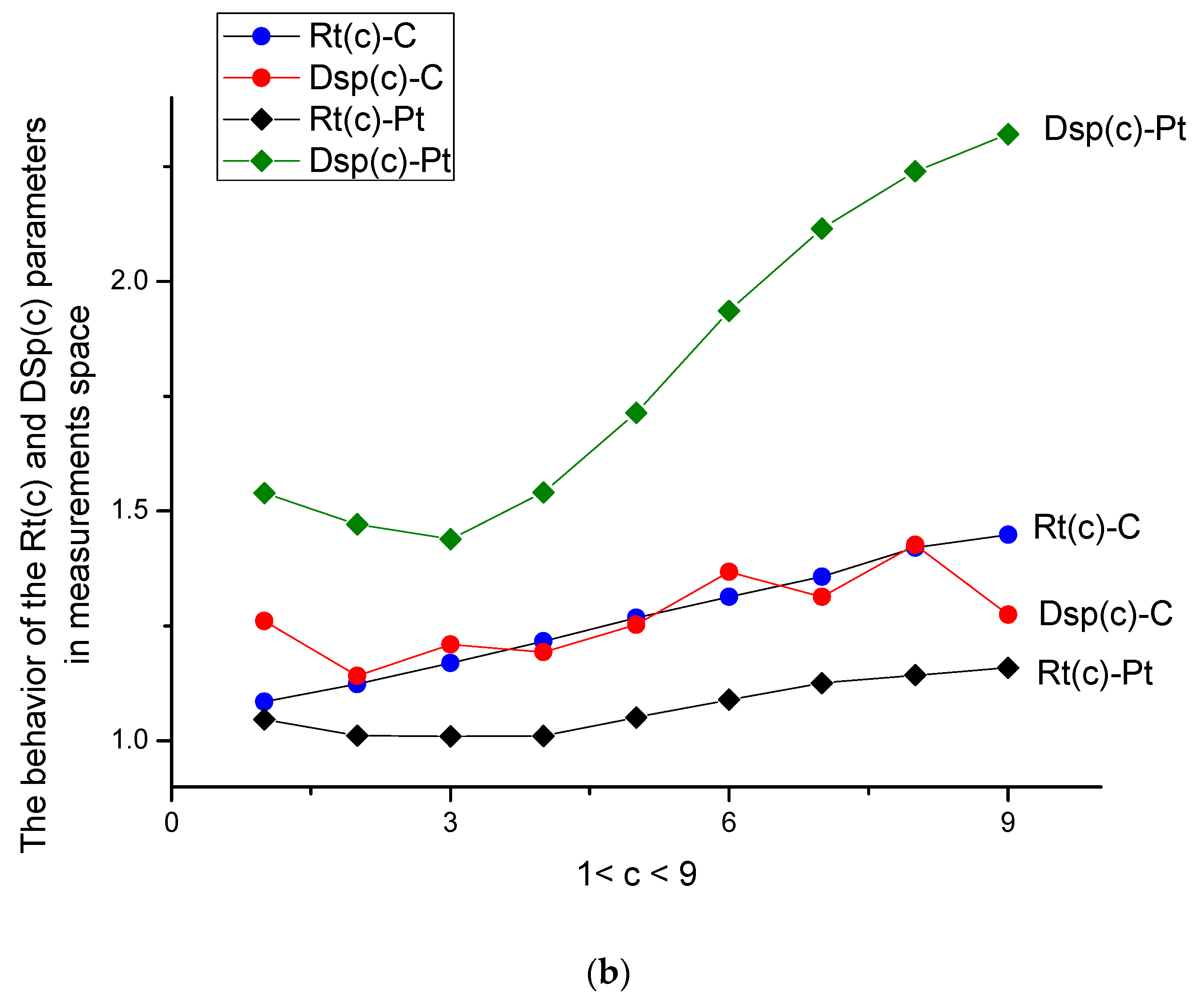
- The ratio Rt(c) = <y(c)>/<x> (remember that the variable <x> = <y(0)> is always related to the buffer solution), while dispersion Dsp(c) = <DY2>/ <DX2>, depicted in Figure 4a, characterizes the center of gravity and its standard deviation. They are more sensitive to the surface changes evoked by the aging phenomenon. Both parameters have monotone behavior for C- and Pt-electrodes in the presence/absence of the amino acid. For the Pt-electrode, this monotone dependence is weak; it confirms the high stability of the Pt-electrode during electro oxidation processes. We should stress here that Figure 4a,b are more informative and allow characterizing the d-tryptophan behavior, as well as comparing its electroactivity with the interaction of C- and Pt-electrodes.
- (a)
- In data space, the dispersion curves Dsp for C- and Pt-electrodes differ by their mutual location with respect to each other; the value Dsp(c) (C-electrode) exceeds the curve Dsp(c) (Pt-electrode) for all values of c. The gravity ratios Rt(c) for both types of electrodes are strongly deviated from each other as well.
- (b)
- In measurement space, a significant difference is observed for the curve Dsp(c) (Pt). It is explained by the excess electroactivity of the tryptophan molecule in its interaction with the Pt-electrode. One can stress here that the clearly expressed peaks on the initial VAGs were not observed.
- (5)
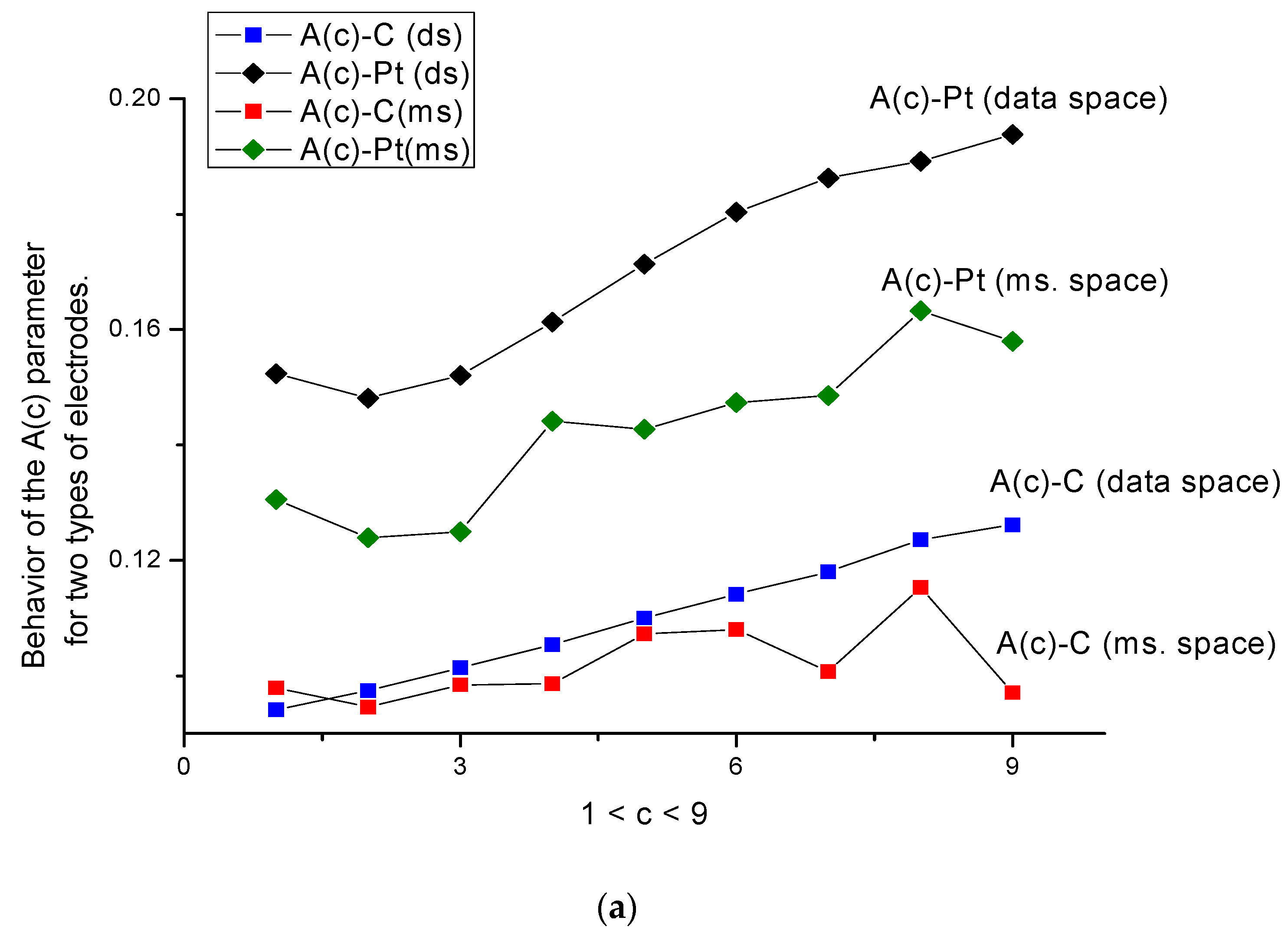
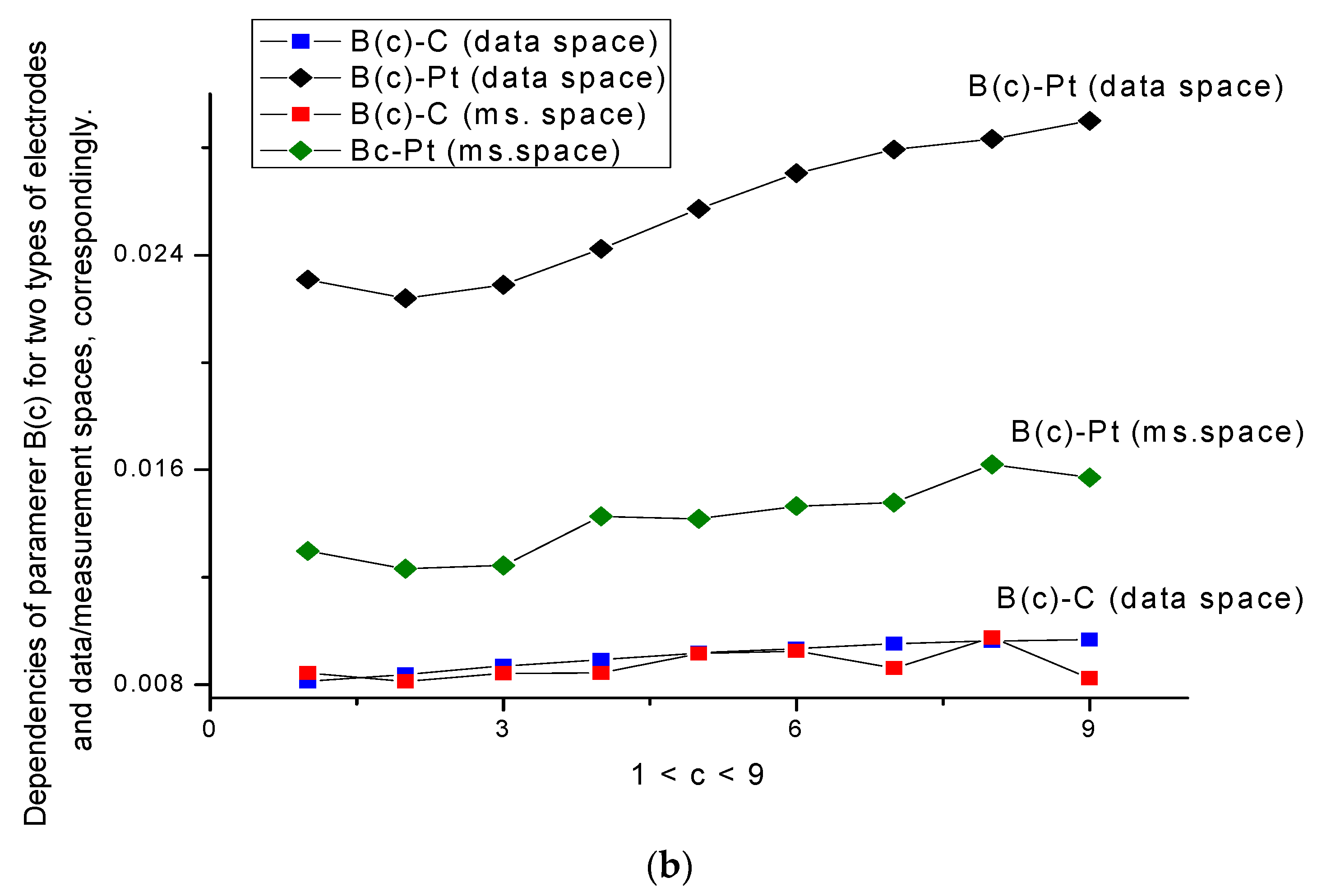
- The behavior of parameters A(c) and B(c) (Figure 5a,b) is more sensitive to the surface changes; the behavior of parameter A(c) for both types of electrodes is characterized by a monotone behavior, which increases in the presence/absence of the amino acid.
- (6)
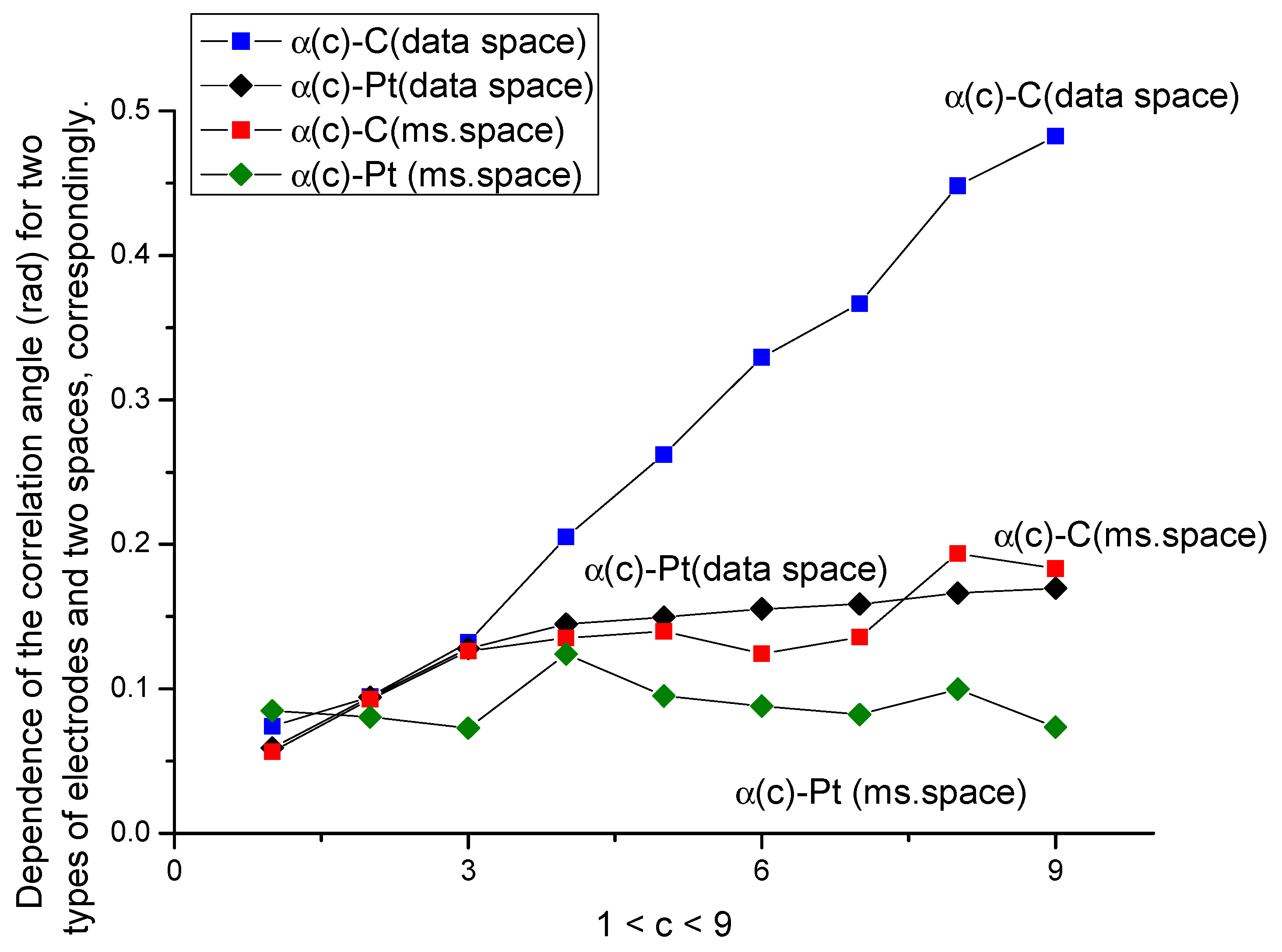
- The ellipse rotation (correlation) angle α for the C-electrode in the absence of amino acid does not correlate with the number of oxidation/reduction cycles; furthermore, it does not depend on the sensor duration and cannot be associated with the aging phenomenon. However, as shown in Figure 6, this parameter correlates strongly with d-tryptophan concentration. The calculated curve is monotone with respect to increasing the concentration of d-tryptophan. For the Pt-electrode after 300 cycles in the presence/absence of amino acid, this curve has an increasing trend. We relate this increasing tendency with additional processes of oxidation in the background solution. From our point of view, this parameter can be used for calibration purposes of some non-electroactive components of the background solute.
- (7)
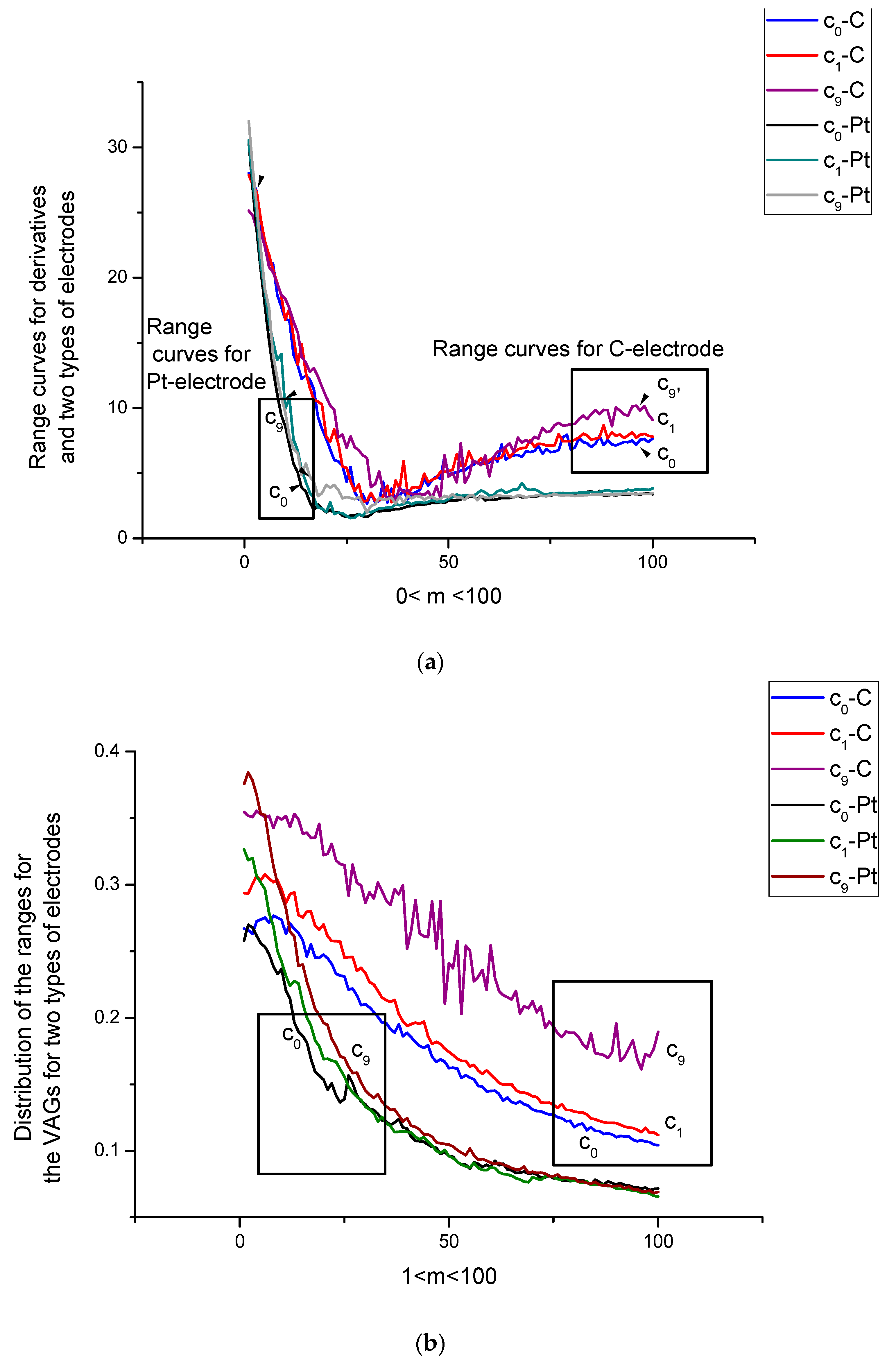
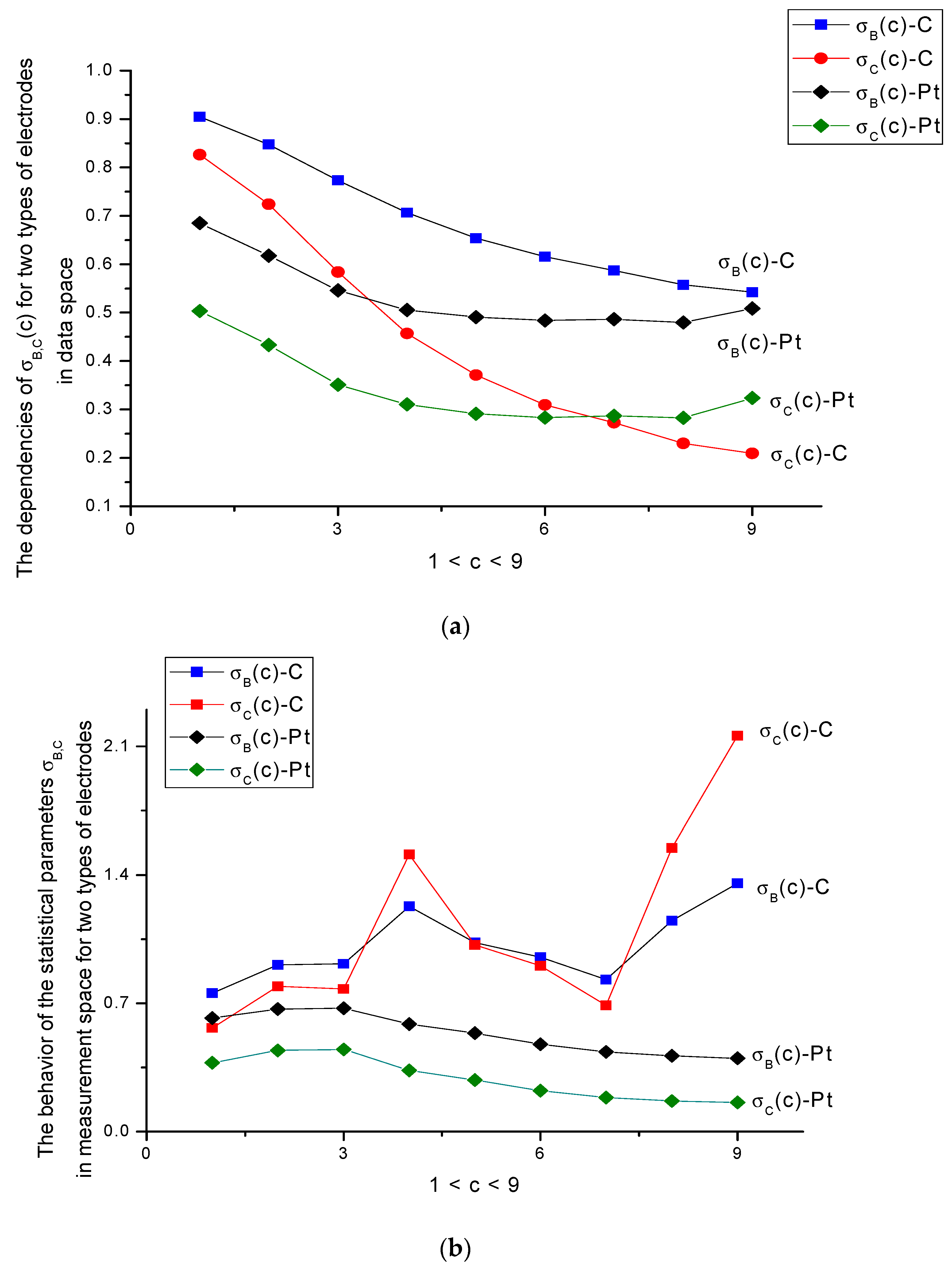
- The parameters analyzed above reflect the specificity of electrode processes from different points of view. For the more active C-electrode, which has better oxidation characteristics, many output DGI parameters changed more intensively in comparison with the more inertial Pt-electrode. The Pt-electrode is more stable in oxidation processes. Being a more “stable” material with respect to the transfer electron process related to the oxidation of amino acids, it requires the calculation of an extended set of “fine” parameters that are sensitive to concentration variations of the electroactive substance. For the given case, we associate these variations with the behavior of parameter σB,C. As it follows from the analysis of Figure 7a, these parameters have monotone and decreasing characteristics against the concentration in data space. In the measurement space shown in Figure 7b, these parameters characterize again the electroactivity of the C-electrode, which has variable characteristics, compared to the relatively stable behavior of the Pt-electrode. Again, the 300-cycle boundary is conserved. Only after this limit does the Pt-electrode tend to the aging regime.
- (8)
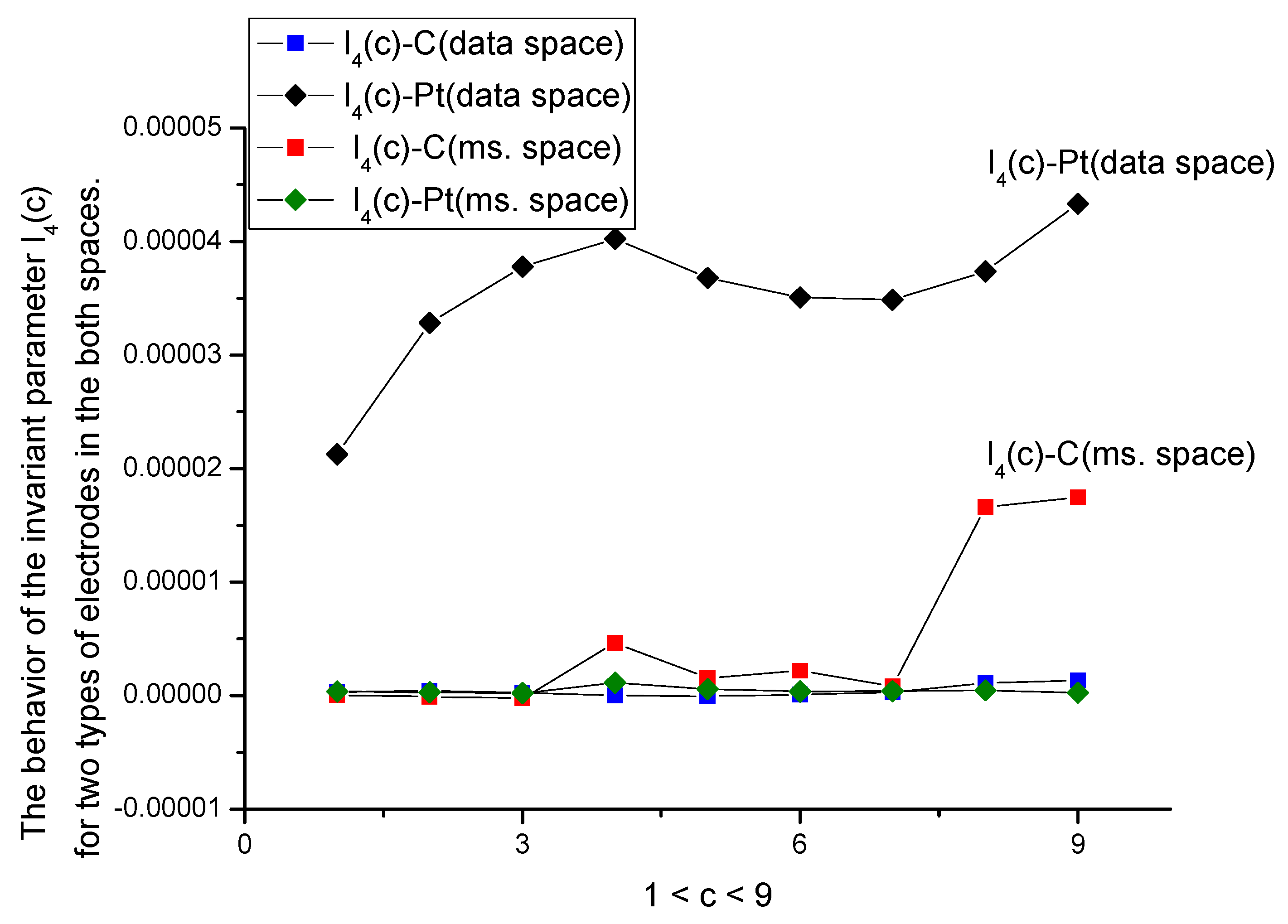
- The proposed DGI approach is rather general and enables comparing random curves with different samples, using the same set of parameters expressed in terms of moments up to the fourth order. We showed also that these parameters have different sensitivity with respect to the input factor (in our case, this factor is associated with concentration of the amino acid). Comparing both spaces, one can say that the data space is more stable in comparison with the measurement space; the latter actually demonstrates the temporal evolution (each measurement together with “idle” time occupies 13 s) of the whole process of the interaction of the amino acid with the given electrode. The DGI approach allows obtaining a set of calibration curves and establishing a natural limit associated with parameter I4(c) (see Figure 8 for details). This parameter has a small value and loses its monotone behavior for the both spaces.
Author Contributions
Funding
Conflicts of Interest
The Basic Abbreviations
| DGI | Discrete geometrical invariant |
| SVD | Single-valued decomposition |
| VAG(s) | Voltammogram(s) |
Appendix A
References
- Winquist, F.; Wide, P.; Lundstrom, I. An electronic tongue based on voltammetry. Anal. Chim. Acta 1997, 357, 21–26. [Google Scholar] [CrossRef]
- Ulrich, C.; Petersson, H.; Sundgren, H.; Bjorefors, F.; Krantz-Rulcker, C. Simultaneous estimation of soot and diesel contamination in engine oil using electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2007, 127, 613–618. [Google Scholar] [CrossRef]
- Kissinger, P.T.; Heineman, W.R. Laboratory Techniques in Electroanalytical Chemistry; Marcel Dekker Inc.: New York, NY, USA, 1996. [Google Scholar]
- Ríos, Á.; Zougagh, M.; Avila, M. Miniaturization through lab-on-a-chip: Utopia or reality for routine laboratories? A review. Anal. Chim. Acta 2012, 740, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ramesh, K.; Ahmed, S.; Kakkar, V. Lab-on-Chip Technology: A Review on Design Trends and Future Scope in Biomedical Applications. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 311–322. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Apetrei, C.; Lozano, J.; Anyogu, A. Potential use of electronic noses, electronic tongues and biosensors as multisensor systems for spoilage examination in foods. Trends Food Sci. Technol. 2018, 80, 71–92. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. On-line monitoring of food fermentation processes using electronic noses and electronic tongues: A review. Anal. Chim. Acta 2013, 804, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Corsi, C. Smart Sensors. Infrared Phys. Technol. 2007, 49, 192–197. [Google Scholar] [CrossRef]
- Meixner, H.; Jones, R. Sensors: Micro- and Nanosensor Technology Trends in Sensor Markets; Wiley-VCH: Hoboken, NJ, USA, 2008; Volume 8, 566p. [Google Scholar]
- Esbensen, K.H. Multivariate Data Analysis—In Practice. An Introduction to Multivariate Data Analysis and Experimental Design, 5th ed.; CAMO AS: Oslo, Norway, 2001. [Google Scholar]
- Jameson, L.; Waseda, T. Error Estimation Using Wavelet Analysis for Data Assimilation: EEWADA. Am. Meteorol. Soc. J. Atmos. Ocean. Technol. 2000, 17, 1235–1246. [Google Scholar] [CrossRef]
- Timashev, S.F.; Polyakov, Y.S. Review of Flicker-noise spectroscopy in electrochemistry. Fluct. Noise Lett. 2007, 7, R15–R47. [Google Scholar] [CrossRef]
- Richards, E.; Bessant, C.; Saini, S. Simultaneous quantification of analytes in quaternary mixtures using dual pulse staircase voltammetry and genetically optimised neural networks. Analyst 2004, 129, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Nigmatullin, R.R.; Budnikov, H.C.; Sidelnikov, A.V.; Maksyutova, E.I. Application of the Discrete Geometrical Invariants to the Quantitative Monitoring of the Electrochemical Background. Res. J. Math. Comput. Sci. (RJMCS) 2017, 1, 7. [Google Scholar]
- Babenko, Y.I. Power-Law Invariants of Discrete Sets; Professional Publishing House: S-Petersburg, Russian, 2014. [Google Scholar]

| Method | Basic Limitations | Comments |
|---|---|---|
| Principal component analysis [10] | In single-valued decomposition (SVD), only 3–4 basic components are used. The influence of other components is not evaluated and explained properly. | This is a widely used method in chemometrics for the detection of possible pair correlations between two random sequences. |
| Wavelet decomposition [11] | The criterion of the selection of the proper wavelet from the wide wavelet family is absent. Each chosen wavelet has its own treatment error, and, in many cases, it cannot be evaluated and applied for the analysis of nanonoises in the interval 10−9–10−6 A. | There exist uncontrollable errors related to the application of continuous wavelets to discrete data. This method is important in the detection of small signals associated with the detection of “trace” substances. |
| Timashev’s method (flicker-noise spectroscopy) [12]. | The basic supposition is related to a continuous representation of an initial sequence (with imposed definitions of intermittency, spikes, outliers, etc.) | The final expressions are derived from the apparatus of continuous mathematics and integral F-transform. These treatment errors are not properly evaluated in analysis of the chosen discrete data. |
| Artificial neural network (ANN) [13] | There is a limited possibility for the physical interpretation of the model parameters. | Analytical possibilities of ANN are stipulated by a wide choice of different transfer functions, and by a wide variation of the number of “neurons” in the intermediate layer, which limits the possibilities of the optimal (well-educated) selection of the desired transfer function. |
| Projection on latent structures, partial least squares (PLS) [14] | The PLS method does not allow deriving the reliable calibration model based on the measured voltammograms (VAGs) at multiple sensors because of the appearance of ellipse-like clusters in the score plots. | PLS regression is the generalization of the usual one-dimensional (1D) linear regression for the case of many independent variables. The fitting error cannot be properly evaluated. |
| Notation of the Solution Affected by d-Tryptophan Additive | Volume of Additive (mL) | d-Tryptophan Concentration (10−5 mol·L−1) |
|---|---|---|
| “0”-buffer solution | 0 | 0 |
| “1” | 0.7 | 6.54 |
| “2” | 1.4 | 12.3 |
| “3” | 2.1 | 17.4 |
| “4” | 2.8 | 21.9 |
| “5” | 3.5 | 25.9 |
| “6” | 4.2 | 29.6 |
| “7” | 4.9 | 32.9 |
| “8” | 5.6 | 35.9 |
| “9” | 6.3 | 38.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigmatullin, R.; Vorobev, A.; Budnikov, H.; Sidelnikov, A.; Maksyutova, E. Detection of Additives with the Help of Discrete Geometrical Invariants. Appl. Sci. 2019, 9, 926. https://doi.org/10.3390/app9050926
Nigmatullin R, Vorobev A, Budnikov H, Sidelnikov A, Maksyutova E. Detection of Additives with the Help of Discrete Geometrical Invariants. Applied Sciences. 2019; 9(5):926. https://doi.org/10.3390/app9050926
Chicago/Turabian StyleNigmatullin, Raoul, Artem Vorobev, Herman Budnikov, Artem Sidelnikov, and Elza Maksyutova. 2019. "Detection of Additives with the Help of Discrete Geometrical Invariants" Applied Sciences 9, no. 5: 926. https://doi.org/10.3390/app9050926
APA StyleNigmatullin, R., Vorobev, A., Budnikov, H., Sidelnikov, A., & Maksyutova, E. (2019). Detection of Additives with the Help of Discrete Geometrical Invariants. Applied Sciences, 9(5), 926. https://doi.org/10.3390/app9050926






