PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations
Abstract
:1. Introduction
2. Materials and Methods
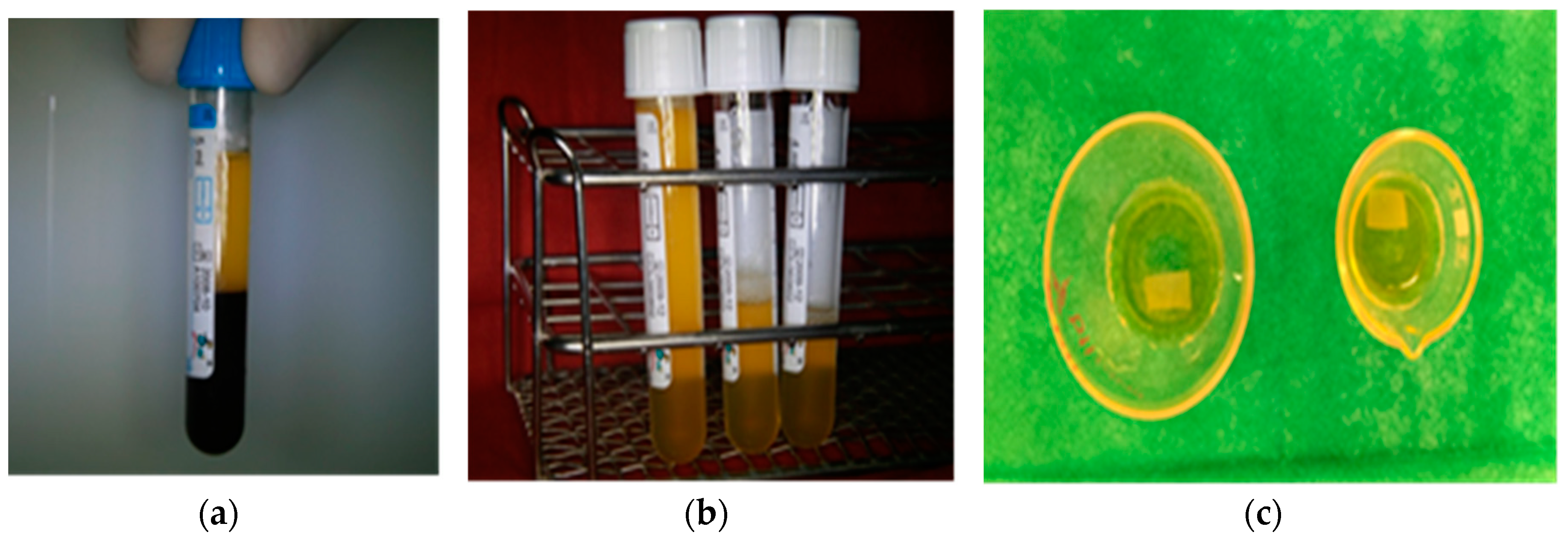
2.1. Preparation of Plasma Rich in Growth Factors (PRGF), Hematology Parameters, and Growth Factor Content
2.2. PRGF Modified Collagen Membranes
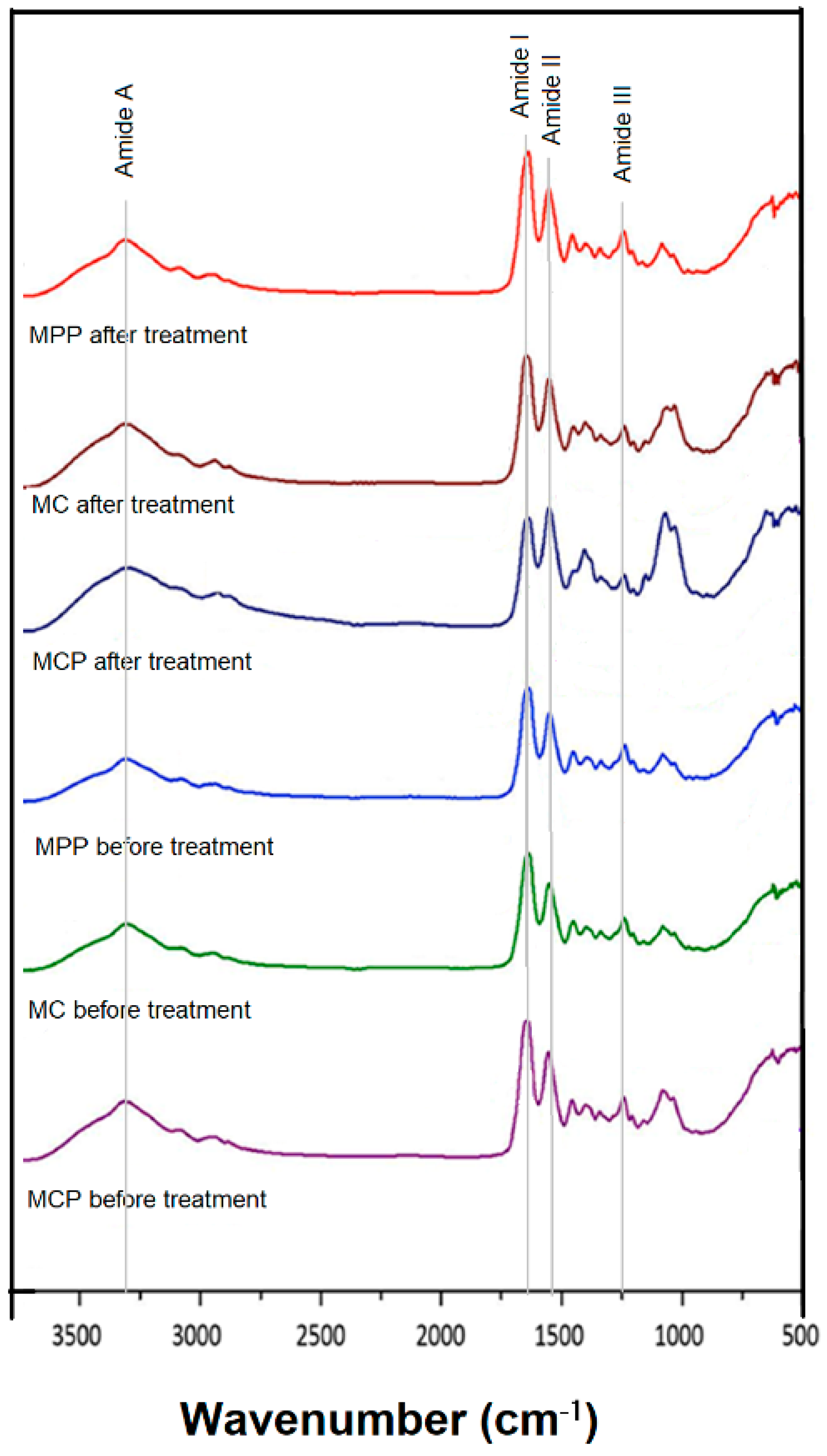
2.3. FTIR Spectroscopy
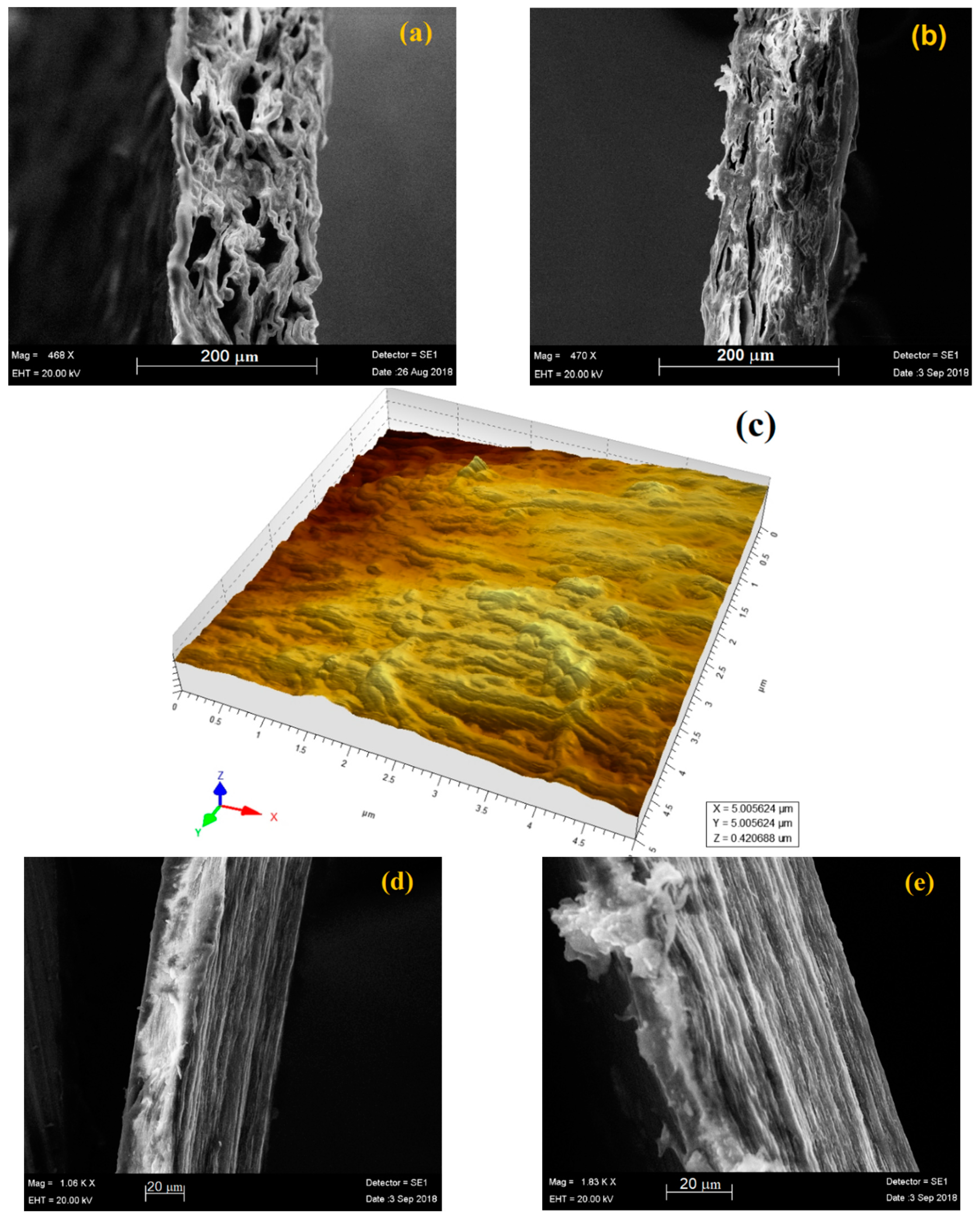
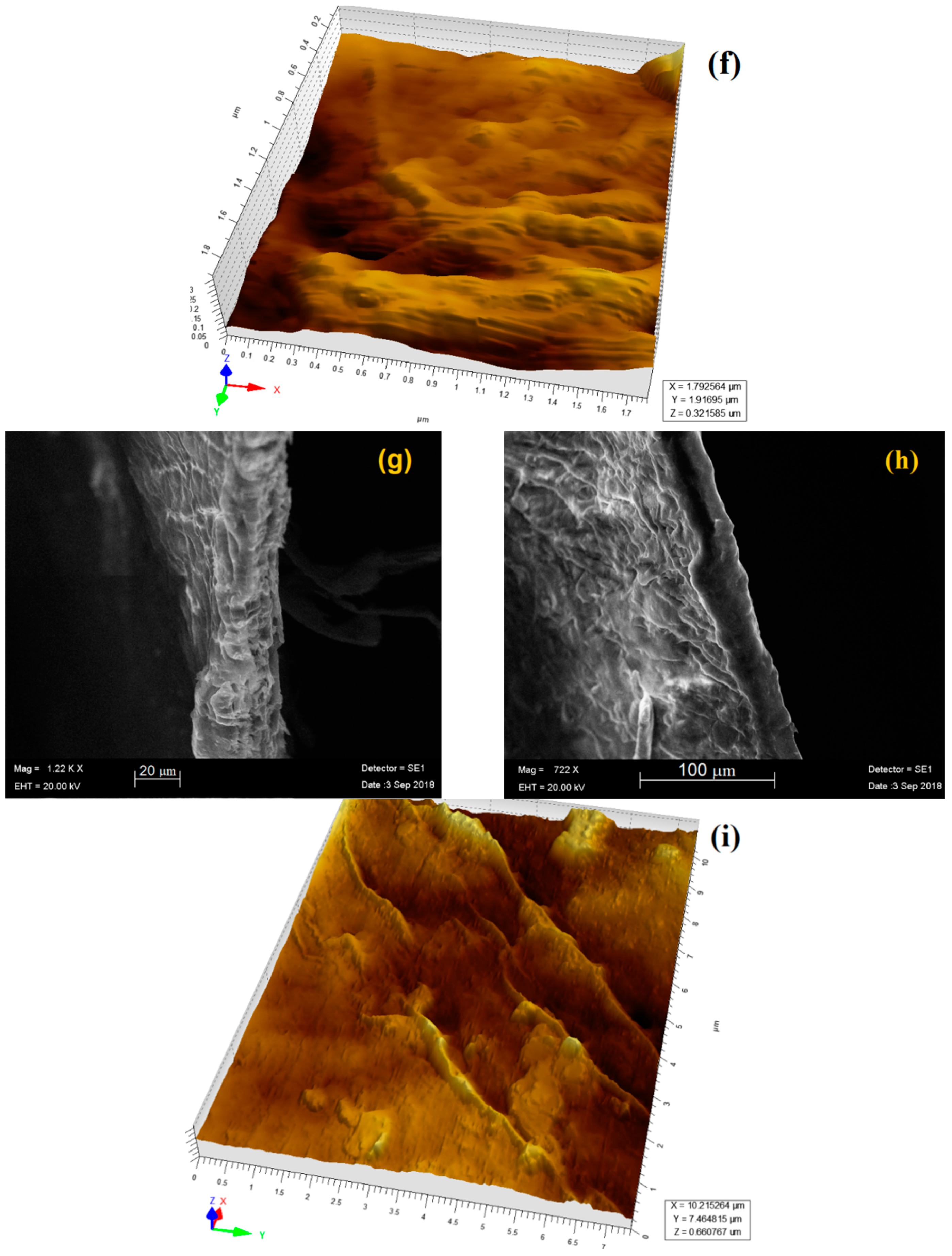
2.4. AFM and SEM Analysis
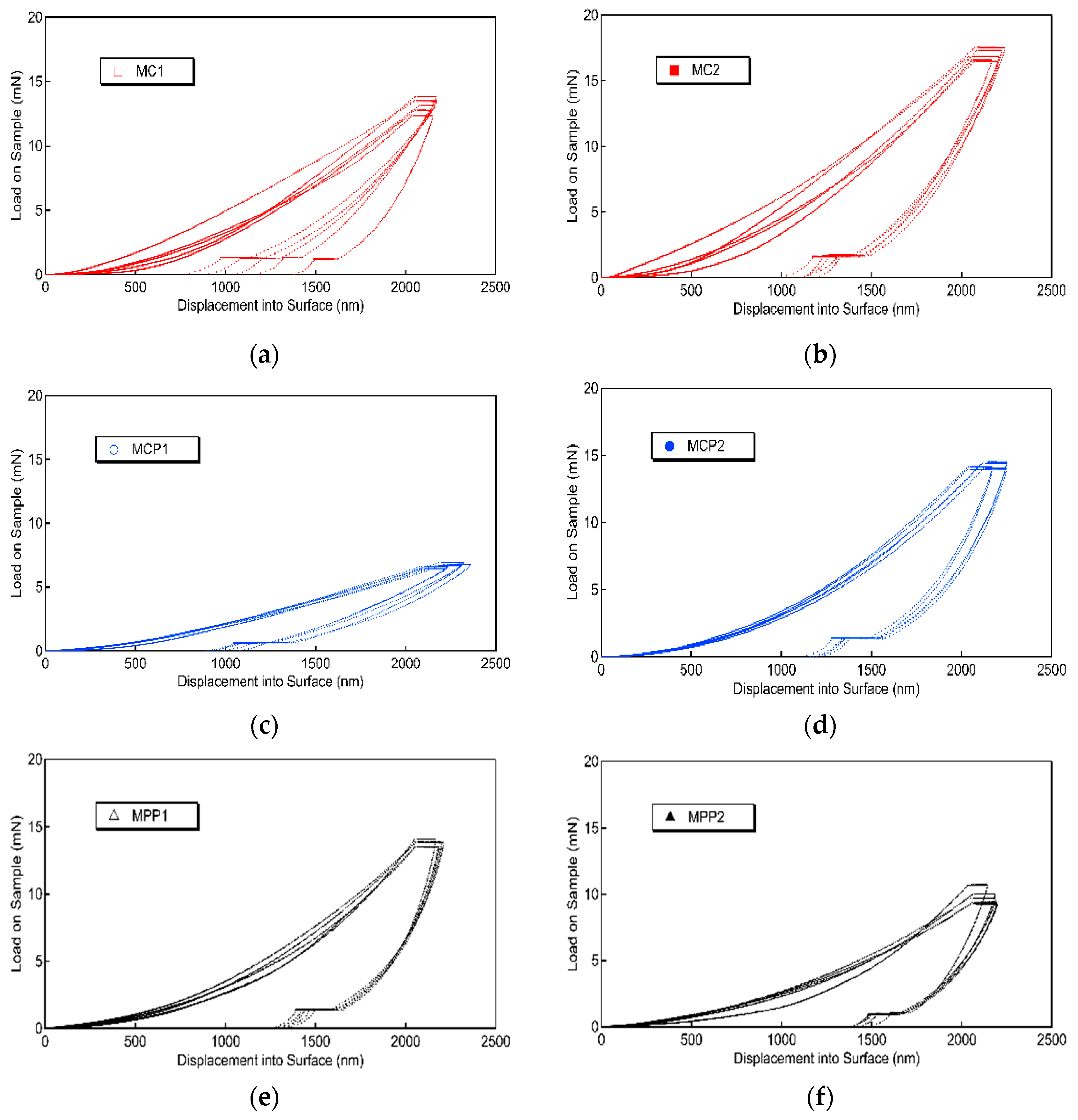
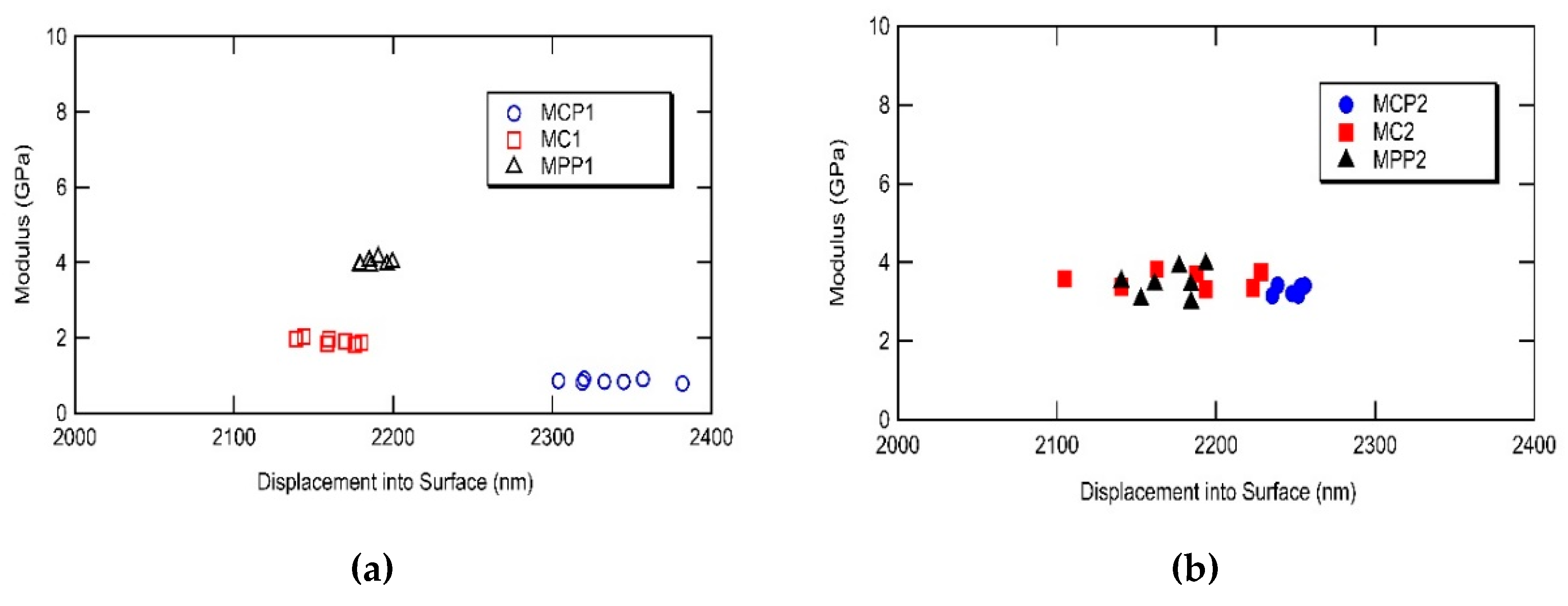
2.5. Nanoindentation Measurements
2.6. Enzymatic Degradation Test
3. Results
3.1. Preparation of Plasma Rich in Growth Factors (PRGF), Hematology Parameters, and Growth Factor Content
3.2. Characterization of PRGF-Modified Collagen Membranes by FTIR Spectroscopy
3.3. Morphological Characterization by SEM and AFM
3.4. Nanoindentation Measurements
3.5. Enzymatic Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Oh, J.-H. New resorbable membrane materials for guided bone regeneration. Appl. Sci. 2018, 8, 2157. [Google Scholar] [CrossRef]
- Choi, N.-R.; Sándor, G.K.; Kim, Y.D. Efficacy of collagen-based membranes in alveolar bone augmentation. Appl. Sci. 2018, 8, 2048. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982–1992: A decade of technology development for guided tissue regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.; Scantlebury, T.V.; Sanchez, R.; Whitley, N.; Ambruster, J. Membrane design criteria for guided bone regeneration of the alveolar ridge. In Guided Bone Regeneration in Implant Dentistry; Buser, D., Ed.; Quintessence Publishing Co. Inc.: Batavia, IL, USA, 1994; pp. 101–136. [Google Scholar]
- Monteiro, A.S.; Macedo, L.G.; Macedo, N.L.; Balducci, I. Polyurethane and PTFE membranes for guided bone regeneration: Histopathological and ultrastructural evaluation. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, e401–e406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Huang, C.; Mo, A.; Li, J.; Zuo, Y. Biological properties of an anti-bacterial membrane for guided bone regeneration: An experimental study in rats. Clin. Oral Implants Res. 2010, 21, 321–327. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, M.; Larsen, P.; Miloro, M.; Beck, M. Comparison of resorbable and nonresorbable guided bone regeneration materials: A preliminary study. Int. J. Oral Maxillofac. Implants 1998, 13, 30–35. [Google Scholar] [PubMed]
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implants Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Reichert, C.; Götz, H.; Röhrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral Maxillofac. Implants 2012, 27, 146–154. [Google Scholar] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagen at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membrane used in dentistry. Annali dell’Istituto Superiore di Sanità 2015, 51, 229–235. [Google Scholar] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chua, T.-M.G.; Kowolikd, M.J.; Janowskie, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration. A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R.; Troya, M.; Zalduendo, M.; De la Fuente, M.; Pino, A.; Muruzabal, F.; Orive, G. Implementation of a more physiological plasma rich in growth factor (PRGF) protocol: Anticoagulant removal and reduction in activator concentration. Platelets 2016, 27, 459–466. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Orive, G.; Andia, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Pino, A.; Jaen, P.; Orive, G. Plasma Rich in Growth Factors enhances wound healing and protects from photo-oxidative stress in dermal fibroblasts and 3D skin models. Curr. Pharm. Biotechnol. 2016, 17, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E. Plasma Rich in Growth Factors: Preliminary Results of Use in the Preparation of Future Sites for Implants. Int. J. Oral Maxillofac. Implants 1999, 14, 529–535. [Google Scholar] [PubMed]
- Nishiyama, K.; Okudera, T.; Watanabe, T.; Isobe, K.; Suzuki, M.; Masuki, H.; Okudera, H.; Uematsu, K.; Nakata, K.; Kawase, T. Basic characteristics of plasma rich in growth factors (PRGF): Blood cell components and biological effects. Clin. Exp. Dent. Res. 2016, 18, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Pharr, G.; Oliver, W.C.; Brotzen, F. On the generality of the relationship among contact stiffness, contact area and elastic modulus during indentation. J. Mater. Res. 1992, 7, 613–617. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Hou, Y.; Xiang, L.; Wu, Y.; Qu, Y.; Man, Y. Application of PEG and EGCG modified collagen-base membrane to promote osteoblasts proliferation. Mater. Sci. Eng. C 2017, 76, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; An, S.-J.; Lim, Y.-M.; Huh, J.-B. The efficacy of electron beam irradiated bacterial cellulose membranes as compared with collagen membranes on guided bone regeneration in peri-implant bone defects. Materials 2017, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Bielli, A.; Bernardini, R.; Varvarasc, D.; Rossic, P.; Di Blasi, G.; Petrella, G.; Buonomo, O.C.; Mattei, M.; Orlandi, A. Characterization of a new decellularized bovine pericardial biological mesh: Structural and mechanical properties. J. Mech. Behav. Biomed. Mater. 2018, 78, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- Tabor, A.J.; Robinson, A.; Pinto, B.I.; Kellar, R.S. Platelet Rich Plasma combined with electrospun collagen scaffold: In vivo and in vitro wound healing effects. Clin. Res. Dermal Open Access 2016, 3, 1–8. [Google Scholar]
- Shiga, Y.; Kubota, G.; Orita, S.; Inage, K.; Kamoda, H.; Yamashita, M.; Yseki, T.; Ito, M.; Yamauchi, K.; Eguchi, Y.; et al. Freeze-dried human platelet-rich plasma retains activation and growth factor expression after eight week preservation period. Asian Spine J. 2017, 11, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.H.; Klepeis, V.E.; Nugent, M.A.; Trinkaus-Randall, V. TGF-β1 regulates TGF-β1 and FGF-2 mRNA expression during fibroblast wound healing. Mol. Pathol. 2002, 55, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; Merayo-Lloves, J.; De la Fuente, M.; Muruzabal, F.; Orive, G. Plasma Rich in Growth Factors (PRGF-Endoret) stimulates proliferation and migration of primary keratocytes and conjunctival fibroblasts and inhibits and reverts TGF-1–induced myodifferentiation. Inves. Ophthal. Vis. Sci. 2011, 52, 6066–6073. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, N.; Yazdi, I.K.; Nabavinia, M.; Gemma, A.; Fanelli, A.; Caizzone, A.; Ptaszek, L.M.; Sinha, I.; Khademhosseini, A.; Ruskin, J.N.; et al. Patient-specific bioinks for 3D bioprinting of tissue engineering scaffolds. Adv. Healthc. Mater. 2018, 7, e1701347. [Google Scholar] [CrossRef] [PubMed]
- Zellin, G.; Linde, A. Effect of different osteopromotive membranes porosities on experimental bone neogenesis in rats. Biomaterials 1996, 17, 695–702. [Google Scholar] [CrossRef]
- Zabrowska, M.; Bodin, A.; Backdahl, H.; Popp, J.; Golstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Yova, D.; Stylianou, A.; Balagiannis, G. The effect of UV irradiation on collagen D-band revealed by Atomic Force Microscopy. Scanning 2015, 37, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Yova, D. Surface nanoscale imaging of collagen thin films by Atomic Force Microscopy. Mater. Sci. Eng. C 2013, 33, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Murdock, K.; Martin, C.; Sun, W. Characterization of mechanical properties of pericardium tissue using planar biaxial tension and flexural deformation. J. Mech. Behav. Biomed. Mater. 2018, 77, 148–156. [Google Scholar] [CrossRef]
- Chung, K.-Y.; Bhadriraju, K.; Spurlin, T.A.; Cook, R.F.; Plant, A.L. Nanomechanical properties of thin films of type I collagen fibrils. Langmuir 2010, 26, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Lopez-Garcia, M.D.C.; Beebe, D.J.; Crone, W.C. Young’s modulus of collagen at slow displacement rates. Biomed. Mater. Eng. 2010, 20, 361–369. [Google Scholar] [PubMed]
- An, Y.-Z.; Kim, Y.-K.; Lim, S.-M.; Heo, Y.-K.; Kwon, M.-K.; Cha, J.-K.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Physicochemical properties and resorption progress of porcine skin-derived collagen membranes: In vitro and in vivo analysis. Dent. Mater. J. 2018, 37, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Tuo, F.Y.; Song, L.; Liu, Y.X.; Dong, X.P.; Li, D.M.; Zhou, D.Y.; Shahidi, F. Action of trypsin on structural changes of collagen fibres from sea cucumber (Stichopus japonicus). Food Chem. 2018, 256, 113–118. [Google Scholar] [CrossRef] [PubMed]
 CovaTMMax;
CovaTMMax;  Jason®;
Jason®;  Biocollagen®.
Biocollagen®.
 CovaTMMax;
CovaTMMax;  Jason®;
Jason®;  Biocollagen®.
Biocollagen®.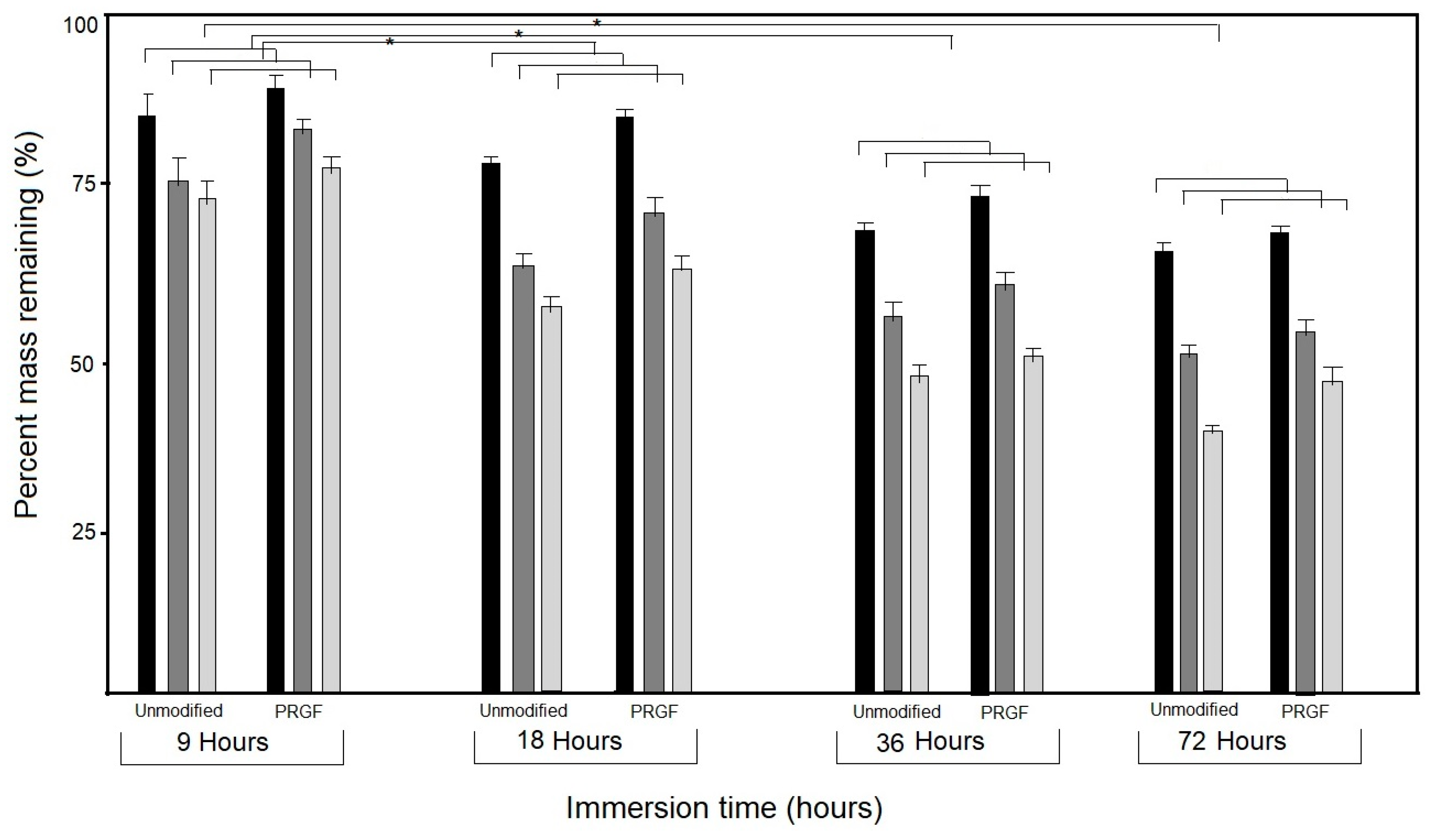
| Component | Whole Blood/Normal Range | PRGF |
|---|---|---|
| Leukocytes (×10−3/μL) | 5.9 ± 0.2/4–10 | 0.4 ± 0.1 |
| Erythrocytes (×10−6/μL) | 4.5 ± 0.4/3.8–5 | 0.01 ± 0.01 |
| Platelets (×10−3/μL) | 210 ± 20/150–300 | 655 ± 85 |
| Growth Factor Content | Value |
|---|---|
| Transforming growth factor TGFβ1—enhances the proliferative activity of fibroblasts and stimulates the biosynthesis of collagen and fibronectin | 43 ng/mL ± 8.2 |
| Vascular endothelial growth factor VEGF—induces angiogenesis via migrating endothelial cells | 220 pg/mL ± 20 |
| Insulin-like growth factor IGF-1—a primary mediator of the effects of growth hormone; can also regulate cellular DNA synthesis | 105 ng/mL ± 15.5 |
| Platelet-derived growth factor PDGR—enhances collagen synthesis and bone cell proliferation | 14 ng/mL ± 3.5 |
| RANTES chemokine (CCL5)—role in regulating T-cell responses and immunity during chronic and acute infection | 520 pg/mL ± 100 |
| Interleukin IL-1β cytokine—regulates and initiates inflammatory responses | 400 pg/mL ± 80 |
| Monocyte chemotactic proteins (MCP-1)—regulates the expression of cell surface antigens; can induce the proliferation and activation of killer cells | 20 pg/mL ± 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035. https://doi.org/10.3390/app9051035
Ratiu C, Brocks M, Costea T, Moldovan L, Cavalu S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Applied Sciences. 2019; 9(5):1035. https://doi.org/10.3390/app9051035
Chicago/Turabian StyleRatiu, Cristian, Marcel Brocks, Traian Costea, Liviu Moldovan, and Simona Cavalu. 2019. "PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations" Applied Sciences 9, no. 5: 1035. https://doi.org/10.3390/app9051035
APA StyleRatiu, C., Brocks, M., Costea, T., Moldovan, L., & Cavalu, S. (2019). PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Applied Sciences, 9(5), 1035. https://doi.org/10.3390/app9051035






