Aerodynamics from Cursorial Running to Aerial Gliding for Avian Flight Evolution
Abstract
:1. Introduction
2. Paleoenvironment of the Earliest Feathered Paravian Dinosaurs
3. Gliding Aerodynamics in Basal Paravian Dinosaurs
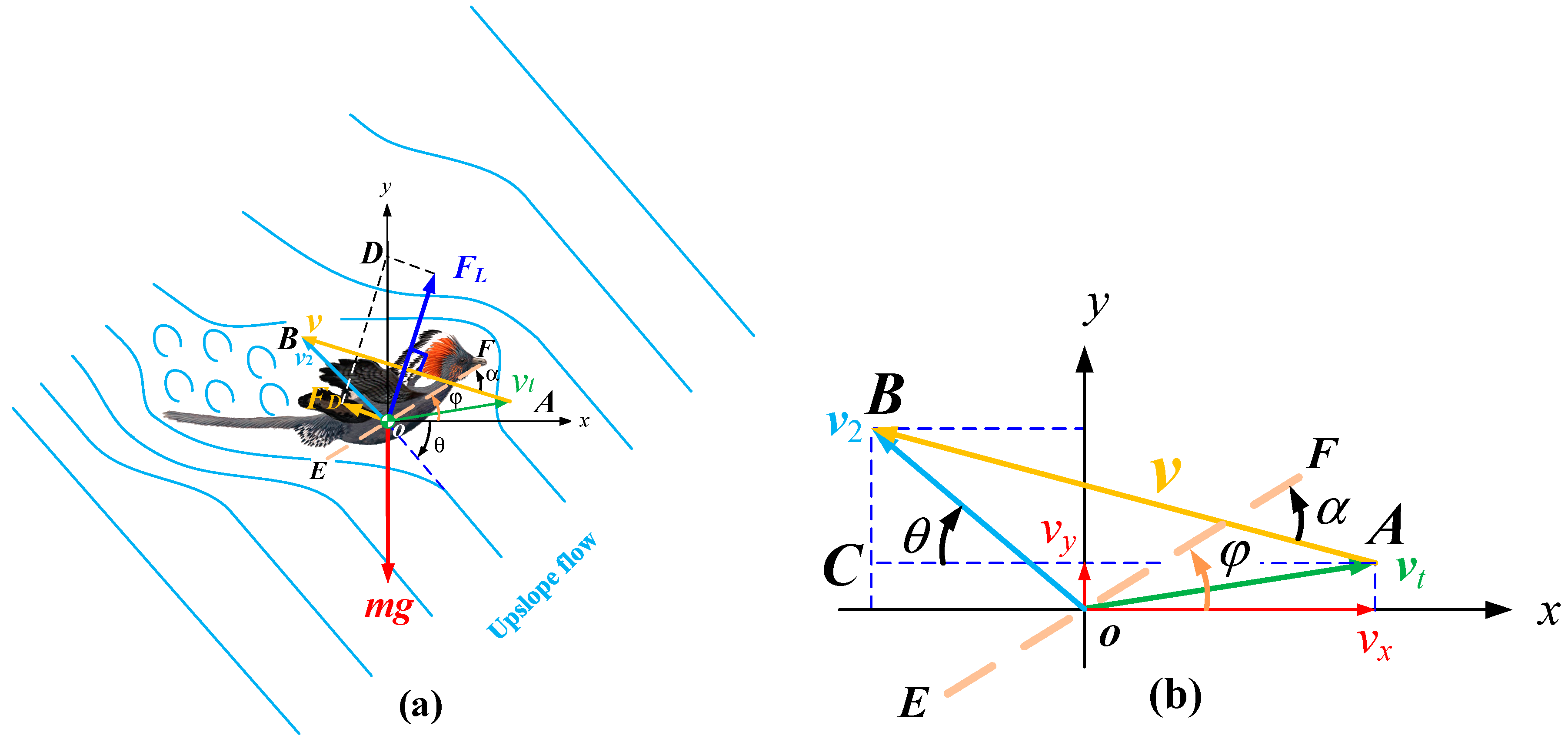
4. Materials and Methods
4.1. Lift and Drag Coefficients
4.2. Mass and Size Parameters of the Ancestors of the Bird
5. Results and Discussion
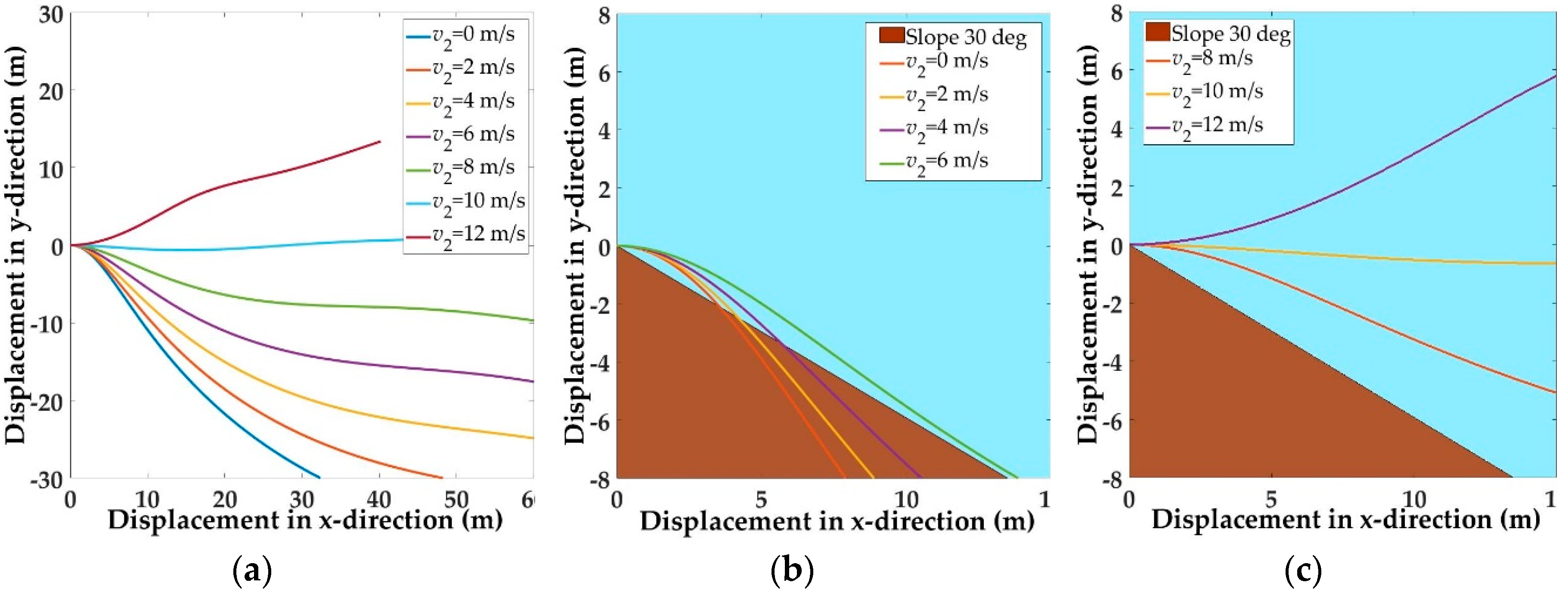
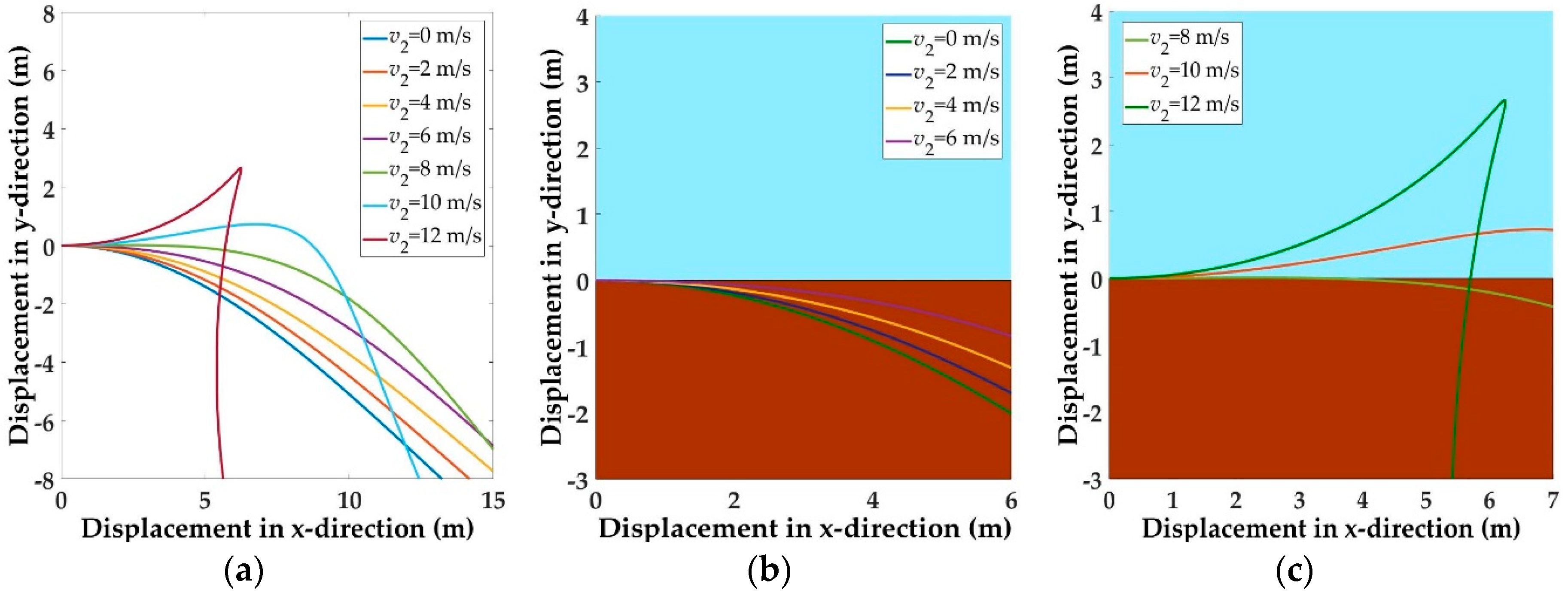
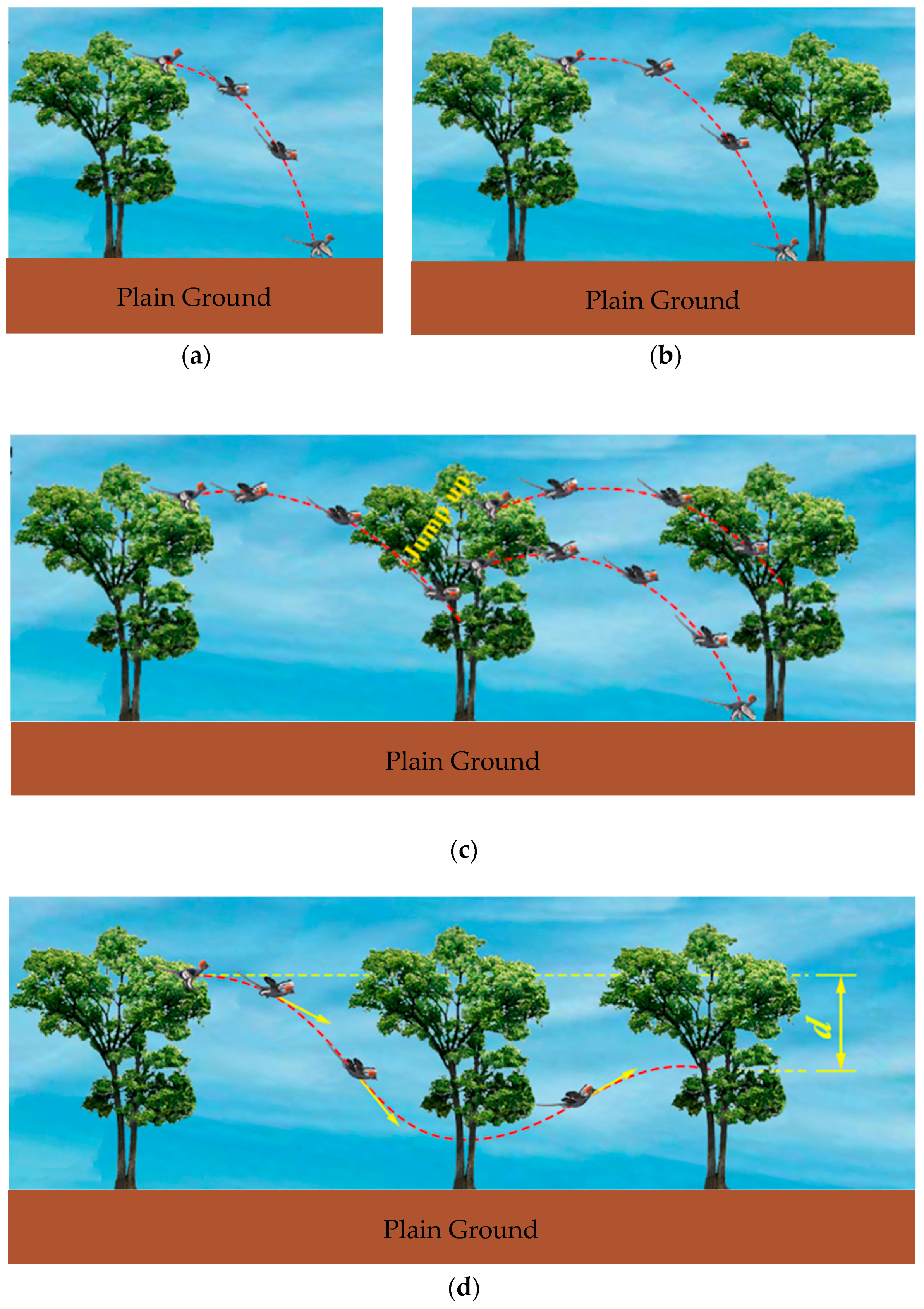
5.1. Trajectories of a Basal Paravian Dinosaur from Cursorial Running to Aerial Gliding
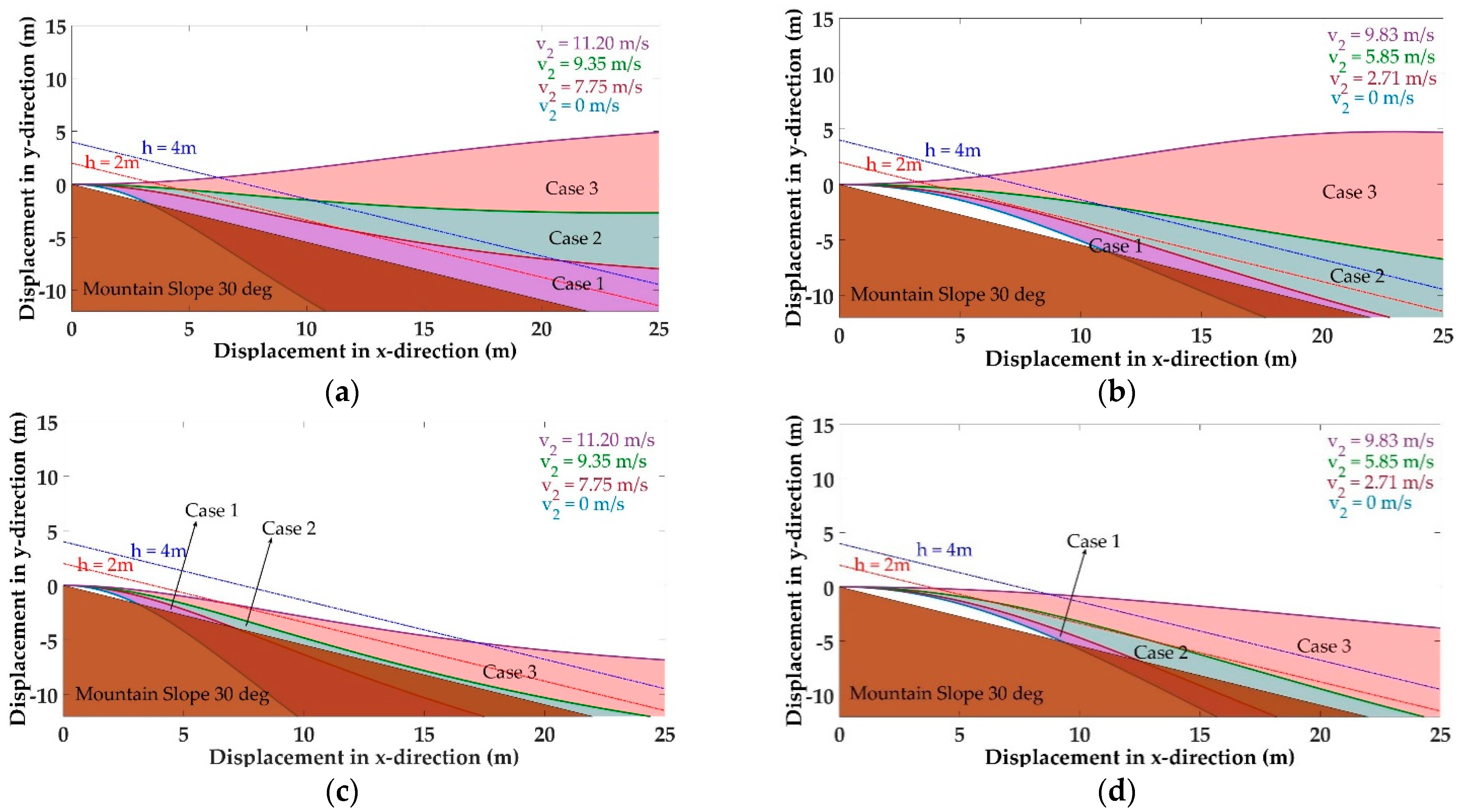
5.2. Evolution of Arboreal Gliding
5.3. Stability Control During Gliding
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sreetharan, P.S.; Wood, R.J. Passive Aerodynamic Drag Balancing in a Flapping-Wing Robotic Insect. J. Mech. Des. 2010, 132, 051006. [Google Scholar] [CrossRef]
- Marden, J.H. Maximum Lift Production During Takeoff in Flying Animals. J. Exp. Btol. 1987, 130, 235–258. [Google Scholar]
- Chin, D.D.; Matloff, L.Y.; Stowers, A.K.; Tucci, E.R.; Lentink, D. Inspiration for wing design: How forelimb specialization enables active flight in modern vertebrates. J. R. Soc. Interface 2017, 14, 20170240. [Google Scholar] [CrossRef] [PubMed]
- McKellar, R.C.; Chatterton, B.D.E.; Wolfe, A.P.; Currie, P.J. A diverse assemblage of late cretaceous dinosaur and bird feathers from Canadian Amber. Science 2011, 333, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J. Feathers before flight. Science 2013, 340, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, J.H. Archaeopteryx: Notice of a “New” Specimen. Science 1970, 170, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, J.H. Bird flight: How did it begin? Did birds begin to fly “from the trees down” or “from the ground up”? Reexamination of Archaeopteryx adds plausibility to an “up from the ground” origin of flight. Am. Sci. 1979, 67, 46–56. [Google Scholar] [CrossRef]
- Forster, C.A.; Sampson, S.D.; Chiappe, L.M.; Krause, D.W. The theropod ancestry of birds: New evidence from the Late Cretaceous of Madagascar. Science 1998, 279, 1915–1919. [Google Scholar] [CrossRef]
- Garner, J.P.; Taylor, G.K.; Thomas, A.L.R. On the origins of birds: The sequence of character acquisition in the evolution of avian flight. Proc. R. Soc. B Biol. Sci. 1999, 266, 1259–1266. [Google Scholar] [CrossRef]
- Sereno, P.C. The evolution of dinosaurs. Science 1999, 284, 2137–2147. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Z.; Wang, X. The smallest known non-avian theropod dinosaur. Nature 2000, 408, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Prum, R.O. Dinosaurs take to the air. Nature 2003, 421, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.S. Comment on “Narrow Primary Feather Rachises in Confuciusornis and Archaeopteryx Suggest Poor Flight Ability”. Science 2010, 328, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, X.; Zhou, Z.; Miao Desui, Z.F. Comment on “Narrow Primary Feather Rachises in Confuciusornis and Archaeopteryx Suggest Poor Flight Ability”. Science 2010, 330, 320. [Google Scholar] [CrossRef] [PubMed]
- Nudds, R.L.; Dyke, G.J. Response to Comments on “‘Narrow Primary Feather Rachises inConfuciusornis and Archaeopteryx Suggest Poor Flight Ability’”. Science 2010, 330, 320. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z. Leg feathers in an early cretaceous bird. Nature 2004, 431, 925. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, A. New Feathered and Dinosaurs Fossil Birds Brings Closer. Am. Assoc. Adv. Sci. 1996, 274, 720–721. [Google Scholar] [CrossRef]
- Dial, K.P. Wing-assisted incline running and the evolution of flight. Science 2003, 299, 402–404. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z. A primitive enantiornithine bird and the origin of feathers. Science 2000, 290, 1955–1959. [Google Scholar] [CrossRef]
- Zelenitsky, D.K.; Therrien, F.; Erickson, G.M.; DeBuhr, C.L.; Kobayashi, Y.; Eberth, D.A.; Hadfield, F. Feathered non-avian dinosaurs from North America provide insight into wing origins. Science 2012, 338, 510–514. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Z.; Wang, X.; Kuang, X.; Zhang, F.; Du, X. Four-winged dinosaurs from China. Nature 2003, 421, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Templin, R.J. The flight of Archaeopteryx. Naturwissenschaften 2003, 90, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z. The origin and early evolution of birds: Discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften 2004, 91, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Feduccia, A. Evidence from Claw Geometry Indicating Arboreal Habits of Archaeopteryx. Science 1993, 259, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, Z.; Wang, X.; Zhang, F.; Zhang, X.; Wang, Y.; Wei, G.; Wang, S.; Xu, X. Hind wings in basal birds and the evolution of leg feathers. Science 2013, 339, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Ruben, J.A. Reptilian physiology and the flight capacity of Archaeopteryx. Evolution 1991, 45, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J. Flight capabilities of Archaeopteryx. Int. J. Org. Evol. 1993, 47, 336–340. [Google Scholar] [CrossRef]
- Dong, S.W.; Zhang, Y.Q.; Long, C.X.; Yang, Z.Y.; Ji, Q.; Wang, T.; Hu, J.M.; Chen, X.H. Jurassic tectonic revolution in China and new interpretation of the “Yanshan Movement.”. Acta Geol. Sin. Ed. 2008. [Google Scholar] [CrossRef]
- Yongdong, W.; Ken’ ichi, S.; Wu, Z.; Shaolin, Z. Biodiversity and palaeoclimate of the Middle Jurassic floras from the Tiaojishan Formation in western Liaoning, China. Prog. Nat. Sci. 2006, 16, 222–230. [Google Scholar] [CrossRef]
- Sullivan, C.; Wang, Y.; Hone, D.W.E.; Wang, Y.; Xu, X.; Zhang, F. The vertebrates of the Jurassic Daohugou Biota of northeastern China. J. Vertebr. Paleontol. 2014. [Google Scholar] [CrossRef]
- Vergeiner, I.; Dreiseitl, E. Valley winds and slope winds—Observations and elementary thoughts. Meteorol. Atmos. Phys. 1987, 36, 264–286. [Google Scholar] [CrossRef]
- Kleissl, J.; Honrath, R.E.; Dziobak, M.P.; Tanner, D.; Val Martín, M.; Owen, R.C.; Helmig, D. Occurrence of upslope flows at the Pico mountaintop observatory: A case study of orographic flows on a small, volcanic island. J. Geophys. Res. Atmos. 2007, 112, 1–16. [Google Scholar] [CrossRef]
- Whiteman, C.D.; Doran, J.C. The relationship between overlying synopic-scale flows and winds within a valley. J. Appl. Meteorol. 1993, 32, 1669–1682. [Google Scholar] [CrossRef]
- Whiteman, C.D. Mountain Meteorology: Fundamentals and Applications; Oxford University Press: Oxford, UK, 2000; ISBN 0-19-513271-8. [Google Scholar]
- Longrich, N.R.; Vinther, J.; Meng, Q.; Li, Q.; Russell, A.P. Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Curr. Biol. 2012, 22, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Hou, L.; Zhang, L.; Xu, X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 2009, 461, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, Z.; Xu, X.; Wang, X.; Sullivan, C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 2008, 455, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Kurochkin, E.N.; Dyke, G.J.; Saveliev, S.V.; Pervushov, E.M.; Popov, E.V. A fossil brain from the Cretaceous of European Russia and avian sensory evolution. Biol. Lett. 2007, 3, 309–313. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Norell, M.; Sullivan, C.; Hone, D.; Erickson, G.; Wang, X.; Han, F.; Guo, Y. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin. Sci. Bull. 2009, 54, 430–435. [Google Scholar] [CrossRef]
- Nudds, R.L.; Dyke, G.J. Narrow primary feather rachises in Confuciusornis and Archaeopteryx suggest poor flight ability. Science 2010, 328, 887–889. [Google Scholar] [CrossRef]
- Carney, R.M.; Vinther, J.; Shawkey, M.D.; D’Alba, L.; Ackermann, J. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nature Commun. 2012, 3, 637. [Google Scholar] [CrossRef]
- Xu, X.; Norell, M.A. Non-avian dinosaur fossils from the Lower Cretaceous Jehol Group of western Liaoning, China. Geol. J. 2006, 41, 419–437. [Google Scholar] [CrossRef]
- O’Connor, J.; Zhou, Z.; Xu, X. Additional specimen of Microraptor provides unique evidence of dinosaurs preying on birds. Proc. Natl. Acad. Sci. USA 2011, 108, 19662–19665. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, K.; Vinther, J.; Shawkey, M.D.; Clarke, J.; D’alba, L.; Meng, Q.; Briggps, D.E.G.; Prum, R.O. Plumage Color Patterns of an. Science 2010, 327, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Sereno, P.C.; Chenggang, R. Early Evolution of Avian Flight and Perching: New Evidence from the Lower Cretaceous of China. Science 2014, 255, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Padian, K.; Chiappe, L.M. The origin and early evolution of birds. Biol. Rev. 1998. [Google Scholar] [CrossRef]



| Species | Date (m. years) | Mass, m (Kg) | Area, A (m2) | Figure of Fossil |
|---|---|---|---|---|
| Epidexipteryx | 152–168 [37] | 0.164 [37] | 0.0132 [37] |  [37] |
| Anchiornis | 155 [36] | 0.110 [37] | 0.04212 [36] |  [36] |
| Archaeopteryx | 150 [38] | 0.276 [40] | 0.0496 [41] |  [41] |
| Microraptor | 110–120 [42] | 1.50 [43] | 0.155 [21] |  [21] |
| Confuciusornis | 120 [40] | 0.500 [40] | 0.0402 [40] |  [40] |
| Species | ||||
|---|---|---|---|---|
| Epidexipteryx | 5 | 0–7.75 | 7.75–9.35 | 9.35–11.20 |
| Anchiornis | 5 | 0–7.75 | 7.75–9.35 | 9.35–11.20 |
| Epidexipteryx | 8 | 0–2.71 | 2.71–5.85 | 5.85–9.83 |
| Anchiornis | 8 | 0–2.71 | 2.71–5.85 | 5.85–9.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, F.; Zhao, J.; Godefroit, P. Aerodynamics from Cursorial Running to Aerial Gliding for Avian Flight Evolution. Appl. Sci. 2019, 9, 649. https://doi.org/10.3390/app9040649
Shahid F, Zhao J, Godefroit P. Aerodynamics from Cursorial Running to Aerial Gliding for Avian Flight Evolution. Applied Sciences. 2019; 9(4):649. https://doi.org/10.3390/app9040649
Chicago/Turabian StyleShahid, Farzeen, Jingshan Zhao, and Pascal Godefroit. 2019. "Aerodynamics from Cursorial Running to Aerial Gliding for Avian Flight Evolution" Applied Sciences 9, no. 4: 649. https://doi.org/10.3390/app9040649
APA StyleShahid, F., Zhao, J., & Godefroit, P. (2019). Aerodynamics from Cursorial Running to Aerial Gliding for Avian Flight Evolution. Applied Sciences, 9(4), 649. https://doi.org/10.3390/app9040649





