N-Doped Carbon Aerogels Obtained from APMP Fiber Aerogels Saturated with Rhodamine Dye and Their Application as Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of APMP Fiber Aerogel
2.3. Adsorption Performance of APMP Aerogels
2.4. Preparation of N-Doped Carbon Aerogels
2.5. Electrochemical Measurements
2.6. Characterization
3. Results
3.1. RB Sorption Capacity of APMP Aerogels
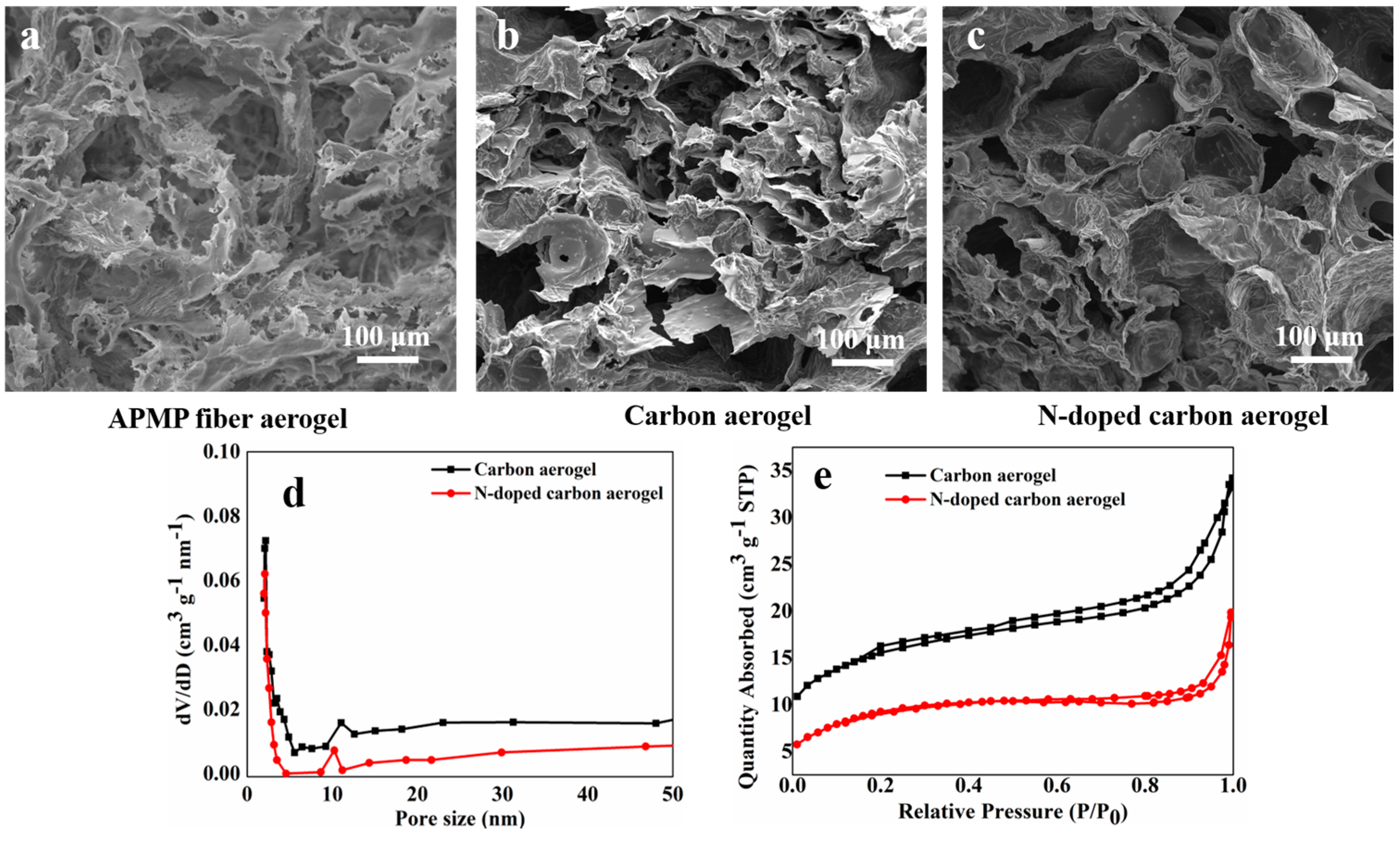
3.2. SEM Observations and Porous Analysis
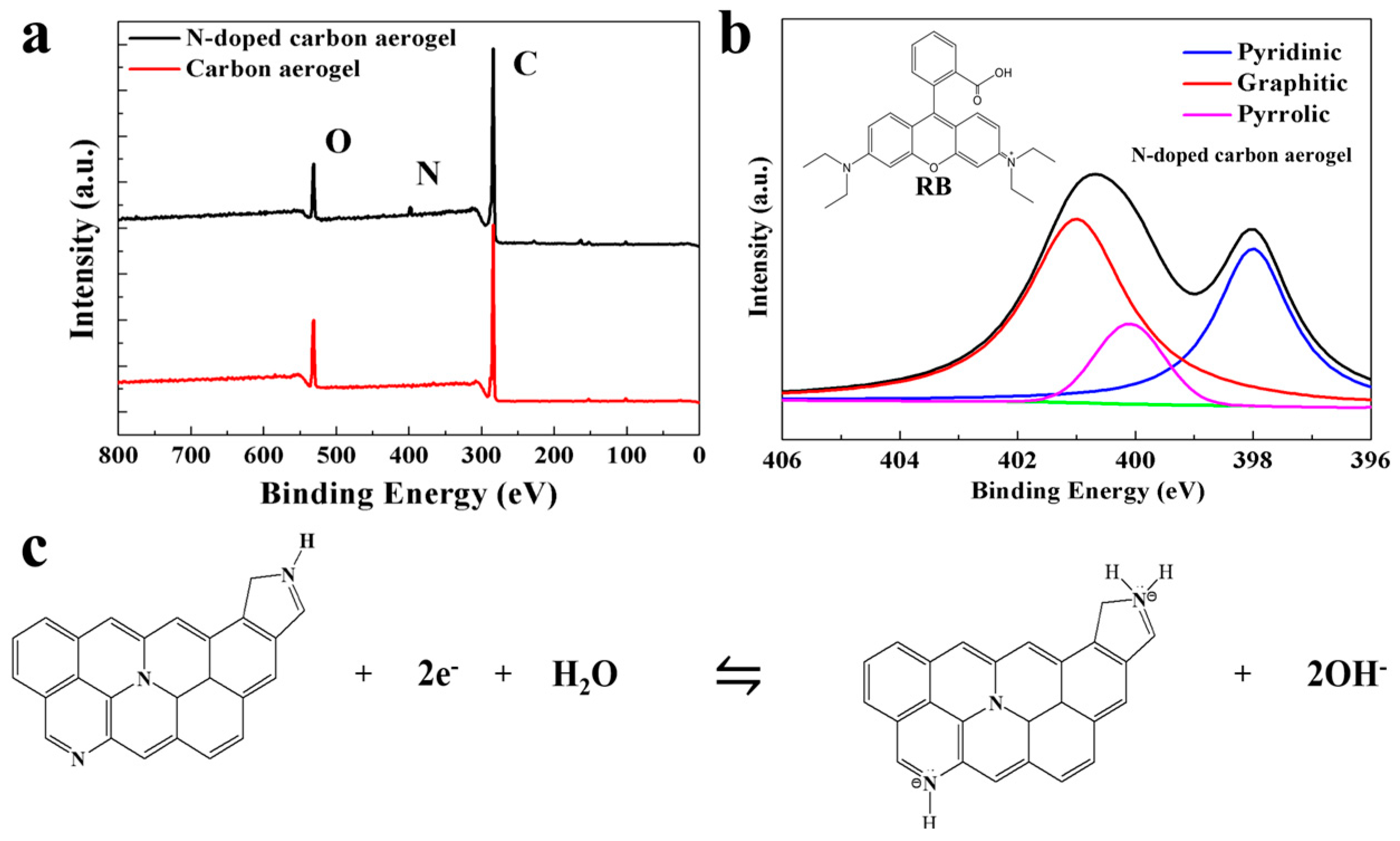
3.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
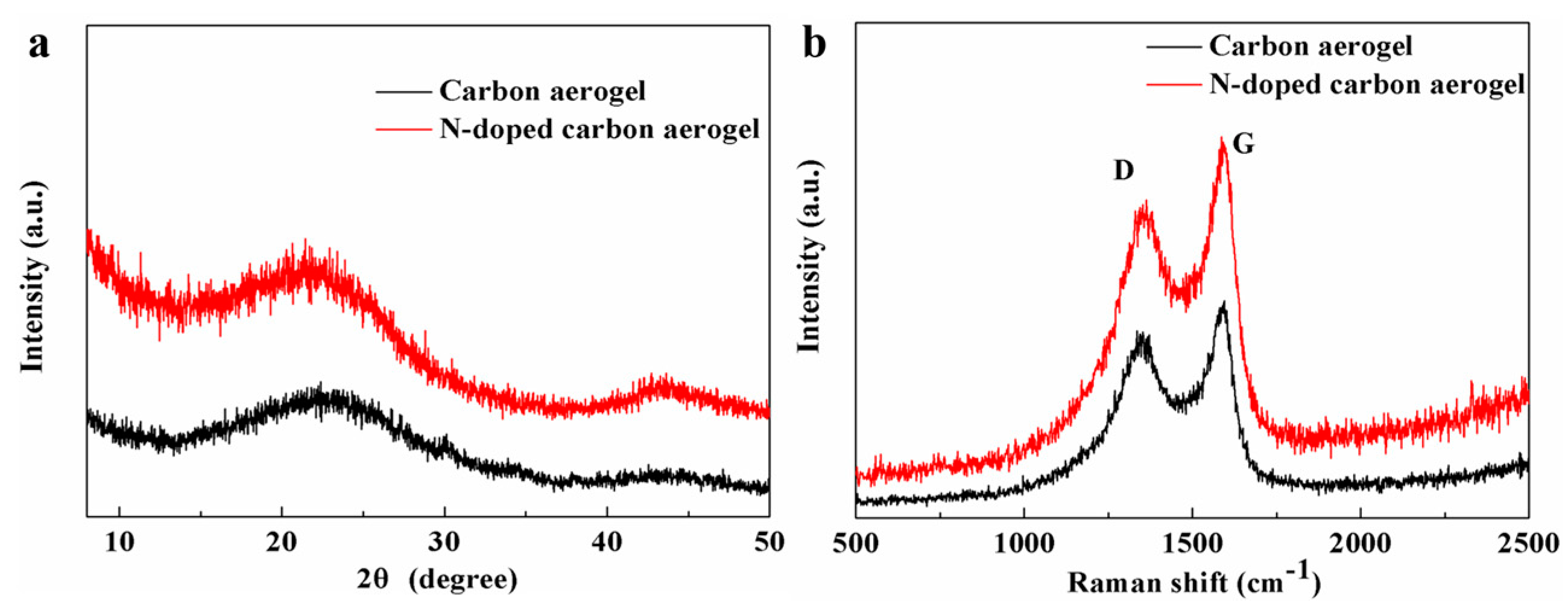
3.4. XRD and Raman Analysis
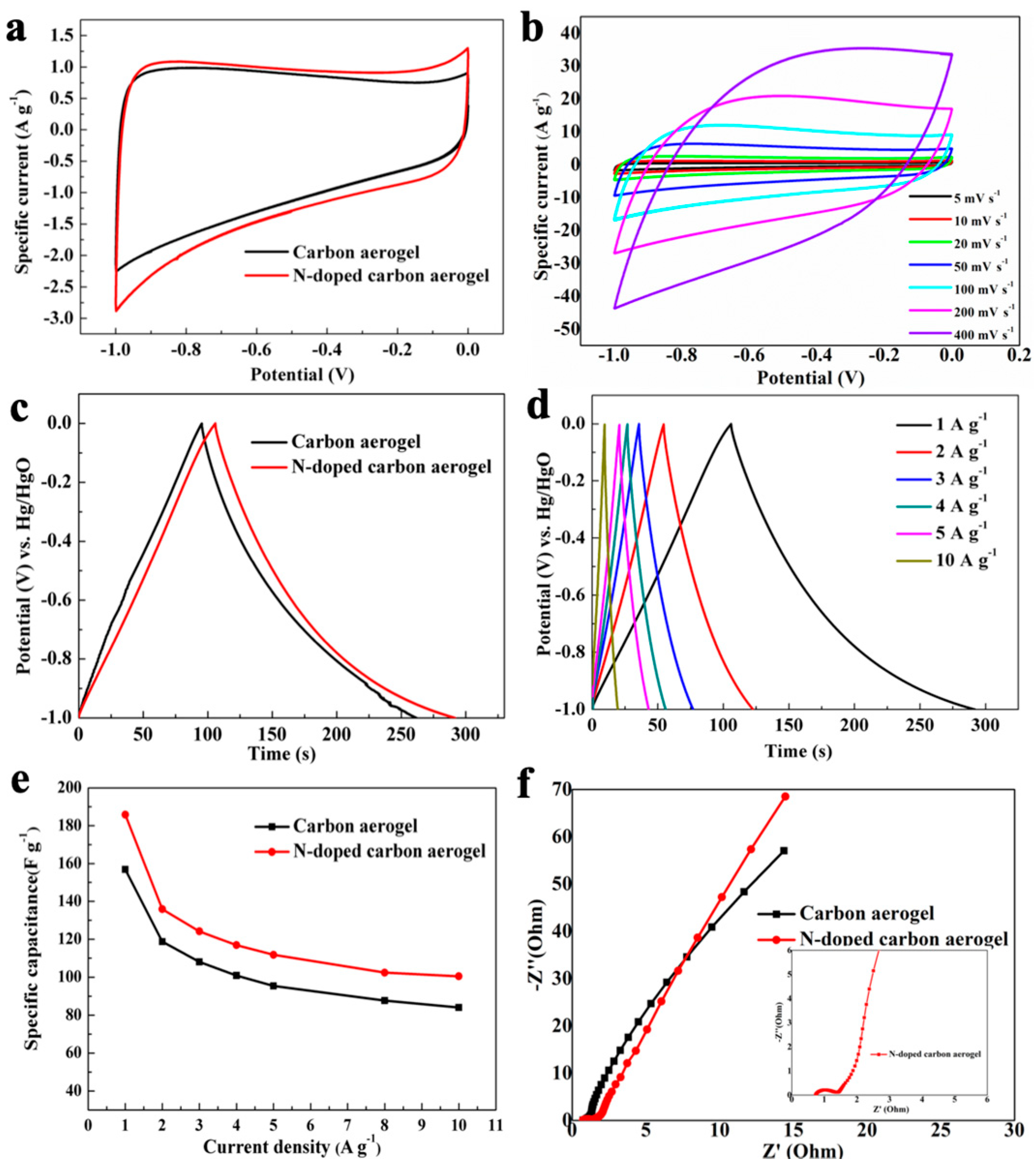
3.5. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Chen, S.; Pan, W.; Zhang, J. A flexible solid-state supercapacitor based on graphene/polyaniline paper electrodes. J. Energy Chem. 2018. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Yi, T.; He, Y.; Li, X. Review and prospect of NiCo2O4-based composite materials for supercapacitor electrodes. J. Energy Chem. 2018. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous electrode materials for supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yang, I.; Kwon, D.; Kim, M.-S.; Jung, J.C. A comparative study of activated carbon aerogel and commercial activated carbons as electrode materials for organic electric double-layer capacitors. Carbon 2018, 132, 503–511. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, X.; Wei, J.; Gao, S. Functional groups and pore size distribution do matter to hierarchically porous carbons as high-rate-performance supercapacitors. Chem. Mater. 2016, 28, 445–458. [Google Scholar] [CrossRef]
- Liu, N.; Shen, J.; Liu, D. Activated high specific surface area carbon aerogels for EDLCs. Microporous Mesoporous Mater. 2013, 167, 176–181. [Google Scholar] [CrossRef]
- Lei, E.; Li, W.; Ma, C.; Xu, Z.; Liu, S. CO2-activated porous self-templated N-doped carbon aerogel derived from banana for high-performance supercapacitors. Appl. Surf. Sci. 2018, 457, 477–486. [Google Scholar]
- Sinan, N.; Unur, E. Hydrothermal conversion of lignocellulosic biomass into high-value energy storage materials. J. Energy Chem. 2017, 26, 783–789. [Google Scholar] [CrossRef]
- Song, L.-T.; Wu, Z.-Y.; Liang, H.-W.; Zhou, F.; Yu, Z.-Y.; Xu, L.; Pan, Z.; Yu, S.-H. Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, D.; Zhao, G.; Du, B.; Tang, W.; Sun, L.; Sun, Y.; Besenbacher, F.; Yu, M. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 2016, 27, 377–389. [Google Scholar] [CrossRef]
- Long, C.; Jiang, L.; Wu, X.; Jiang, Y.; Yang, D.; Wang, C.; Wei, T.; Fan, Z. Facile synthesis of functionalized porous carbon with three-dimensional interconnected pore structure for high volumetric performance supercapacitors. Carbon 2015, 93, 412–420. [Google Scholar] [CrossRef]
- Su, F.; Poh, C.K.; Chen, J.S.; Xu, G.; Wang, D.; Li, Q.; Lin, J.; Lou, X.W. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, B.; Song, H.; Chen, X. Preparation and electrochemical performance of polyaniline-based carbon nanotubes as electrode material for supercapacitor. Electrochim. Acta 2010, 55, 7021–7027. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Qian, W.; Zhu, J.; Yan, F. Host–guest inclusion complexes derived heteroatom-doped porous carbon materials. Carbon 2016, 105, 183–190. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Alinajafi, H.A.; Rezaei, B. Thermally reduced graphene oxide/polymelamine formaldehyde nanocomposite as a high specific capacitance electrochemical supercapacitor electrode. J. Mater. Chem. A 2018, 6, 6045–6053. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Martins, A.C.; Pezoti, O.; Bedin, K.C.; Beltrame, K.K.; Asefa, T.; Almeida, V.C. Synthesis and application of N–S-doped mesoporous carbon obtained from nanocasting method using bone char as heteroatom precursor and template. Chem. Eng. J. 2016, 300, 54–63. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-doping of graphene through electrothermal reactions with ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4264. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Chen, S.; Ding, C. Graphene aerogel prepared through double hydrothermal reduction as high-performance oil adsorbent. Adv. Mater. Sci. Eng. B 2017, 226, 141–150. [Google Scholar] [CrossRef]
- Yang, X.; Shi, K.; Zhitomirsky, I.; Cranston, E.D. Cellulose nanocrystal aerogels as universal 3D lightweight substrates for supercapacitor materials. Adv. Mater. 2015, 27, 6104–6109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, J.; Ren, H.; Bi, Y.; Zhang, L. Facile Synthesis of Nitrogen-Doped Graphene Aerogels for Electrode Materials in Supercapacitors. Chin. J. Chem. 2017, 35, 1069–1078. [Google Scholar] [CrossRef]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Zhang, L.; Huang, Y.; Lu, H.; Fan, W.; Liu, T. Cotton wool derived carbon fiber aerogel supported few-layered MoSe2 nanosheets as efficient electrocatalysts for hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 7077–7085. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-cotton-derived N-doped carbon fiber aerogel as an efficient electrode for electrochemical capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Mu, L.; Hao, B.; Ma, P.-C. Low cost carbon fiber aerogel derived from bamboo for the adsorption of oils and organic solvents with excellent performances. RSC Adv. 2015, 5, 38470–38478. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liang, H.W.; Chen, L.F.; Hu, B.C.; Yu, S.H. Bacterial cellulose: A robust platform for design of three dimensional carbon-based functional nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Li, L.; Hu, T.; Sun, H.; Zhang, J.; Wang, A. Pressure-sensitive and conductive carbon aerogels from poplars catkins for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 18001–18007. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Hódar, F.J.; Moreno-Castilla, C.; Pérez-Cadenas, A.F. Catalytic combustion of toluene on platinum-containing monolithic carbon aerogels. Appl. Catal. B 2004, 54, 217–224. [Google Scholar] [CrossRef]
- Lei, E.; Li, W.; Ma, C.; Liu, S. An ultra-lightweight recyclable carbon aerogel from bleached softwood kraft pulp for efficient oil and organic absorption. Mater. Chem. Phys. 2018, 214, 291–296. [Google Scholar]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.E.; Fan, W.; Liu, T. Polymer/carbon-based hybrid aerogels: Preparation, properties and applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef]
- Yin, A.; Xu, F.; Zhang, X. Fabrication of biomass-derived carbon aerogels with high adsorption of oils and organic solvents: Effect of hydrothermal and post-pyrolysis processes. Materials 2016, 9, 758. [Google Scholar] [CrossRef]
- Wu, X.-L.; Wen, T.; Guo, H.-L.; Yang, S.; Wang, X.; Xu, A.-W. Biomass-Derived Sponge-like Carbonaceous Hydrogels and Aerogels for Supercapacitors. J. Am. Chem. Soc. 2013, 7, 3589–3597. [Google Scholar] [CrossRef]
- Katanyoota, P.; Chaisuwan, T.; Wongchaisuwat, A.; Wongkasemjit, S. Novel polybenzoxazine-based carbon aerogel electrode for supercapacitors. Adv. Mater. Sci. Eng. B 2010, 167, 36–42. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, Y.; Li, J.; Wang, L. Polypyrrole-anchored cattail biomass-derived carbon aerogels for high performance binder-free supercapacitors. Carbohydr. Polym. 2018, 199, 555–562. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Han, S.; Li, J.; Sun, Q. Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym. 2015, 123, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Zhu, T. Application of activated carbon derived from scrap tires for adsorption of Rhodamine, B. J. Environ. Sci. 2010, 22, 1273–1280. [Google Scholar] [CrossRef]
- Da Silva Lacerda, V.; Lopez-Sotelo, J.B.; Correa-Guimaraes, A.; Hernandez-Navarro, S.; Sanchez-Bascones, M.; Navas-Gracia, L.M.; Martin-Ramos, P.; Martin-Gil, J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manage. 2015, 155, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.M.; El-Sayed, A.A. Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Yao, S.; Wu, X.; Feng, Q.; Wang, Z.; Geng, B. High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures. J. Mater. Chem. A 2014, 2, 15958–15963. [Google Scholar] [CrossRef]
- Hou, M.F.; Ma, C.X.; Zhang, W.D.; Tang, X.Y.; Fan, Y.N.; Wan, H.F. Removal of rhodamine B using iron-pillared bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Xie, S.; Li, H. Hexagonal single crystal growth of WO3 nanorods along a [110] axis with enhanced adsorption capacity. Chem. Commun. 2011, 47, 4403–4405. [Google Scholar] [CrossRef]
- Bian, X.; Lu, X.; Xue, Y.; Zhang, C.; Kong, L.; Wang, C. A facile one-pot hydrothermal method to produce SnS2/reduced graphene oxide with flake-on-sheet structures and their application in the removal of dyes from aqueous solution. J. Colloid Interface Sci. 2013, 406, 37–43. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.; Lei, M.; Zeng, Q.; Song, H.; Yang, J.; Shao, J.; Liao, B.; Gu, J. Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J. Hazard. Mater. 2012, 209, 193–198. [Google Scholar] [CrossRef]
- Ding, L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from aqueous solution using treated rice husk-based activated carbon. Colloids Surf. A 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Panda, G.C.; Das, S.K.; Guha, A.K. Jute stick powder as a potential biomass for the removal of congo red and rhodamine B from their aqueous solution. J. Hazard. Mater. 2009, 164, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Mishra, S.B. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydr. Polym. 2014, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-L.; Li, Y.-X.; Liu, Z. Fabrication of graphene oxide/silicalite-1 composites with hierarchical porous structure and investigation on their adsorption performance for rhodamine B. J. Ind. Eng. Chem. 2017, 55, 234–243. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Liu, Y. Synthesis of a hierarchical SnS2 nanostructure for efficient adsorption of Rhodamine B dye. J. Colloid Interface Sci. 2017, 507, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xiao, F.; Yang, C.; Wang, J.; Su, X. Hydrothermal fabrication of W18O49 nanowire networks with superior performance for water treatment. J. Mater. Chem. A 2013, 1, 5831. [Google Scholar] [CrossRef]
- Selvam, P.P.; Preethi, S.; Basakaralingam, P.; Thinakaran, N.; Sivasamy, A.; Sivanesan, S. Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J. Hazard. Mater. 2008, 155, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, X.; Feng, S.; Guo, Z.; Liang, S. Enhancement of rhodamine B removal by modifying activated carbon developed from Lythrum salicaria L. with pyruvic acid. Colloids Surf. A 2016, 489, 154–162. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Liang, H.-W.; Li, C.; Hu, B.-C.; Xu, X.-X.; Wang, Q.; Chen, J.-F.; Yu, S.-H. Dyeing bacterial cellulose pellicles for energetic heteroatom doped carbon nanofiber aerogels. Nano Res. 2014, 7, 1861–1872. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1774. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
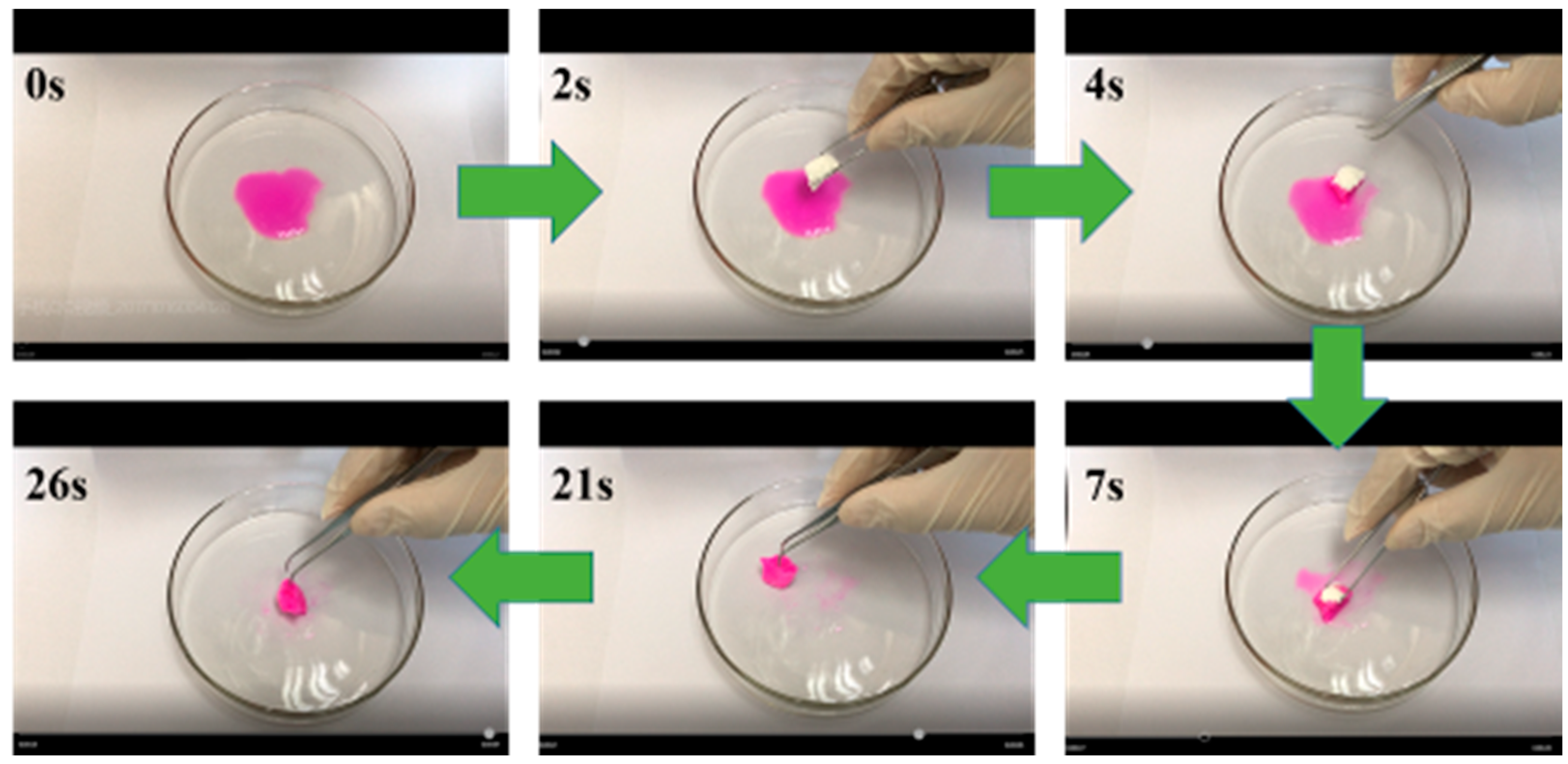
| Adsorbents | Adsorption Capacity (mg g−1) | Saturation Time (min) | Ref. |
|---|---|---|---|
| Activated carbon from scrap tires | 280.1 | 300 | [43] |
| Activated carbon from lignocellulosic waste | 39.2 | [44] | |
| Activated carbon from agricultural by-products | 263.8 | 240 | [45] |
| MoS2 | 49.2 | 35 | [46] |
| Iron-pillared bentonite | 98.62 | 40 | [47] |
| WO3 | 64 | [48] | |
| SnS2/rGO | 94.07 | 480 | [49] |
| Fe3O4/HA | 161.8 | 15 | [50] |
| Rice husk-based activated carbon | 234 | 120 | [51] |
| Jute stick powder | 87.7 | 60 | [52] |
| Gg-cl-P(AA-co-AAm)/Fe3O4 nanocomposite | 529.1 | 50 | [53] |
| GO/silicalite−1 | 56.55 | 60 | [54] |
| SnS2 | 200 | 65 | [55] |
| W18O49 | 120 | [56] | |
| Sodium montmorillonite | 38.27 | 320 | [57] |
| Activated carbon from Lythrum salicaria | 384.62 | 480 | [58] |
| Kaolinite | 46.08 | 80 | [59] |
| APMP fiber aerogels | 250 | 0.5 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
E, L.; Li, W.; Sun, J.; Wu, Z.; Liu, S. N-Doped Carbon Aerogels Obtained from APMP Fiber Aerogels Saturated with Rhodamine Dye and Their Application as Supercapacitor Electrodes. Appl. Sci. 2019, 9, 618. https://doi.org/10.3390/app9040618
E L, Li W, Sun J, Wu Z, Liu S. N-Doped Carbon Aerogels Obtained from APMP Fiber Aerogels Saturated with Rhodamine Dye and Their Application as Supercapacitor Electrodes. Applied Sciences. 2019; 9(4):618. https://doi.org/10.3390/app9040618
Chicago/Turabian StyleE, Lei, Wei Li, Jiaming Sun, Zhenwei Wu, and Shouxin Liu. 2019. "N-Doped Carbon Aerogels Obtained from APMP Fiber Aerogels Saturated with Rhodamine Dye and Their Application as Supercapacitor Electrodes" Applied Sciences 9, no. 4: 618. https://doi.org/10.3390/app9040618
APA StyleE, L., Li, W., Sun, J., Wu, Z., & Liu, S. (2019). N-Doped Carbon Aerogels Obtained from APMP Fiber Aerogels Saturated with Rhodamine Dye and Their Application as Supercapacitor Electrodes. Applied Sciences, 9(4), 618. https://doi.org/10.3390/app9040618






