Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products
Abstract
1. Introduction
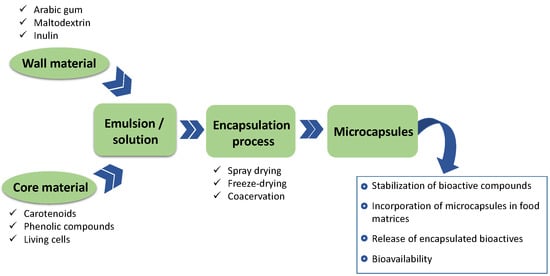
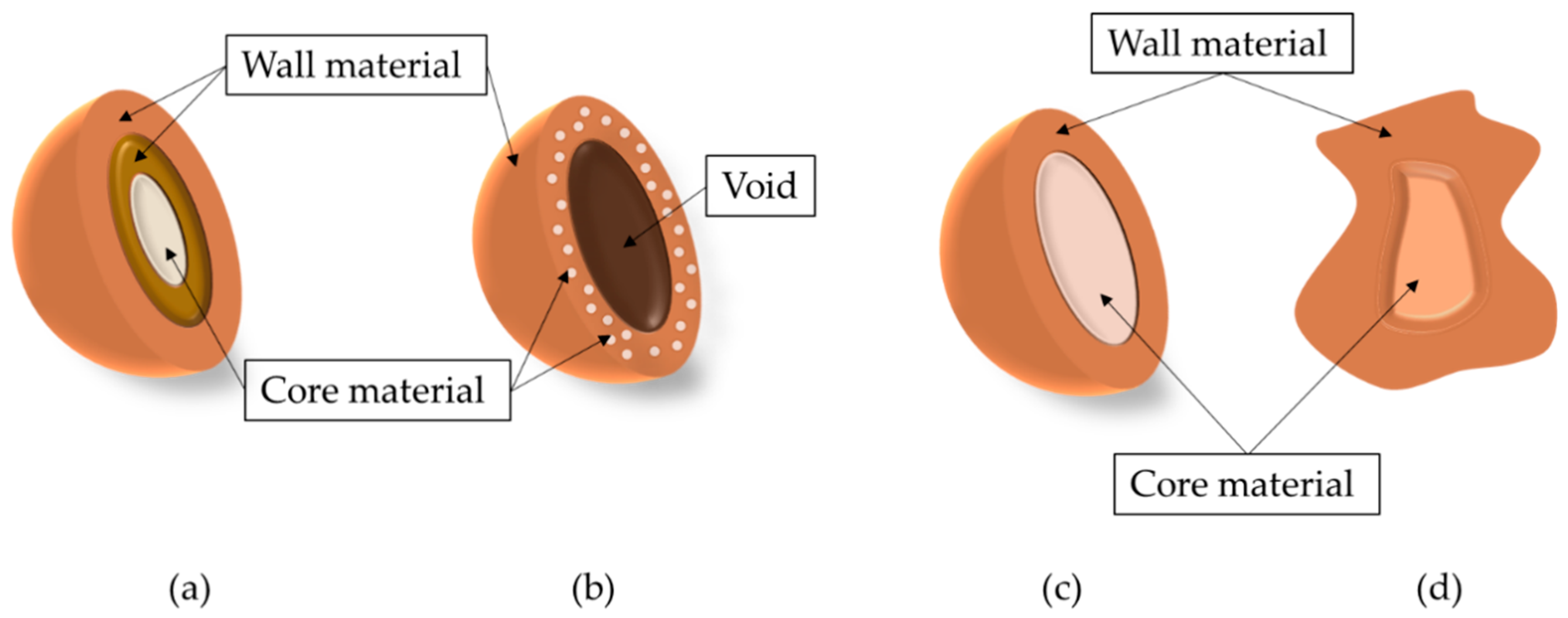
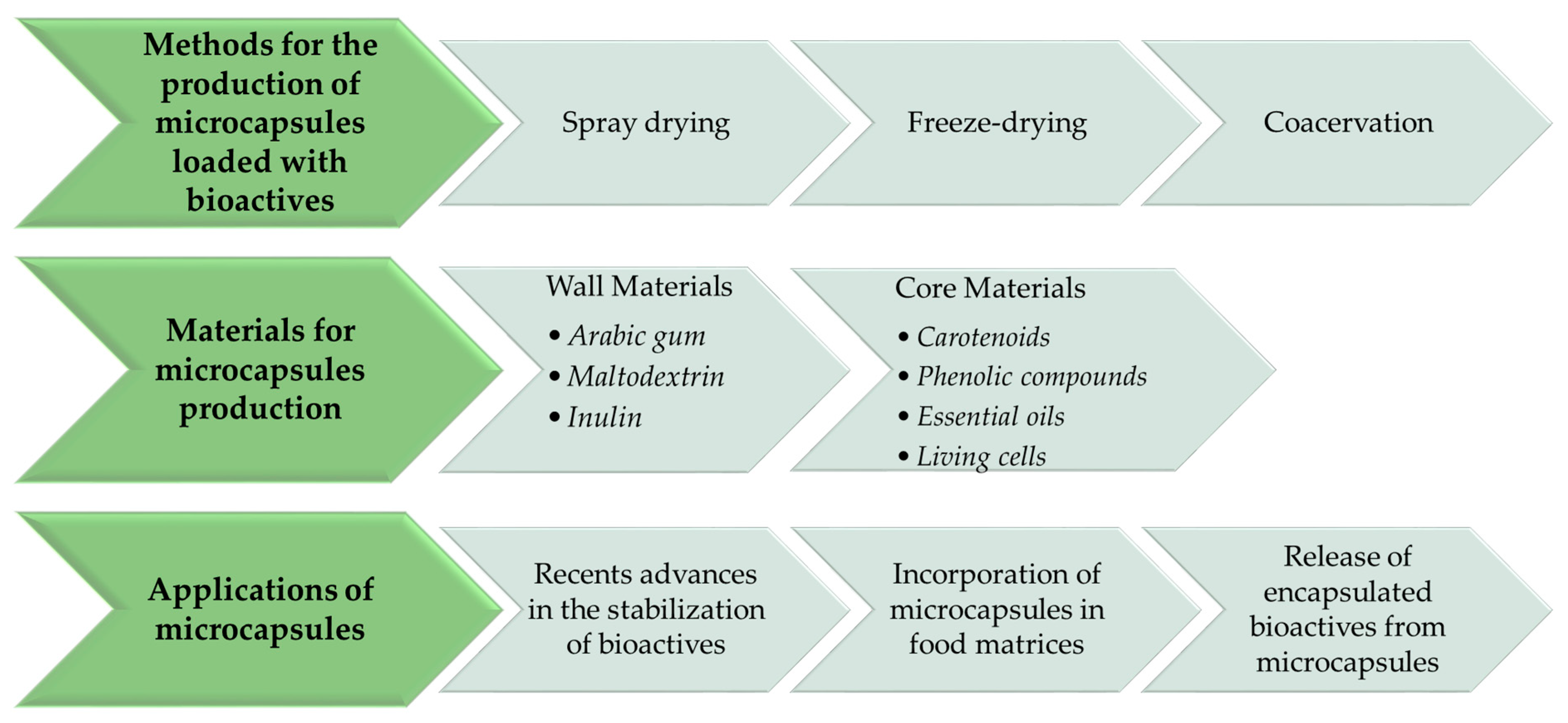
2. Methods for the Production of Microcapsules Loaded with Bioactive Compounds
3. Wall Materials
4. Core Materials
5. Recent Advances in the Stabilization of Bioactive Compounds
6. Incorporation of Microcapsules in Food Matrices
7. Release of Encapsulated Bioactives from Microcapsules
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Reineccius, G. Use of proteins for the delivery of flavors and other bioactive compounds. Food Hydrocoll 2019, 86, 62–69. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids microencapsulation by spray drying method and supercritical micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Moschakis, T.; Biliaderis, C.G. Biopolymer-based coacervates: Structures, functionality and applications in food products. Curr. Opin. Colloid Interface Sci. 2017, 28, 96–109. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Jyothi, S.; Seethadevi, A.; Prabha, K.S.; Muthuprasanna, P.; Pavitra, P. Microencapsulation: A review. Int. J. Pharm. Bio Sci. 2012, 3, 509–531. [Google Scholar]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- De Souza, V.B.; Thomazini, M.; Echalar Barrientos, M.A.; Nalin, C.M.; Ferro-Furtado, R.; Genovese, M.I.; Favaro-Trindade, C.S. Functional properties and encapsulation of a proanthocyanidin-rich cinnamon extract (Cinnamomum zeylanicum) by complex coacervation using gelatin and different polysaccharides. Food Hydrocoll. 2018, 77, 297–306. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, O.; Ruiz-Espinosa, H.; Luna-Guevara, J.J.; Ochoa-Velasco, C.E.; Luna Vital, D.; Luna-Guevara, M.L. A potential natural coloring agent with antioxidant properties: Microencapsulates of Renealmia alpinia (Rottb.) Maas fruit pericarp. NFS J. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- García, J.M.; Giuffrida, D.; Dugo, P.; Mondello, L.; Osorio, C. Development and characterisation of carotenoid-rich microencapsulates from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technol. 2018, 339, 702–709. [Google Scholar] [CrossRef]
- Lee, W.J.; Tan, C.P.; Sulaiman, R.; Smith, R.L.; Chong, G.H. Microencapsulation of red palm oil as an oil-in-water emulsion with supercritical carbon dioxide solution-enhanced dispersion. J. Food Eng. 2018, 222, 100–109. [Google Scholar] [CrossRef]
- Nalawade, P.B.; Gajjar, A.K. Microencapsulation of lutein extracted from marigold flowers (Tagetes erecta L.) using full factorial design. J. Drug Deliv. Sci. Technol. 2016, 33, 75–87. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Shi, J.; Huang, X.; Peng, Q.; Xue, F. Encapsulation of tomato oleoresin using soy protein isolate-gum aracia conjugates as emulsifier and coating materials. Food Hydrocoll. 2015, 45, 301–308. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sanches, E.A.; Fernandes, R.V.d.B.; Botrel, D.A.; Borges, S.V. Stability of lime essential oil microparticles produced with protein-carbohydrate blends. Food Res. Int. 2018, 105, 936–944. [Google Scholar] [CrossRef]
- Dima, C.; Pătraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The kinetics of the swelling process and the release mechanisms of Coriandrum sativum L. essential oil from chitosan/alginate/inulin microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.L.; Etchepare, M.d.A.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; Flores, É.M.d.M.; da Silva, C.d.B.; de Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Dias, C.O.; dos Santos Opuski de Almeida, J.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; de Mello Castanho Amboni, R.D. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Sultana, A.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Stability and release behavior of encapsulated flavor from spray-dried Saccharomyces cerevisiae and maltodextrin powder. Food Res. Int. 2018, 106, 809–816. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth adaptation of probiotics in biopolymer-based coacervate structures to enhance cell viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Poshadri, A.; Aparna, K. Microencapsulation technology: A review. J. Res. ANGRAU 2010, 38, 86–102. [Google Scholar]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. The influence of carrier material on some physical and structural properties of carrot juice microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. A storage study of encapsulated gac (Momordica cochinchinensis) oil powder and its fortification into foods. Food Bioprod. Process. 2015, 96, 113–125. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, M.M.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Beirão-da-Costa, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Januário, M.I.N.; Vicente, A.A.; Beirão-da-Costa, M.L.; Delgadillo, I. Inulin potential for encapsulation and controlled delivery of Oregano essential oil. Food Hydrocoll. 2013, 33, 199–206. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by spray drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Yinbin, L.; Wu, L.; Weng, M.; Tang, B.; Lai, P.; Chen, J. Effect of different encapsulating agent combinations on physicochemical properties and stability of microcapsules loaded with phenolics of plum (Prunus salicina lindl.). Powder Technol. 2018, 340, 459–464. [Google Scholar] [CrossRef]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.A.; Calderas, F.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Ochoa-Martínez, L.A.; Bernad-Bernad, M.J. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef]
- Moreno, T.; Cocero, M.J.; Rodríguez-Rojo, S. Storage stability and simulated gastrointestinal release of spray dried grape marc phenolics. Food Bioprod. Process. 2018, 112, 96–107. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Mendoza, M.R.; García, O.; Jiménez-Fernández, V.M.; Jiménez, M. Microencapsulation of vanilla (Vanilla planifolia Andrews) and powder characterization. Powder Technol. 2018, 323, 416–423. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT Food Sci. Technol. 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Reyes, V.; Chotiko, A.; Chouljenko, A.; Sathivel, S. Viability of Lactobacillus acidophilus NRRL B-4495 encapsulated with high maize starch, maltodextrin, and gum arabic. LWT 2018, 96, 642–647. [Google Scholar] [CrossRef]
- Verruck, S.; de Carvalho, M.W.; de Liz, G.R.; Amante, E.R.; Vieira, C.R.W.; Amboni, R.D.d.M.C.; Prudencio, E.S. Survival of Bifidobacterium BB-12 microencapsulated with full-fat goat’s milk and prebiotics when exposed to simulated gastrointestinal conditions and thermal treatments. Small Rumin. Res. 2017, 153, 48–56. [Google Scholar] [CrossRef]
- Liao, L.-K.; Wei, X.-Y.; Gong, X.; Li, J.-H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Design and characterization of controlled-release vitamin A microparticles prepared by a spray-drying process. Powder Technol. 2017, 305, 411–417. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, L.; Turturică, M.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleț, M.; Stănciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. Technol. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of palm oil by complex coacervation for application in food systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Boillon, M.R.G.; Thomazini, M.; Nogueira, M.S.; de Castro, I.A.; Favaro-Trindade, C.S. Protection of echium oil by microencapsulation with phenolic compounds. Food Res. Int. 2016, 88, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.M.; García, F.; Calvo, P.; Bernalte, M.J.; González-Gómez, D. Optimization of broccoli microencapsulation process by complex coacervation using response surface methodology. Innov. Food Sci. Emerg. Technol. 2016, 34, 243–249. [Google Scholar] [CrossRef]
- Marques da Silva, T.; Jacob Lopes, E.; Codevilla, C.F.; Cichoski, A.J.; Flores, É.M.d.M.; Motta, M.H.; da Silva, C.d.B.; Grosso, C.R.F.; de Menezes, C.R. Development and characterization of microcapsules containing Bifidobacterium Bb-12 produced by complex coacervation followed by freeze drying. LWT 2018, 90, 412–417. [Google Scholar] [CrossRef]
- Santos, M.G.; Bozza, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Microencapsulation of xylitol by double emulsion followed by complex coacervation. Food Chem. 2015, 171, 32–39. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Carazo-Díaz, C.; Gabaldón, J.A.; Núñez-Delicado, E. Optimization of the microencapsulation of synthetic strawberry flavor with different blends of encapsulating agents using spray drying. Powder Technol. 2018, 338, 591–598. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Rodríguez-Marín, M.L.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent advances in microencapsulation of natural sources of antimicrobial compounds used in food—A review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Kang, Y.-R.; Lee, Y.-K.; Kim, Y.J.; Chang, Y.H. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum Arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef]
- Chang, C.; Varankovich, N.; Nickerson, M.T. Microencapsulation of canola oil by lentil protein isolate-based wall materials. Food Chem. 2016, 212, 264–273. [Google Scholar] [CrossRef]
- Kuang, S.S.; Oliveira, J.C.; Crean, A.M. Microencapsulation as a tool for incorporating bioactive ingredients into food. Crit. Rev. Food Sci. Nutr. 2010, 50, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Orsat, V. Spray drying for the production of nutraceutical ingredients—A review. Food Bioprocess Technol. 2011, 5, 3–14. [Google Scholar] [CrossRef]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Alves, A.R.C. Drying by spray drying in the food industry: Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ordoñez, M.; Herrera, A. Morphologic and stability cassava starch matrices for encapsulating limonene by spray drying. Powder Technol. 2014, 253, 89–97. [Google Scholar] [CrossRef]
- Krishnan, S.; Bhosale, R.; Singhal, R.S. Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr. Polym. 2005, 61, 95–102. [Google Scholar] [CrossRef]
- Chronakis, I.S. On the molecular characteristics, compositional properties, and structural-functional mechanisms of maltodextrins: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 599–637. [Google Scholar] [CrossRef] [PubMed]
- Dokic, L.; Jakovljevic, J.; Dokic, P. Relation between Viscous Characteristics and Dextrose Equivalent of Maltodextrins. Starch Stärke 2004, 56, 520–525. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Y.; Ye, J.; Jia, J.; Ma, J.; Ge, F. Preparation of walnut oil microcapsules employing soybean protein isolate and maltodextrin with enhanced oxidation stability of walnut oil. LWT Food Sci. Technol. 2017, 83, 292–297. [Google Scholar] [CrossRef]
- Botrel, D.A.; Rodrigues, I.C.B.; Souza, H.J.B.d.; Fernandes, R.V.d.B. Application of inulin in thin-layer drying process of araticum (Annona crassiflora) pulp. LWT Food Sci. Technol. 2016, 69, 32–39. [Google Scholar] [CrossRef]
- Jain, A.K.; Sood, V.; Bora, M.; Vasita, R.; Katti, D.S. Electrosprayed inulin microparticles for microbiota triggered targeting of colon. Carbohydr. Polym. 2014, 112, 225–234. [Google Scholar] [CrossRef]
- Niness, K.R. Inulin and oligofructose: What are they? J. Nutr. 1999, 129, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Fukami, K.; Thanatuksorn, P.; Viriyarattanasak, C.; Kajiwara, K. Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr. Polym. 2011, 83, 934–939. [Google Scholar] [CrossRef]
- Lopes, S.M.S.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M. Food Polysaccharides and Their Applications; CRC Press: Boca Raton, FL, USA, 1995; Volume 67. [Google Scholar]
- González-Herrera, S.M.; Herrera, R.R.; López, M.G.; Rutiaga, O.M.; Aguilar, C.N.; Esquivel, J.C.C.; Martínez, L.A.O. Inulin in food products: Prebiotic and functional ingredient. Br. Food J. 2015, 117, 371–387. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Xu, B.; Ren, G.; Li, P.; Li, X.; Han, S.; Liu, J. Effects of inulin with different degree of polymerization on gelatinization and retrogradation of wheat starch. Food Chem. 2017, 229, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, L.; Sangronis, E. La inulina y derivados como ingredientes claves en alimentos funcionales. Archivos latinoamericanos de nutrición 2007, 57, 387. [Google Scholar]
- Fernandes, R.V.d.B.; Botrel, D.A.; Silva, E.K.; Borges, S.V.; Oliveira, C.R.d.; Yoshida, M.I.; Feitosa, J.P.d.A.; de Paula, R.C.M. Cashew gum and inulin: New alternative for ginger essential oil microencapsulation. Carbohydr. Polym. 2016, 153, 133–142. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Dias, D.R.; Botrel, D.A.; Fernandes, R.V.D.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. TrAC Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst Cvejić, J.; Atanacković Krstonošić, M.; Bursać, M.; Miljić, U. Chapter 7—Polyphenols. In Nutraceutical and Functional Food Components; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 203–258. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Morales, M.E.; Ruiz, M.A. 16—Microencapsulation of probiotic cells: Applications in nutraceutic and food industry. In Nutraceuticals; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 627–668. [Google Scholar] [CrossRef]
| Wall Material | Core Material | Core Material Source | Main Properties/Applications Studied | Refs. |
|---|---|---|---|---|
| Spray Drying | ||||
| Arabic gum Gelatin Maltodextrin | Anthocyanins | Barberry extract | Stabilization of active ingredient | [31] |
| Arabic gum Maltodextrin | Anthocyanins Carotenoids | Tamarillo | Stabilization of active ingredient Storage stability | [32] |
| Arabic gum Maltodextrin Whey protein | Carotenoids | Carrot | Stabilization of active ingredient | [33] |
| Arabic gum Whey protein | Carotenoids | Gac oil | Storage stability Incorporation in food matrix | [34] |
| Maltodextrin | Carotenoids | Mango Banana Tamarillo | Stabilization of active ingredient Storage stability | [16] |
| Maltodextrin Sodium caseinate | Carotenoids | Red palm oil | Stabilization of active ingredient | [17] |
| Arabic gum Soy protein | Carotenoids | Tomato oleoresin | Stabilization of active ingredient Storage stability Controlled release of bioactives from microcapsules | [19] |
| Arabic gum | Carotenoids | β-carotene | Stabilization of active ingredient | [35] |
| Alginate Chitosan Inulin | Essential oil | Coriander | Controlled release of bioactives from microcapsules | [23] |
| Inulin | Essential oil | Oregano | Controlled release of bioactives from microcapsules | [36] |
| Maltodextrin Whey protein | Essential oil | Lime | Controlled release of bioactives from microcapsules | [22] |
| Maltodextrin | Lutein | Marigold flowers | Stabilization of active ingredient | [18] |
| Maltodextrin | Phenolic compounds | Laurel infusions | Controlled release of bioactives from microcapsules | [37] |
| Arabic gum Maltodextrin | Phenolic compounds | Renealmia alpinia | Stabilization of active ingredient Storage stability | [14] |
| Arabic gum Maltodextrin Skimmed milk Whey protein | Phenolic compounds | Pomegranate peels | Stabilization of active ingredient Incorporation in food matrix | [13] |
| Arabic gum Maltodextrin β-Cyclodextrin Chitosan Gelatin | Phenolic compounds | Plum | Storage stability | [38] |
| Maltodextrin | Phenolic compounds | Cinnamon infusions | Stabilization of active ingredient Controlled release of bioactives from microcapsules | [39] |
| Maltodextrin | Phenolic compounds | Averrhoa carambola pomace | In vitro simulated gastrointestinal digestion release of bioactives from microcapsules | [40] |
| Maltodextrin Pea protein Whey protein | Phenolic compounds | Grape marc | Storage stability In vitro simulated gastrointestinal digestion release of bioactives from microcapsules | [41] |
| Whey protein | Polyphenols | Vanilla | Stabilization of active ingredient Storage stability | [42] |
| Arabic gum Inulin | Probiotic bacteria | Lactobacillus acidophilus | Storage stability In vitro simulated gastrointestinal digestion release of probiotics from microcapsules | [24] |
| Arabic gum Maltodextrin Modified starch Whey protein | Probiotic bacteria | Saccharomyces cerevisiae | In vitro simulated gastric digestion release of probiotics from microcapsules | [43] |
| Arabic gum High maize starch Maltodextrin | Probiotic bacteria | Lactobacillus acidophilus | Storage stability | [44] |
| Goat’s milk Inulin Oligofructose | Probiotic bacteria | Bifidobacterium | In vitro simulated gastrointestinal digestion release of the probiotics from microcapsules | [45] |
| Inulin Maltodextrin | Probiotic bacteria | Bifidobacterium | Stabilization of active ingredient Storage stability | [25] |
| Maltodextrin Skim milk Trehalose | Probiotic bacteria | Lactobacillus casei | In vitro simulated gastrointestinal digestion release of the probiotics from microcapsules Storage stability | [46] |
| Arabic gum | Vitamin A | Retinol | Controlled release of bioactives from microcapsules | [47] |
| Freeze-drying | ||||
| Maltodextrin | Anthocyanins | Blackberry pulp pomace | Stabilization of active ingredient | [12] |
| Arabic gum Whey protein | Anthocyanins | Sour cherries | In vitro simulated gastrointestinal digestion release of bioactives from microcapsules Incorporation in food matrix | [15] |
| Maltodextrin | Phenolic compounds | Averrhoa carambola pomace | In vitro simulated gastrointestinal digestion release of bioactives from microcapsules | [40] |
| Maltodextrin β-cyclodextrin | Polyphenols | Green tea | Incorporation in food | [48] |
| Denatured whey protein Fructooligosaccharide Sodium alginateWhey protein | Probiotic bacteria | Lactobacillus plantarum | Stabilization of active ingredient Storage stability | [49] |
| Inulin Persian gum Whey protein | Probiotic bacteria | Lactobacillus rhamnosus | In vitro simulated gastrointestinal digestion release of probiotics from microcapsules Storage stability | [26] |
| Maltodextrin | Probiotic bacteria | Saccharomyces cerevisiae | Controlled release of bacteria from microcapsules Storage stability | [27] |
| Coacervation | ||||
| Arabic gum | Anthocyanins | Black raspberry | Stabilization of active ingredient Storage stability | [50] |
| Arabic gum Whey protein | Astaxanthins | Haematococcus pluvialis | Storage stability In vitro and in vivo simulated gastrointestinal digestion release of bioactives from microcapsules | [51] |
| Arabic gum Whey protein | Carotenoids | Sea buckthorn | Stabilization of active ingredient | [52] |
| Arabic gum Whey protein | Carotenoids | Sea buckthorn | Incorporation in food matrix | [21] |
| Chitosan Pectin Xanthan gum | Carotenoids | Palm oil | Incorporation in food matrix In vitro simulated gastrointestinal digestion release of bioactives from food matrix | [53] |
| Carboxymethylcellulose Chitosan Sodium tripolyphosphate | Carotenoids | Palm oil Soybean oil with β-carotene | Incorporation in food matrix In vitro simulated gastrointestinal digestion release of bioactives from microcapsules and food matrix | [54] |
| Arabic gum Gelatin | Phenolic compounds | Echium oil | Stabilization of active ingredient | [55] |
| Arabic gum Gelatin | Phenolic compounds | Broccoli | Stabilization of active ingredient | [56] |
| Arabic gum Gelatin | Proanthocyanidin | Cinnamon | Stabilization of active ingredient | [11] |
| Arabic gum Gelatin | Probiotic bacteria | Bifidobacterium | In vitro simulated gastrointestinal digestion release of probiotics from microcapsules Storage stability | [57] |
| Arabic gum Whey protein | Probiotic bacteria | Lactobacillus paracasei Lactobacillus paraplantarum | Stabilization of active ingredient In vitro simulated gastrointestinal digestion release of probiotics from microcapsules | [28] |
| Arabic gum Gelatin | Xylitol | Commercial | Stabilization of active ingredient Controlled release of bioactives from microcapsules | [58] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. https://doi.org/10.3390/app9030571
Corrêa-Filho LC, Moldão-Martins M, Alves VD. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Applied Sciences. 2019; 9(3):571. https://doi.org/10.3390/app9030571
Chicago/Turabian StyleCorrêa-Filho, Luiz C., Margarida Moldão-Martins, and Vitor D. Alves. 2019. "Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products" Applied Sciences 9, no. 3: 571. https://doi.org/10.3390/app9030571
APA StyleCorrêa-Filho, L. C., Moldão-Martins, M., & Alves, V. D. (2019). Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Applied Sciences, 9(3), 571. https://doi.org/10.3390/app9030571