Impact of Interactions between Ferulic and Chlorogenic Acids on Enzymatic and Non-Enzymatic Lipids Oxidation: An Example of Bread Enriched with Green Coffee Flour
Abstract
:1. Introduction
- (i)
- a given activity of phenolic compounds studied in vitro (after their isolation from the food) does not have to be in accordance with the same activity demonstrated in the human organism [13]. In order to study the impact of digestion on the biological activity of food and beverages, in vitro models based on human physiology are widely used.
- (ii)
- interactions between bioactive components naturally occur in raw materials.
- (iii)
- interactions occur between nutraceuticals and food matrix components (starch, proteins, lipids).
- (iv)
- processing has an impact during food production (e.g. cooking, baking, storage).
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Bread Preparation
2.4. Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry LC-ESI-MS/MS Analysis of Phenolic Acids
2.5. Extracts Preparation
2.6. Inhibition of Linoleic Acid Peroxidation
2.7. Inhibition of Lipoxygenase Activity (LOXI)
2.8. Isobolographic Analysis of Interaction
2.9. Theoretical Approach
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.E.; Hornbrook, K.R. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta Mol. Basis Dis. 1997, 1362, 116–127. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, J.B.; Strosznajder, R.P. The lipoxygenases: Their regulation and implication in Alzheimer’s disease. Neurochem. Res. 2016, 41, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Khuda-Bukhsh, A.R. 5-Lipoxygenase Antagonist therapy: A new approach towards targeted cancer chemotherapy. Acta Biochim. Biophys. Sin. (Shanghai) 2013, 45, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Suhasini, G.E.; Nirmala, M.; Varalakshmi, V.; Solomon sunder Raj, B. Antioxidant activity of newly synthesised imidazole (2-(2-benzylidenehydrazinyl)-5, 5-diphenyl-1,5-dihydro-4H-imidazol 4-one). Int. J. Pharm. 2012, 2, 386–391. [Google Scholar]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed eview. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Chen, X.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+or ultraviolet light. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 1717–1721. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Różyło, R.; Świeca, M.; Krzykowski, A.; Rudy, S. Ground green coffee beans as a functional food supplement—Preliminary study. LWT Food Sci. Technol. 2015, 63, 691–699. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-dziki, U.; Dziki, D.; Baraniak, B. Wheat bread enriched with green coffee—In vitro bioaccessibility and bioavailability of phenolics and antioxidant activity. Food Chem. 2017, 221, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Goñi, I.; Saura-Calixto, F. Food antioxidant capacity determined by chemical methods may underestimate the physiological antioxidant capacity. Food Res. Int. 2007, 40, 15–21. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT Food Sci. Technol. 2014, 59, 689–694. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitrodigestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.M.; Yeh, D.B.; Pan, B.S. Rapid photometric assay evaluating antioxidative activity in edible plant material. J. Agric. Food Chem. 1999, 47, 3206–3209. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from Soybeans: EC 1.13.11.12 Linoleate: Oxygen oxidoreductase. Methods Enzymol. 1981, 71, 441–451. [Google Scholar]
- Chou, T. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J. Funct. Foods 2012, 4, 872–882. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R. Mechanism of action and interactions between xanthine oxidase inhibitors derived from natural sources of chlorogenic and ferulic acids. Food Chem. 2017, 225, 1451–1457. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.-L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Marinova, E.M.; Yanishlieva, N.V.; Toneva, A.G. Antioxidant activity and mechanism of action of ferulic and caffeic acids in different lipid systems. Riv. Ital. Delle Sostanze Grasse 2006, 83, 6–13. [Google Scholar]
- Medina, I.; Gallardo, J.M.; González, M.J.; Lois, S.; Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Dziki, D.; Swieca, M.; Durak, A.; Czyż, J. Effect of fortification with green coffee beans on chelating power of wheat bread. J. King Saud Univ. Sci. 2018, 30. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chan, H.-C.; Chu, Y.-T.; Ho, H.-Y.; Chen, P.-Y.; Lee, T.-H.; Lee, C.-K. Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules 2009, 14, 2947–2958. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase[sol]cyclooxygenase. Phyther. Res. 2005, 19, 81–102. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Swieca, M.; Gawlik-Dziki, U. Nutritional and health-promoting properties of bean paste fortified with onion skin in the light of phenolic-food matrix interactions. Food Funct. 2015, 6, 3560–3566. [Google Scholar] [CrossRef]
- Seczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Changes of antioxidant potential of pasta fortified with parsley (Petroselinum crispum Mill.) Leaves in the light of protein-phenolics interactions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Kowalska, I.; Pecio, Ł.; Durak, A.; Sęczyk, Ł. Lipoxygenase inhibitors and antioxidants from green coffee—Mechanism of action in the light of potential bioaccessibility. Food Res. Int. 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Hong, J.; Smith, T.J.; Ho, C.-T.; August, D.A.; Yang, C.S. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem. Pharmacol. 2001, 62, 1175–1183. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Baraniak, B.; Tomiło, J.; Czyż, J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013, 138, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
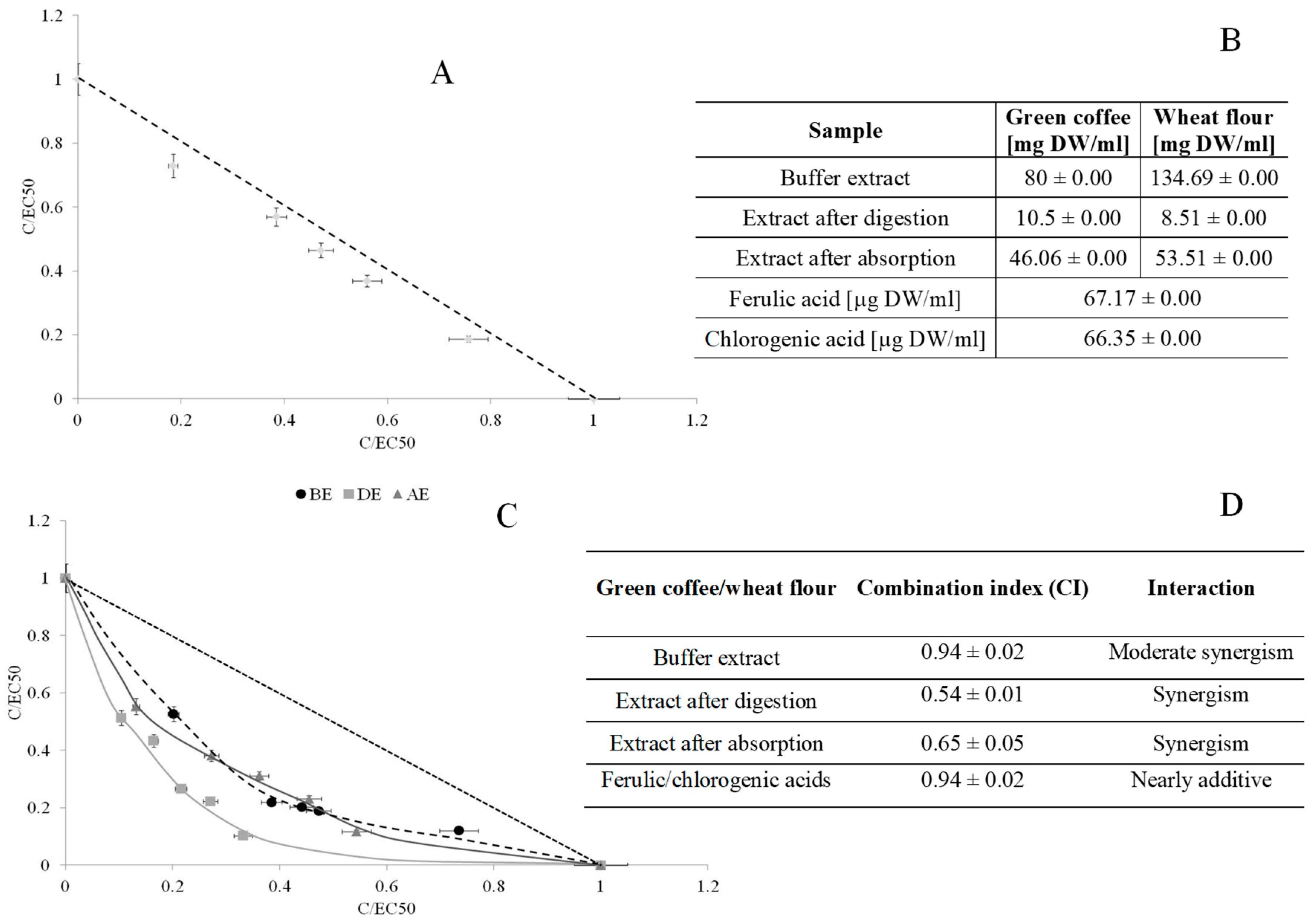
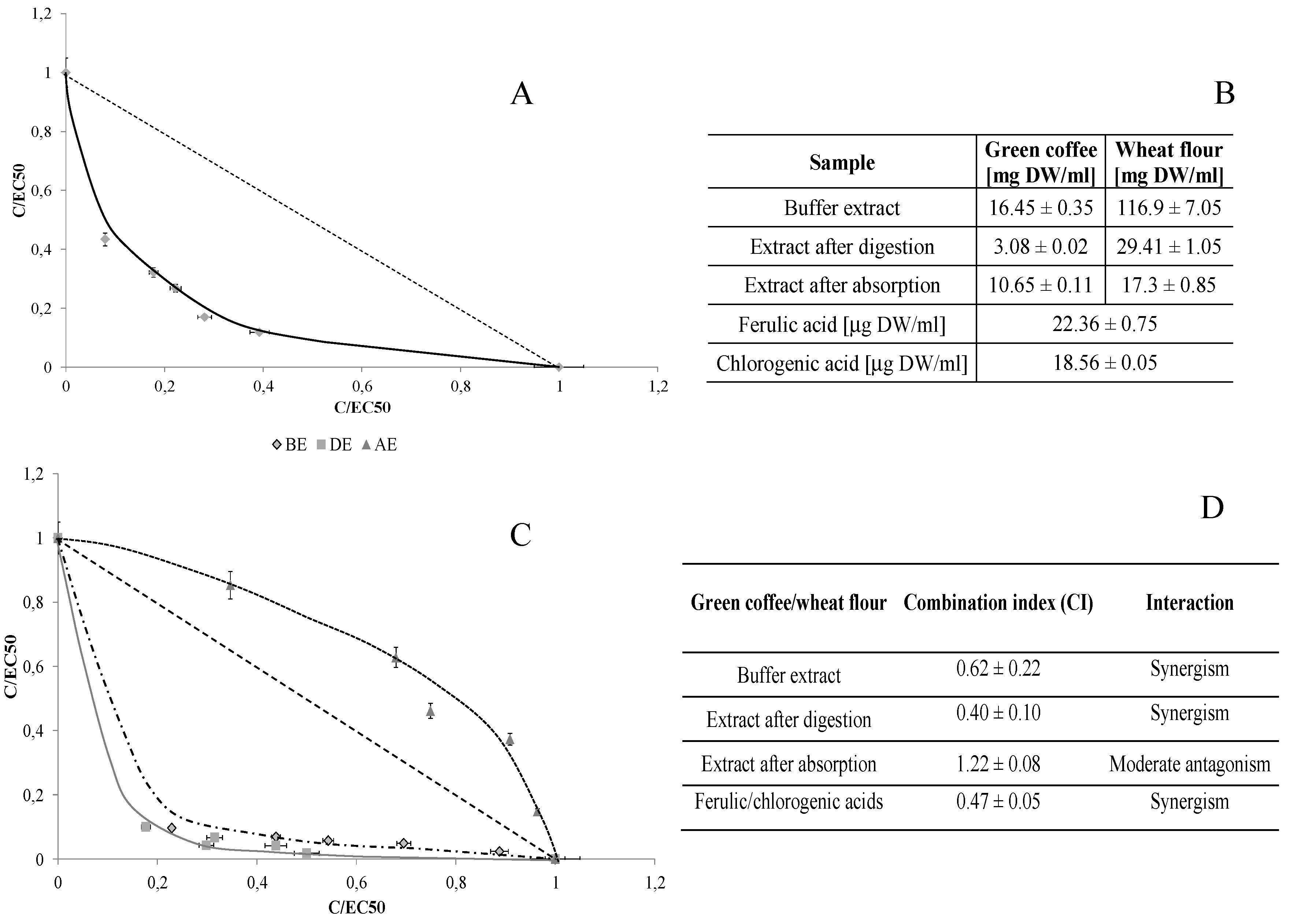

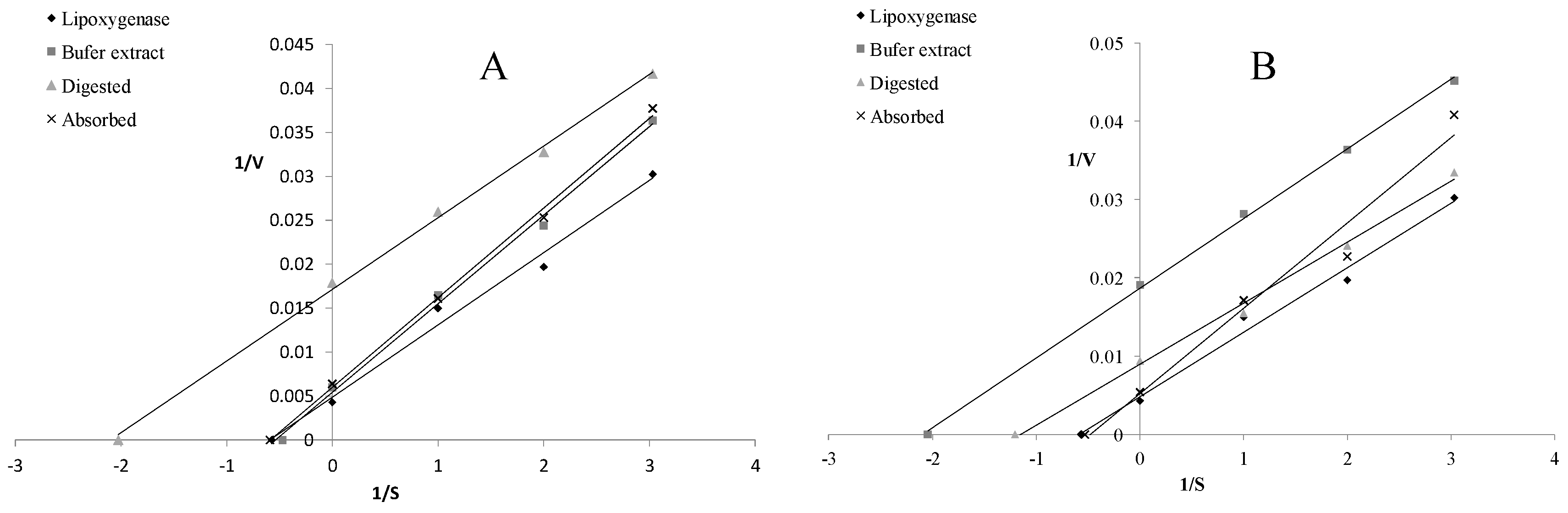
| trans-FA | cis-FA | 5-CQA | |
|---|---|---|---|
| C | 341.81 ± 3.21 a | 220.21 ± 7.09 a | nd |
| GC1 | 338.41 ± 5.88 b | 219.01 ± 6.95 b | 322.38 ± 14.85 a |
| GC2 | 335.97 ± 5.48b c | 214.94 ± 5.82 c | 644.76 ± 15.11 b |
| GC3 | 332.82 ± 5.78 d | 213.58 ± 3.64 d | 895.5 ± 12.57 c |
| GC4 | 325.46 ± 6.01 e | 210.32 ± 3.88 e | 1325.34 ± 34.21 d |
| GC5 | 323.62 ± 4.85 f | 208.56 ± 2.45 f | 1647.72 ± 46.32 e |
| Sample | LPO | LOX | |||||
|---|---|---|---|---|---|---|---|
| AM [mg DW/ml] | AT [mg DW/ml] | IF | AM [mg DW/ml] | AT [mg DW/ml] | IF | ||
| BE | C | 134.69 ± 6.43 a | - | 248.00 ± 5.55 a | - | ||
| GC1 | 130.35 ± 5.98 b | 134.23 | 0.98 ± 0.01 a | 131.33 ± 4.32 b | 262.25 | 0.38 ± 0.09 a | |
| GC2 | 122.86 ± 6.12 c | 133.76 | 115.04 ± 5.12 c | 259.60 | |||
| GC3 | 104.80 ± 5.13 d | 133.29 | 92.28 ± 3.21 d | 256.95 | |||
| GC4 | 103.91 ± 4.88 d | 132.83 | 83.83 ± 3.32 e | 254.30 | |||
| GC5 | 93.42 ± 4.35 e | 132.36 | 71.71 ± 4.15 f | 251.66 | |||
| DE | C | 11.40 ± 0.54 f | - | 44.20 ± 1.17 g | - | ||
| GC1 | 10.25 ± 0.49 g | 11.39 | 0.97 ± 0.01 a | 37.48 ± 0.99 h | 46.81 | 0.77 ± 0.02 b | |
| GC2 | 7.53 ± 0.38 h | 11.39 | 36.78 ± 1.38 h | 46.33 | |||
| GC3 | 6.72 ± 0.25 h,i | 11.38 | 34.48 ± 1.72 i | 45.86 | |||
| GC4 | 6.34 ± 0.31 i | 11.60 | 34.51 ± 1.56 i | 45.39 | |||
| GC5 | 4.97 ± 0.19 j | 11.81 | 33.78 ± 1.33 i | 44.92 | |||
| AE | C | 84.31 ± 4.11 k | - | 20.87 ± 1.19 j | - | ||
| GC1 | 75.73 ± 3.33 l | 83.93 | 0.98 ± 0.01 a | 33.78 ± 1.05 i | 31.20 | 1.04 ± 0.04 c | |
| GC2 | 44.02 ± 2.01 m | 83.54 | 31.86 ± 1.11 k | 30.89 | |||
| GC3 | 39.54 ± 1.93 m | 83.16 | 33.26 ± 1.12 i | 30.57 | |||
| GC4 | 25.46 ± 1.17 n | 82.78 | 31.05 ± 0.94 k | 30.26 | |||
| GC5 | 26.68 ± 1.11 n | 82.40 | 29.64 ± 0.98 k | 29.94 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlik-Dziki, U.; Bryda, J.; Dziki, D.; Świeca, M.; Habza-Kowalska, E.; Złotek, U. Impact of Interactions between Ferulic and Chlorogenic Acids on Enzymatic and Non-Enzymatic Lipids Oxidation: An Example of Bread Enriched with Green Coffee Flour. Appl. Sci. 2019, 9, 568. https://doi.org/10.3390/app9030568
Gawlik-Dziki U, Bryda J, Dziki D, Świeca M, Habza-Kowalska E, Złotek U. Impact of Interactions between Ferulic and Chlorogenic Acids on Enzymatic and Non-Enzymatic Lipids Oxidation: An Example of Bread Enriched with Green Coffee Flour. Applied Sciences. 2019; 9(3):568. https://doi.org/10.3390/app9030568
Chicago/Turabian StyleGawlik-Dziki, Urszula, Jarosław Bryda, Dariusz Dziki, Michał Świeca, Ewa Habza-Kowalska, and Urszula Złotek. 2019. "Impact of Interactions between Ferulic and Chlorogenic Acids on Enzymatic and Non-Enzymatic Lipids Oxidation: An Example of Bread Enriched with Green Coffee Flour" Applied Sciences 9, no. 3: 568. https://doi.org/10.3390/app9030568
APA StyleGawlik-Dziki, U., Bryda, J., Dziki, D., Świeca, M., Habza-Kowalska, E., & Złotek, U. (2019). Impact of Interactions between Ferulic and Chlorogenic Acids on Enzymatic and Non-Enzymatic Lipids Oxidation: An Example of Bread Enriched with Green Coffee Flour. Applied Sciences, 9(3), 568. https://doi.org/10.3390/app9030568










