Preparation and Properties of Poly(imide-siloxane) Copolymer Composite Films with Micro-Al2O3 Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
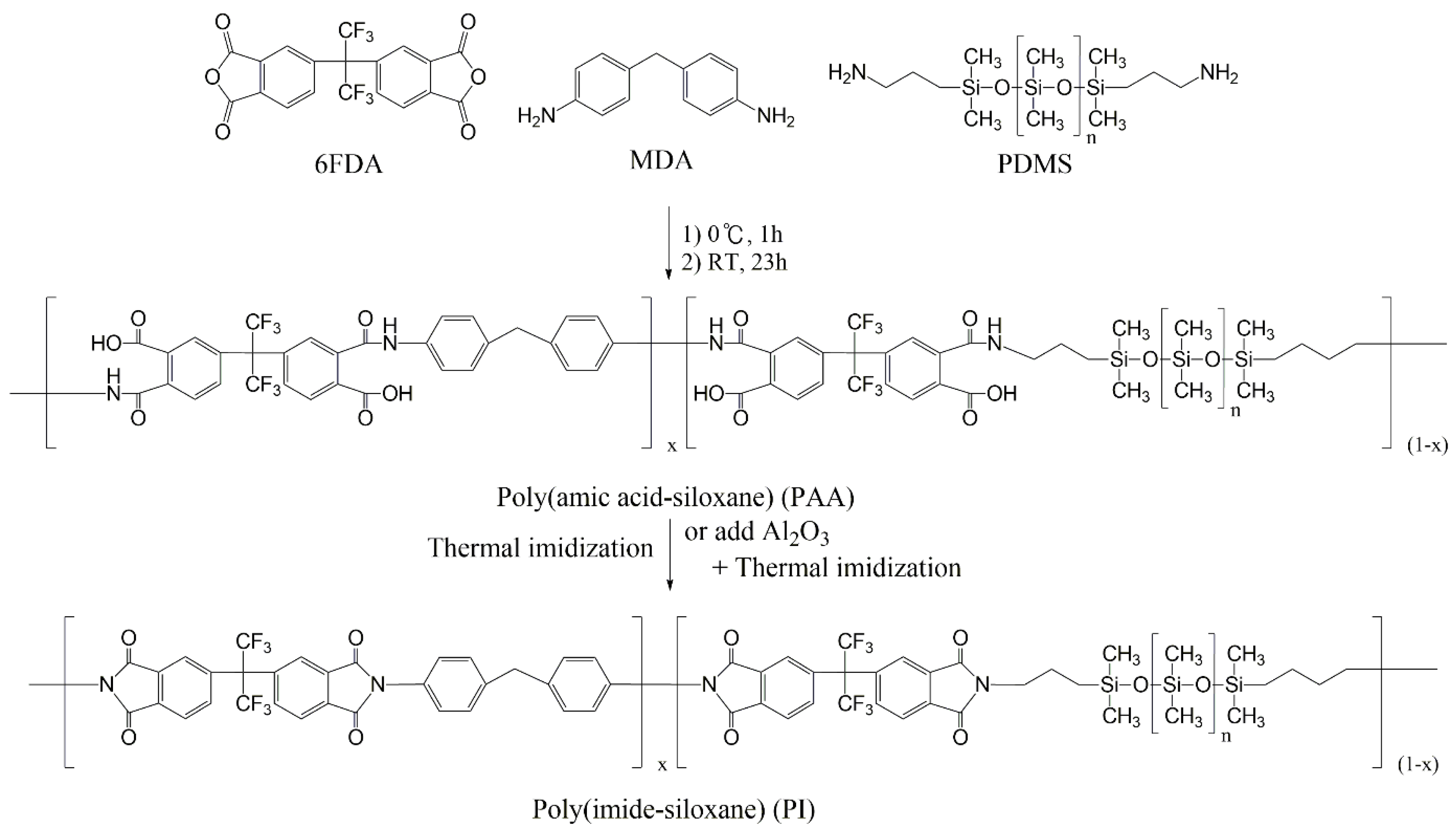
2.3. Preparation of Poly(amic acid-siloxane)s (PAAs)
2.4. Preparation of PI Films
2.5. Preparation of PI Powders
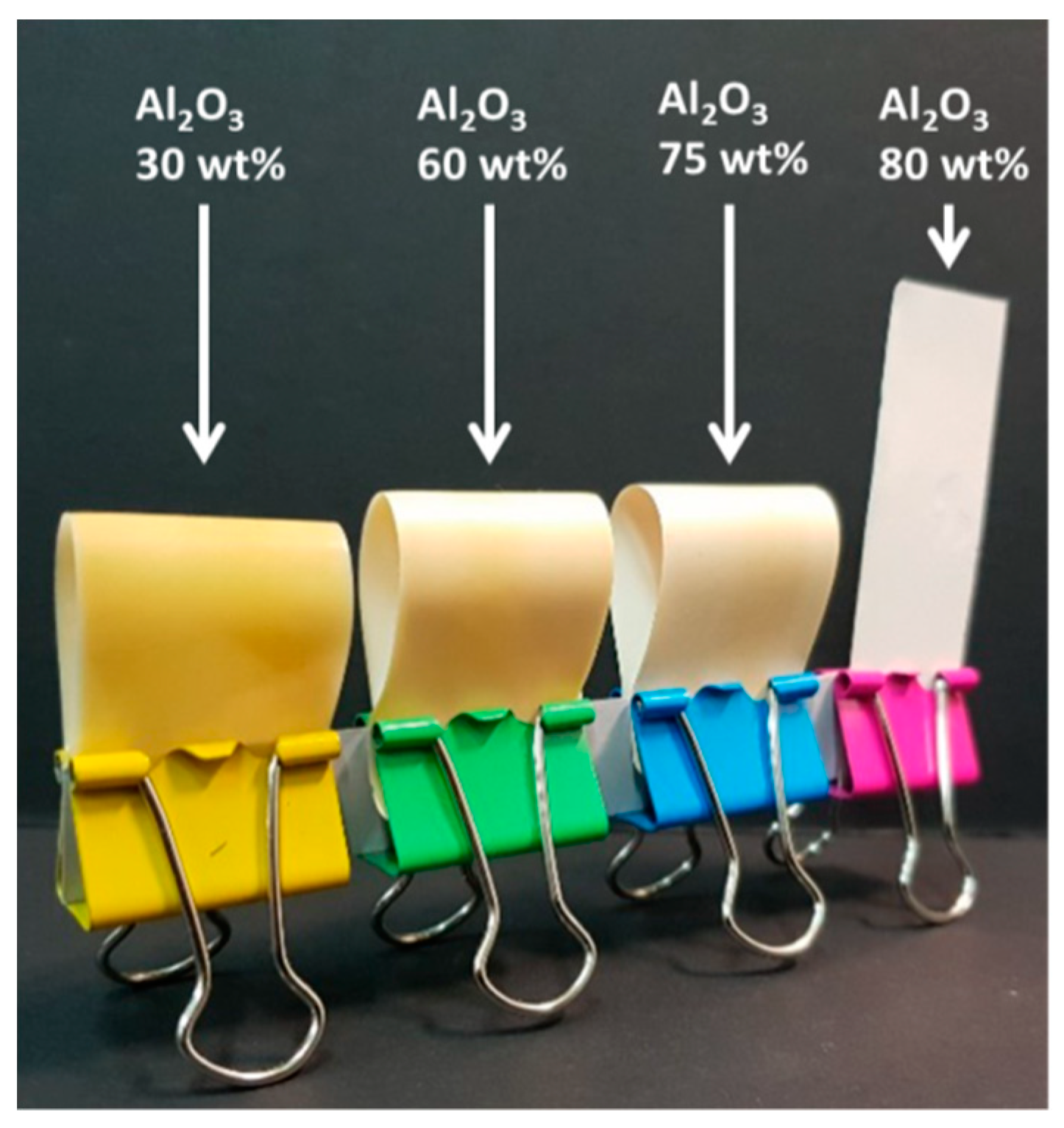
2.6. Preparation of PI Composite Films
3. Results and Discussion
3.1. Preparation of PIs and Their Composite Films
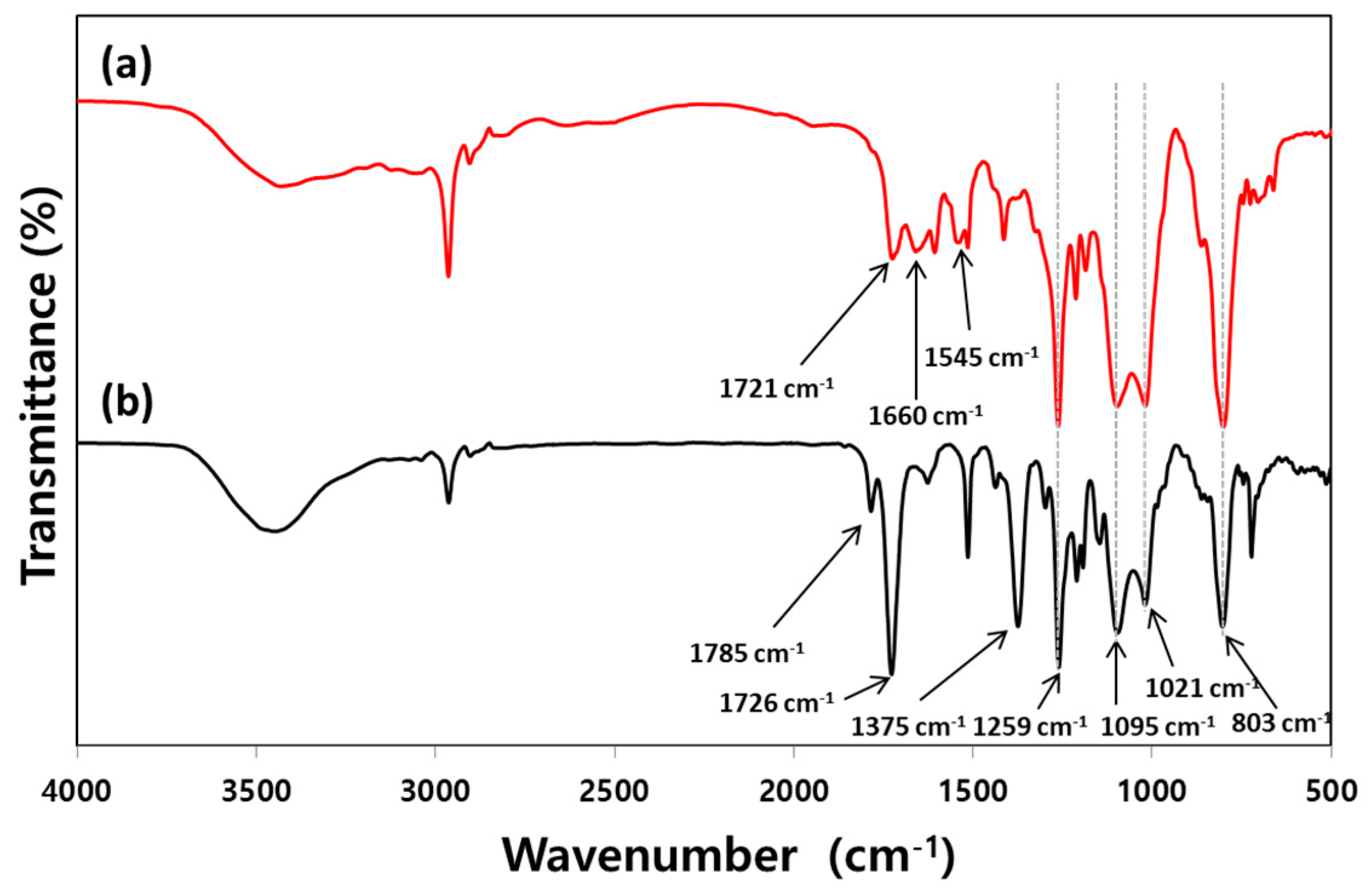
3.2. Characterization of PAAs and PIs
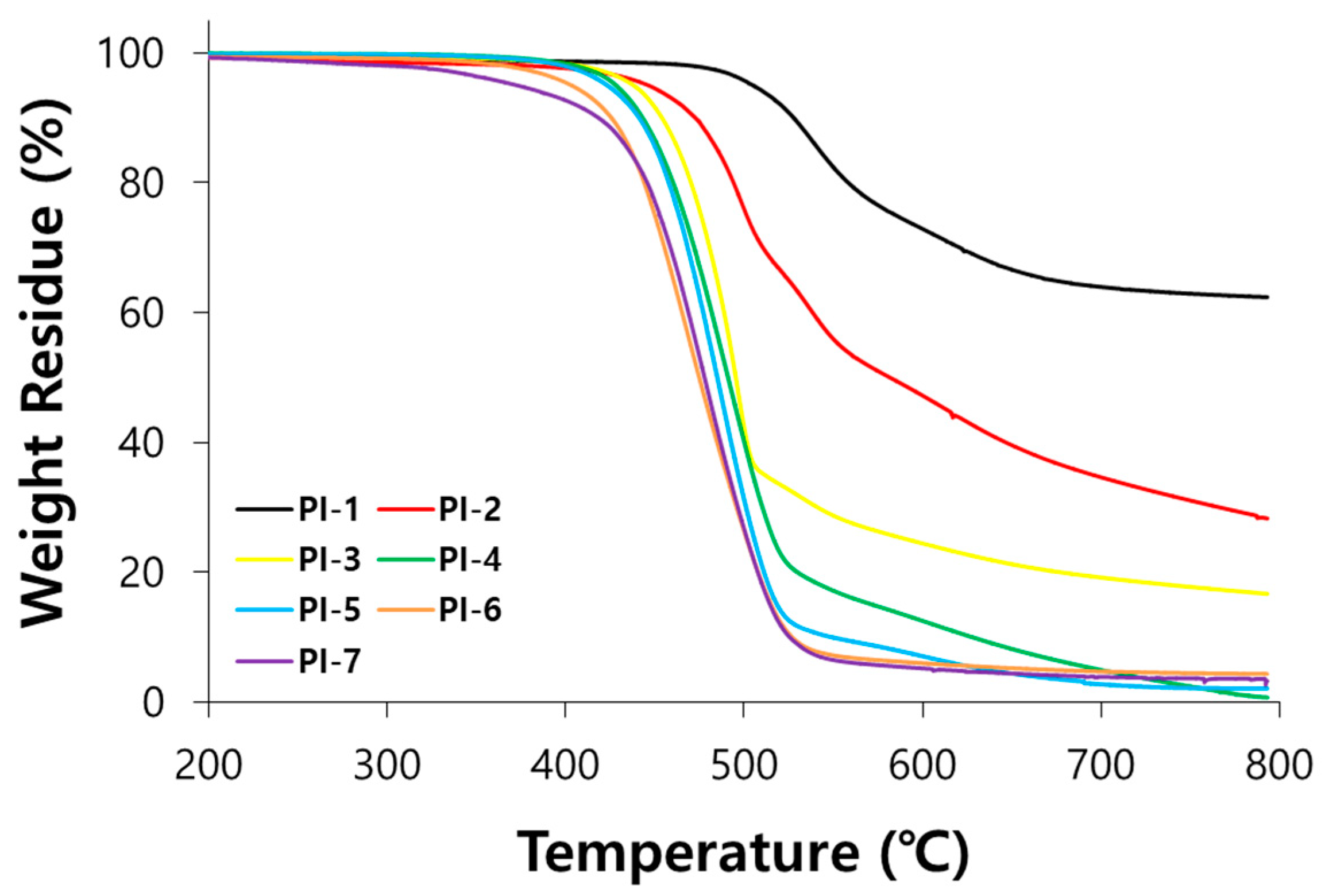
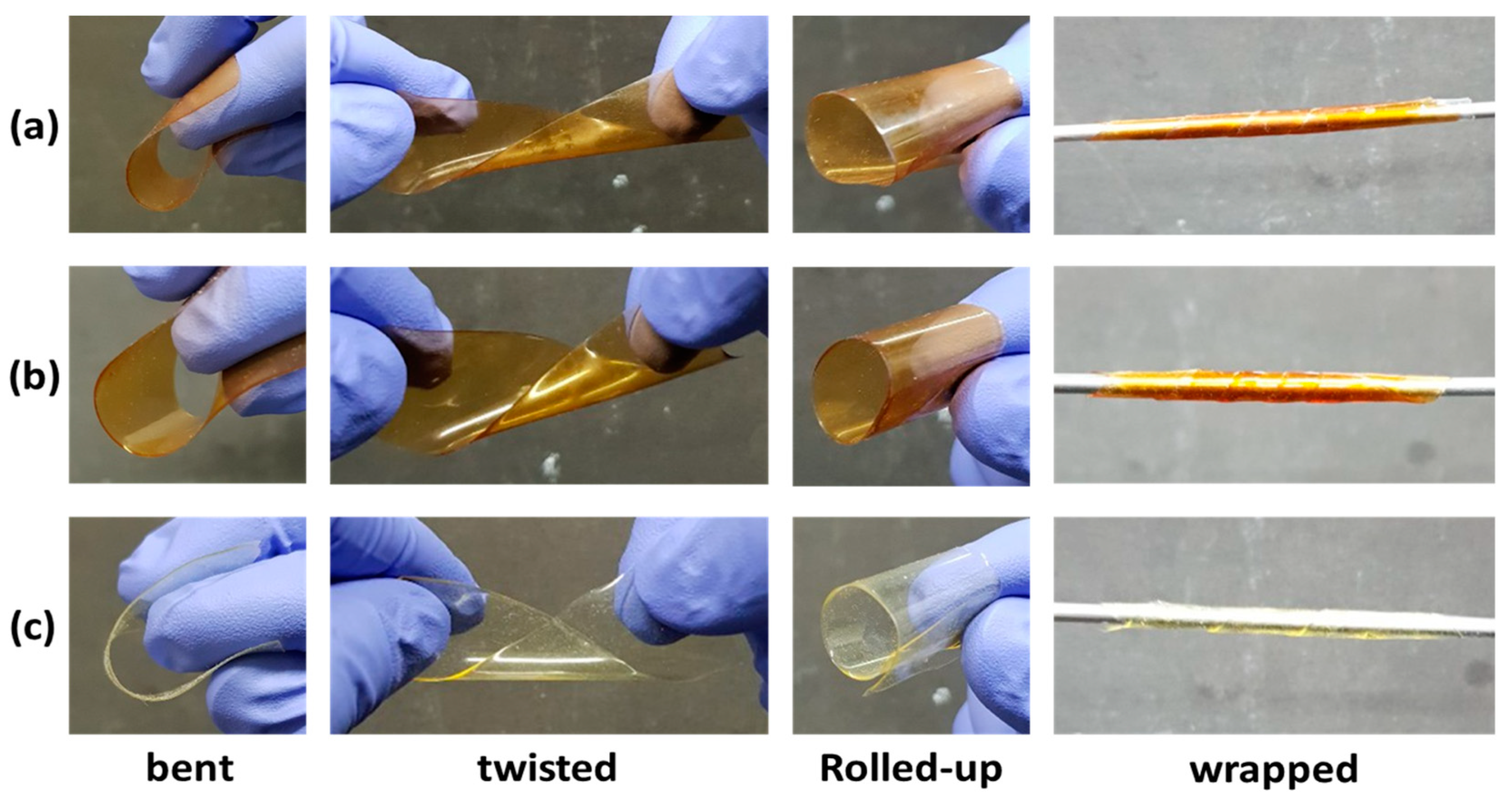
3.3. Properties of PI Films
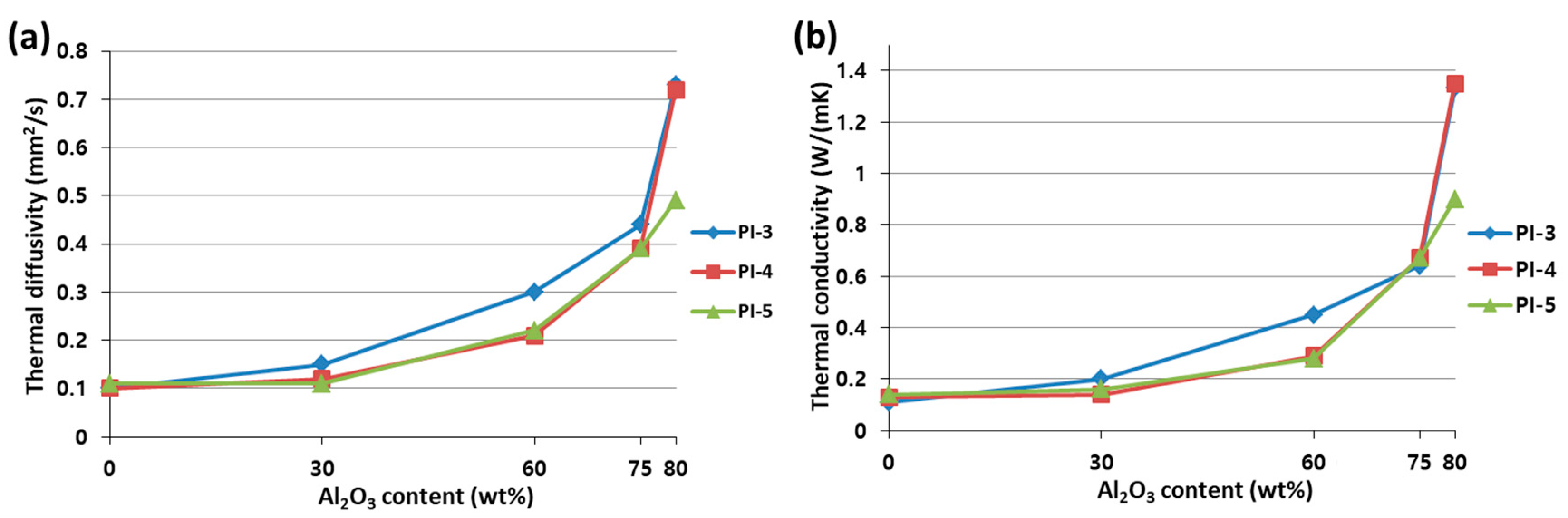
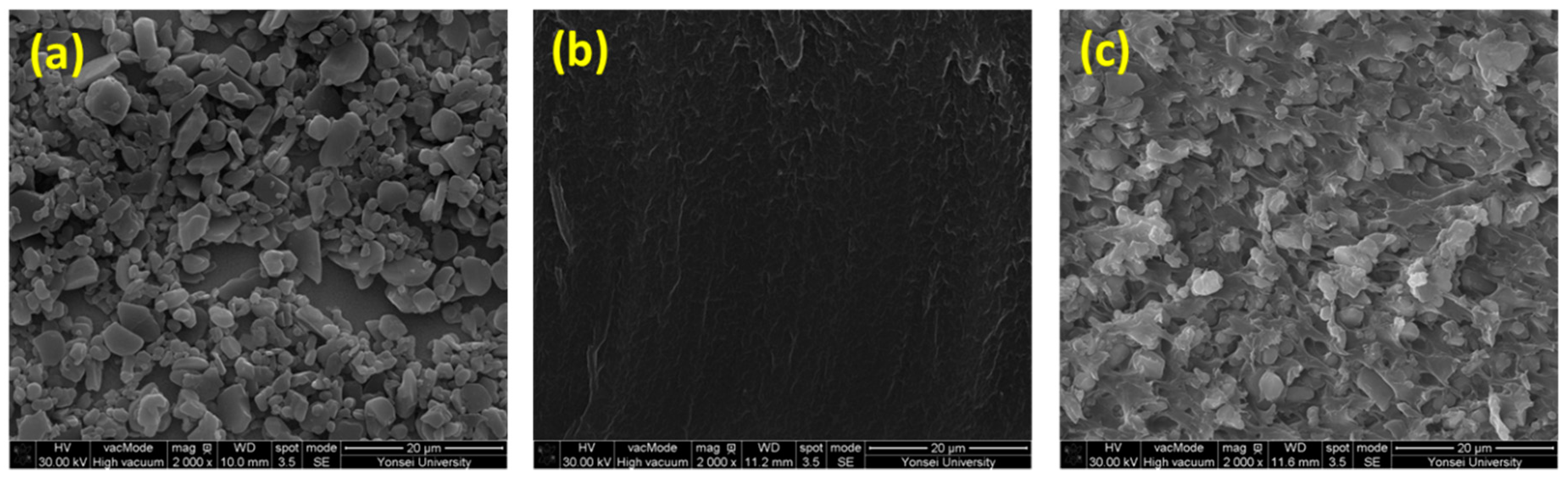
3.4. Properties of PI/Al2O3 Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Gutfleisch, O.; Willard, M.A.; Bruck, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Das, S.K.; Archer, L.A. The rechargeable aluminum-ion battery. Chem. Commun. 2011, 47, 12610–12612. [Google Scholar] [CrossRef] [PubMed]
- Gain, A.K.; Fouzder, T.; Chan, Y.C.; Sharif, A.; Wong, N.B.; Yung, W.K.C. The influence of addition of al nano-particles on the microstructure and shear strength of eutectic Sn–Ag–Cu solder on Au/Ni metallized Cu pads. J. Alloys Comp. 2010, 506, 216–223. [Google Scholar] [CrossRef]
- Li, T.-L.; Hsu, S.L.-C. Enhanced thermal conductivity of polyimide films via a hybrid of micro-and nano-sized boron nitride. J. Phys. Chem. B 2010, 114, 6825–6829. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiao, Z.; Chen, Y.; Hou, X.; Fu, L.; Wu, Y.; Li, S.; Jiang, N.; Yu, J. Enhanced thermal conductivity of poly(vinylidene fluoride)/boron nitride nanosheet composites at low filler content. Compos. Part A Appl. Sci. Manuf. 2018, 109, 321–329. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Trans. Dielectr. Electr. Insul. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, X.; Zhao, Y.; Bai, S.-L. Dense graphene foam and hexagonal boron nitride filled PDMS composites with high thermal conductivity and breakdown strength. Compos. Sci. Technol. 2017, 152, 243–253. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Liu, Y.; Zheng, X.; Kormakov, S.; Sun, J.; Zhuang, J.; Gao, X.; Wu, D.J. Improved thermal conductivity of polydimethylsiloxane/short carbon fiber composites prepared by spatial confining forced network assembly. J. Mater. Sci. 2018, 53, 14299–14310. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; So, J.I.; Kim, C.L.; Kim, M.; Shim, S.E. Treatment of atmospheric-pressure radio frequency plasma on boron nitride for improving thermal conductivity of polydimethylsiloxane composites. Macromol. Res. 2018, 26, 864–867. [Google Scholar] [CrossRef]
- Feng, L.; Iroh, J.O. Polyimide-b-polysiloxane copolymers: Synthesis and properties. J. Inorg. Organomet Polym. Mater. 2013, 23, 477–488. [Google Scholar] [CrossRef]
- Barry, A.; Beck, H. Inorganic Polymers; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Valentini, L.; Bittolo Bon, S.; Pugno, N.M. Severe graphene nanoplatelets aggregation as building block for the preparation of negative temperature coefficient and healable silicone rubber composites. Compos. Sci. Tech. 2016, 134, 125–131. [Google Scholar] [CrossRef]
- Clarson, S.J. Silicones and silicone-modified materials: A concise overview. ChemInform 2004, 35, 1–10. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.; Lu, G.; Boroyevich, D.; Ngo, K.D.T. Characterization of encapsulants for high-voltage high-temperature power electronic packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2012, 2, 539–547. [Google Scholar] [CrossRef]
- Scofield, J.D.; Merrett, J.N.; Richmond, J.; Agarwal, A.; Leslie, S. Performance and Reliability Characteristics of 1200 V, 100 A, 200 °C Half-Bridge SIC MOSFET-JBS Diode Power Modules. In Proceedings of the International Conference on High Temperature Electronics, International Microelectronics & Packaging Society, Albuquerque, NM, USA, 11–13 May 2010; pp. 000289–000296. [Google Scholar]
- Yao, Y.; Lu, G.-Q.; Boroyevich, D.; Ngo, K.D.T. Effect of Al2O3 fibers on the high-temperature stability of silicone elastomer. Polymer 2014, 55, 4232–4240. [Google Scholar] [CrossRef]
- Nakanishi, F. Photoreaction of amphiphilic diolefins in monolayers formed on an air-water interface. J. Polym. Sci. Part C Polym. Lett. 1988, 26, 159–163. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Liaw, B.-Y.; Li, L.-J.; Sillion, B.; Mercier, R.; Thiria, R.; Sekiguchi, H. Synthesis and characterization of new soluble polyimides from 3,3′,4,4′-benzhydrol tetracarboxylic dianhydride and various diamines. Chem. Mater. 1998, 10, 734–739. [Google Scholar] [CrossRef]
- Mathews, A.S.; Kim, I.; Ha, C.S. Fully aliphatic polyimides from adamantane-based diamines for enhanced thermal stability, solubility, transparency, and low dielectric constant. J. Appl. Polym. Sci. 2006, 102, 3316–3326. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Frank, C.W. Effect of cure history on the morphology of polyimide: Fluorescence spectroscopy as a method for determining the degree of cure. Polymer 1988, 29, 1191–1197. [Google Scholar] [CrossRef]
- Ngo, I.-L.; Jeon, S.; Byon, C. Thermal conductivity of transparent and flexible polymers containing fillers: A literature review. Int. J. Heat Mass Transf. 2016, 98, 219–226. [Google Scholar] [CrossRef]
- Kato, R.; Maesono, A.; Tye, R.P. Thermal conductivity measurement of submicron-thick films deposited on substrates by modified ac calorimetry (laser-heating Çngstrom method). Int. J. Thermophys. 2001, 22, 617–629. [Google Scholar] [CrossRef]
- Kurabayashi, K.; Asheghi, M.; Touzelbaev, M.; Goodson, K.E. Measurement of the thermal conductivity anisotropy in polyimide films. J. Microelectromech. Syst. 1999, 8, 180–191. [Google Scholar] [CrossRef]
- Irwin, P.C.; Cao, Y.; Bansal, A.; Schadler, L.S. Thermal and mechanical properties of polyimide nanocomposites. In Proceedings of the 2003 IEEE Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Albuquerque, NM, USA, 19–22 October 2003; pp. 120–123. [Google Scholar]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly enhanced and precisely modeled thermal conductivity in polyimide nanocomposites with chemically modified graphene via in situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 2018, 6, 3004–3015. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Fu, L.; Duan, Z.; Chen, Y.; Hou, X.; Wu, Y.; Li, S.; Guo, L.; Kang, R.; et al. Enhanced thermal conductivity of polyimide composites with boron nitride nanosheets. Sci. Rep. 2018, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- You, L. Preparation and thermal properties of silicon-containing polyimide composite films. Chem. Eng. Trans. 2017, 59, 1051–1056. [Google Scholar]
- Lupinski, J.H.; Moore, R.S. Polymeric Materials for Electronics Packaging and Interconnection; American Chemical Society: Washington, DC, USA, 1989; Volume 407, p. 516. [Google Scholar]
- Mahoney, C.M.; Gardella, J.A.; Rosenfeld, J.C. Surface characterization and adhesive properties of poly (imidesiloxane) copolymers containing multiple siloxane segment lengths. Marcromolecules 2002, 35, 5256–5266. [Google Scholar] [CrossRef]
- Homma, T.; Yamaguchi, M.; Kutsuzawa, Y.; Otsuka, N. Electrical stability of polyimide siloxane films for interlayer dielectrics in multilevel interconnections. Thin Solid Films 1999, 340, 237–241. [Google Scholar] [CrossRef]
- Rogers, M.E.; Rodrigues, D.; Wilkes, G.L.; McGrath, J.E.; Brennan, A. Perfectly alternating segmented polyimide siloxane copolymers. In Advances in New Materials; Springer: Boston, MA, USA, 1992; pp. 47–55. [Google Scholar]
- McGrath, J.E.; Dunson, D.L.; Mecham, S.J.; Hedrick, J.L. Synthesis and characterization of segmented polyimide-polyorganosiloxane copolymers. In Progress in Polyimide Chemistry I; Springer: Berlin/Heidelberg, Germany, 1999; pp. 61–105. [Google Scholar]
- Zou, L.; Anthamatten, M. Synthesis and characterization of polyimide-polysiloxane segmented copolymers for fuel cell applications. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3747–3758. [Google Scholar] [CrossRef]
- Pechar, T.W.; Kim, S.; Vaughan, B.; Marand, E.; Baranauskas, V.; Riffle, J.; Jeong, H.K.; Tsapatsis, M. Preparation and characterization of a poly(imide siloxane) and zeolite L mixed matrix membrane. J. Memb. Sci. 2006, 277, 210–218. [Google Scholar] [CrossRef]
- Bujard, P.; Kuhnlein, G.; Ino, S.; Shiobara, T. Thermal conductivity of molding compounds for plastic packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 1994, 17, 527–532. [Google Scholar] [CrossRef]
- Droval, G.; Feller, J.-F.; Salagnac, P.; Glouannec, P. Thermal conductivity enhancement of electrically insulating syndiotactic poly(styrene) matrix for diphasic conductive polymer composites. Polym. Adv. Technol. 2006, 17, 732–745. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Hojo, F.; Takezawa, Y. Preparation and characterization of thermoconductive polymer nanocomposite with branched alumina nanofiber. Appl. Phys. Lett. 2008, 92, 133309. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Hojo, F.; Takezawa, Y. Highly thermoconductive polymer nanocomposite with a nanoporous α-alumina sheet. ACS Appl. Mater. Interfaces 2009, 1, 225–227. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Liu, B.; Chen, W.; Chen, S. Dielectric properties of polyimide/Al2O3 hybrids synthesized by in-situ polymerization. Mater. Lett. 2007, 61, 1507–1511. [Google Scholar] [CrossRef]
- Ha, S.Y.; Oh, B.-K.; Lee, Y.M. Preparation of poly(amideimide siloxane) from trimellitic anhydride chloride, oxylene diamine and oligo(dimethylsiloxane) diamine. Polymer 1995, 36, 3549–3553. [Google Scholar] [CrossRef]
- Bowens, A.D. Synthesis and Characterization of Poly (Siloxane Imide) Block Copolymers and End-Functional Polyimides for Interphase Applications; Virginia Tech: Blacksburg, VA, USA, 1999. [Google Scholar]
- Deng, X.; Luo, R.; Chen, H.; Liu, B.; Feng, Y.; Sun, Y. Synthesis and surface properties of pdms–acrylate emulsion with gemini surfactant as co-emulsifier. Colloid Polym. Sci. 2007, 285, 923–930. [Google Scholar] [CrossRef]
- Téllez, L.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J.L. FT-IR study of the hydrolysis and polymerization of tetraethyl orthosilicate and polydimethyl siloxane in the presence of tetrabutyl orthotitanate. Spectrosc. Lett. 2004, 37, 11–31. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X.; Xie, H. Effects of calcination temperature on the microstructure and wetting behavior of superhydrophobic polydimethylsiloxane/silica coating. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 111–118. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Q.; Zhu, L.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties. Polymers 2017, 9, 677. [Google Scholar] [CrossRef]
- Yu, H.-C.; Choi, J.-Y.; Jeong, J.-W.; Kim, B.-J.; Chung, C.-M. Simple and easy recycling of poly(Amic Acid) gels through microwave irradiation. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 981–987. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Yu, H.-C.; Lee, J.; Jeon, J.; Im, J.; Jang, J.; Jin, S.-W.; Kim, K.-K.; Cho, S.; Chung, C.-M. Preparation of polyimide/graphene oxide nanocomposite and its application to nonvolatile resistive memory device. Polymers 2018, 10, 901. [Google Scholar] [CrossRef]
- Muratov, D.S.; Kuznetsov, D.V.; Il’inykh, I.A.; Burmistrov, I.N.; Mazov, I.N. Thermal conductivity of polypropylene composites filled with silane-modified hexagonal BN. Compos. Sci. Technol. 2015, 111, 40–43. [Google Scholar] [CrossRef]
- Cinausero, N.; Azema, N.; Cochez, M.; Ferriol, M.; Essahli, M.; Ganachaud, F.; Lopez-Cuesta, J.M. Influence of the surface modification of alumina nanoparticles on the thermal stability and fire reaction of PMMA composites. Polym. Adv. Technol. 2008, 19, 701–709. [Google Scholar] [CrossRef]
- Chen, Y.; Iroh, J.O. Synthesis and characterization of polyimide/silica hybrid composites. Chem. Mater. 1999, 11, 1218–1222. [Google Scholar] [CrossRef]
| PAA Code a | Molar Feed Ratio (6FDA:MDA:PDMS) | PDMS Content (mol%) b | n (×104 g/mol) | w (×104 g/mol) | PDI c |
|---|---|---|---|---|---|
| PAA-1 | 1:1:0 | - | 1.03 | 2.32 | 2.2 |
| PAA-2 | 1:0.9:0.1 | 13 | 10.8 | 37.8 | 3.5 |
| PAA-3 | 1:0.7:0.3 | 32 | 10.8 | 18.1 | 1.6 |
| PAA-4 | 1:0.5:0.5 | 49 | 3.73 | 13.2 | 3.5 |
| PAA-5 | 1:0.3:0.7 | 70 | 21.1 | 48.8 | 2.3 |
| PAA-6 | 1:0.1:0.9 | 89 | 16.3 | 50.9 | 3.1 |
| PAA-7 | 1:0:1 | - | 7.92 | 25.1 | 3.2 |
| PI Code a | Film Quality | T5 (°C) b | T10 (°C) c | Char Yield (%) d |
|---|---|---|---|---|
| PI-1 | Brittle | 505 | 528 | 62.4 |
| PI-2 | Brittle | 446 | 473 | 28.2 |
| PI-3 | Flexible | 438 | 454 | 16.7 |
| PI-4 | Flexible | 428 | 443 | 0.7 |
| PI-5 | Flexible | 423 | 441 | 2.1 |
| PI-6 | Sticky | 403 | 426 | 4.3 |
| PI-7 | Sticky | 373 | 419 | 3.2 |
| PI/Al2O3 Composite Code a | T5 (°C) b | T10 (°C) c | Char Yield (%) d | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| PI-3 | 438 | 461 | 16.7 | 14.6 ± 2.7 | 210.4 ± 38.4 |
| PI-3-30 | 440 | 456 | 30.7 | 7.3 ± 0.2 | 41.7 ± 2.8 |
| PI-3-60 | 454 | 469 | 61.3 | 6.5 ± 0.4 | 10.4 ± 0.6 |
| PI-3-75 | 450 | 474 | 75.2 | 5.7 ± 0.5 | 4.8 ± 0.6 |
| PI-3-80 | 437 | 471 | 79.1 | 5.2 ± 0.5 | 3.7 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-Y.; Nam, K.-N.; Jin, S.-W.; Kim, D.-M.; Song, I.-H.; Park, H.-J.; Park, S.; Chung, C.-M. Preparation and Properties of Poly(imide-siloxane) Copolymer Composite Films with Micro-Al2O3 Particles. Appl. Sci. 2019, 9, 548. https://doi.org/10.3390/app9030548
Choi J-Y, Nam K-N, Jin S-W, Kim D-M, Song I-H, Park H-J, Park S, Chung C-M. Preparation and Properties of Poly(imide-siloxane) Copolymer Composite Films with Micro-Al2O3 Particles. Applied Sciences. 2019; 9(3):548. https://doi.org/10.3390/app9030548
Chicago/Turabian StyleChoi, Ju-Young, Kyeong-Nam Nam, Seung-Won Jin, Dong-Min Kim, In-Ho Song, Hyeong-Joo Park, Sungjin Park, and Chan-Moon Chung. 2019. "Preparation and Properties of Poly(imide-siloxane) Copolymer Composite Films with Micro-Al2O3 Particles" Applied Sciences 9, no. 3: 548. https://doi.org/10.3390/app9030548
APA StyleChoi, J.-Y., Nam, K.-N., Jin, S.-W., Kim, D.-M., Song, I.-H., Park, H.-J., Park, S., & Chung, C.-M. (2019). Preparation and Properties of Poly(imide-siloxane) Copolymer Composite Films with Micro-Al2O3 Particles. Applied Sciences, 9(3), 548. https://doi.org/10.3390/app9030548






