A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes
Abstract
Featured Application
Abstract
1. Introduction
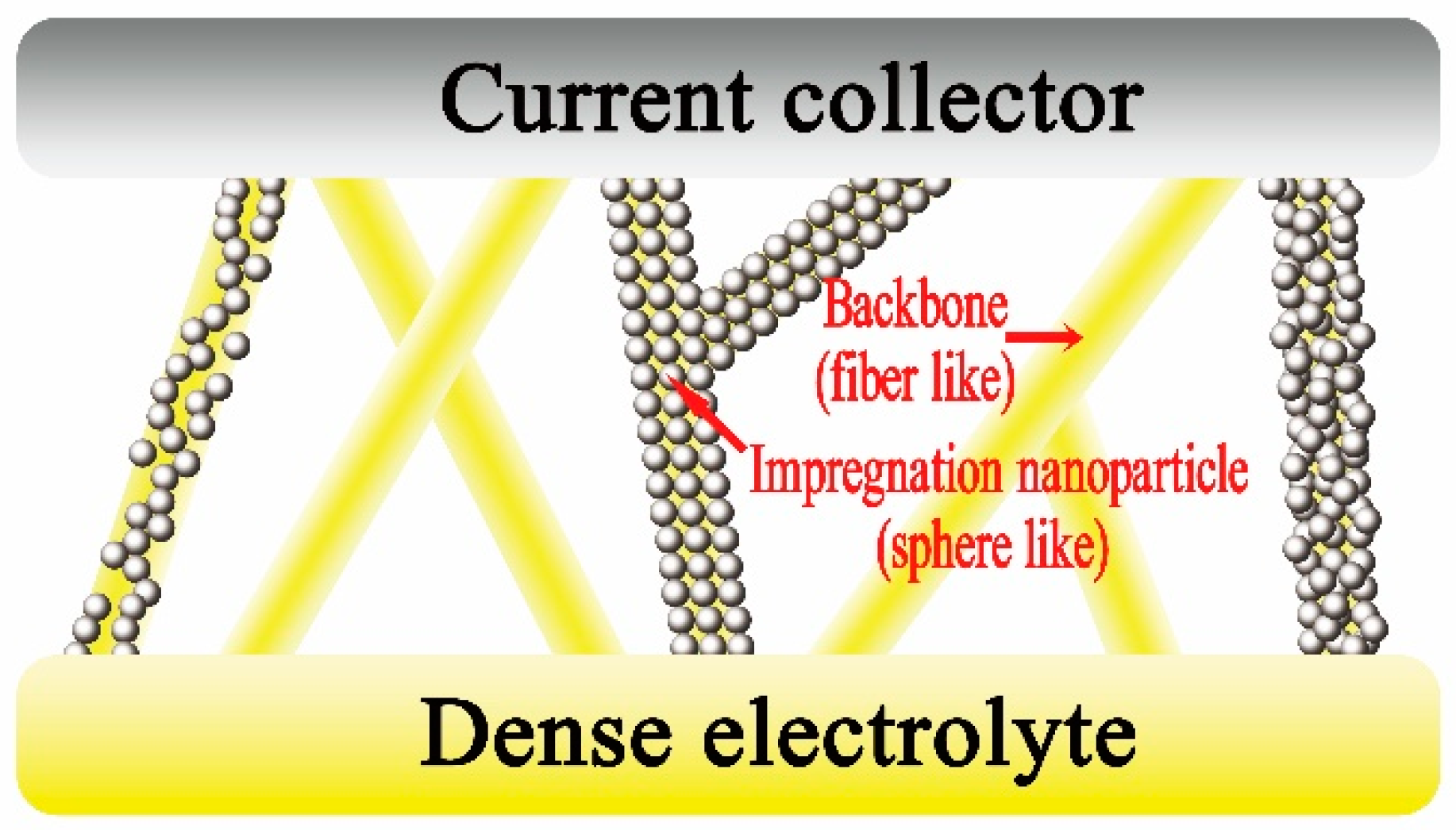
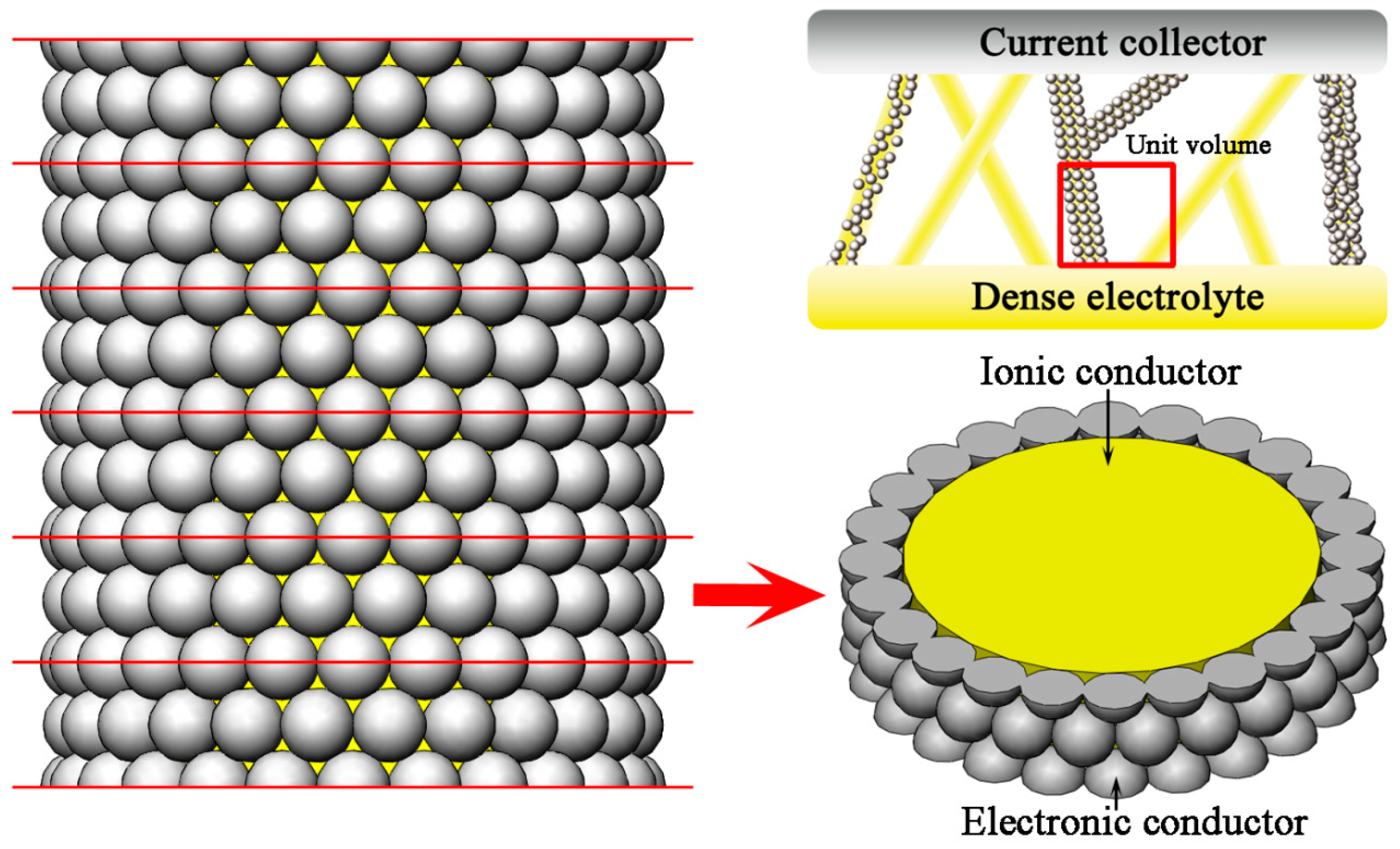
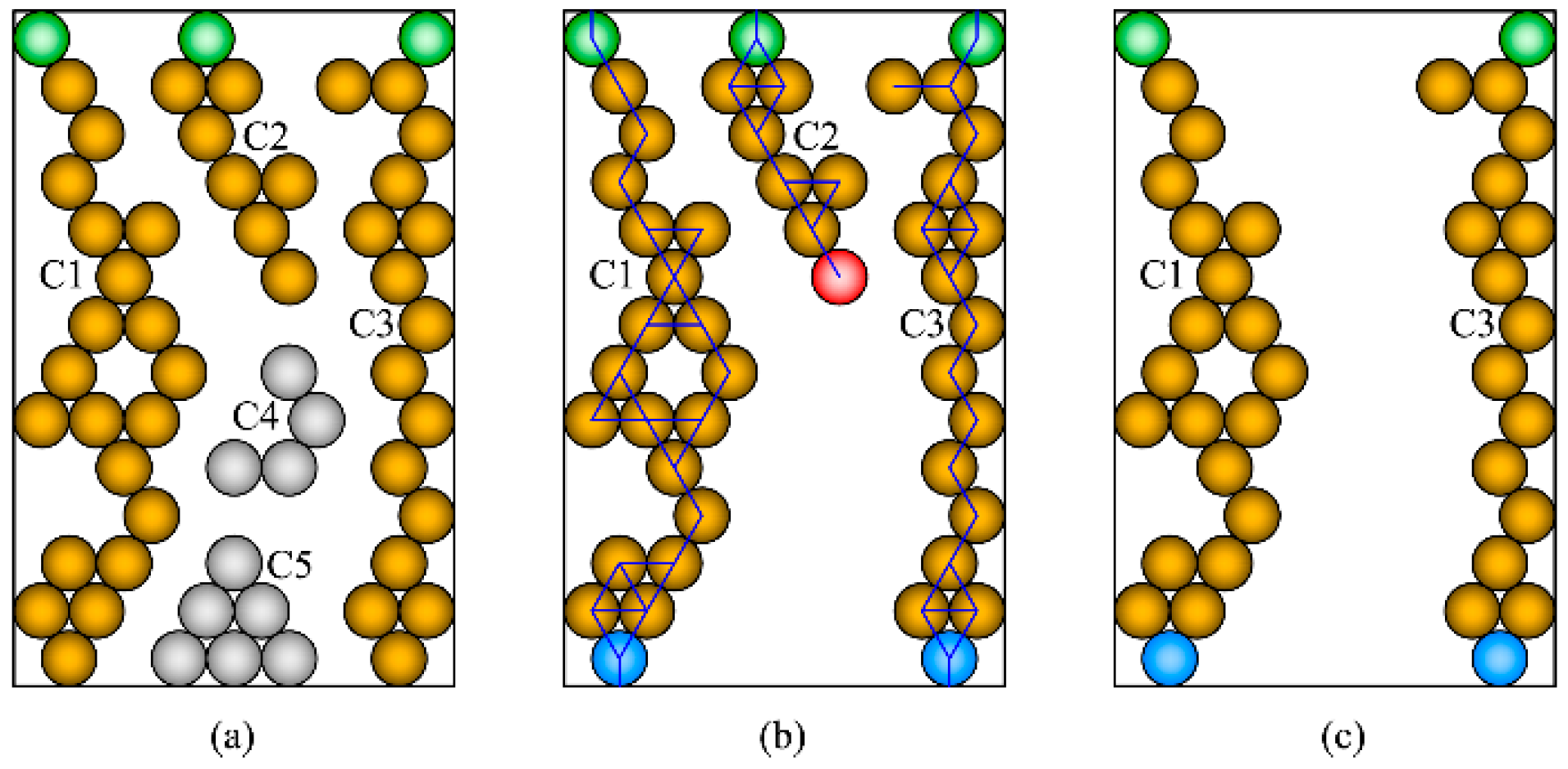
2. Theoretical Model
- All electrospun fibers have constant radii.
- All particles have constant radii.
- The particles are coated on the fiber face; the contact degree is 15°.
- The contact degree between particles is 15°.
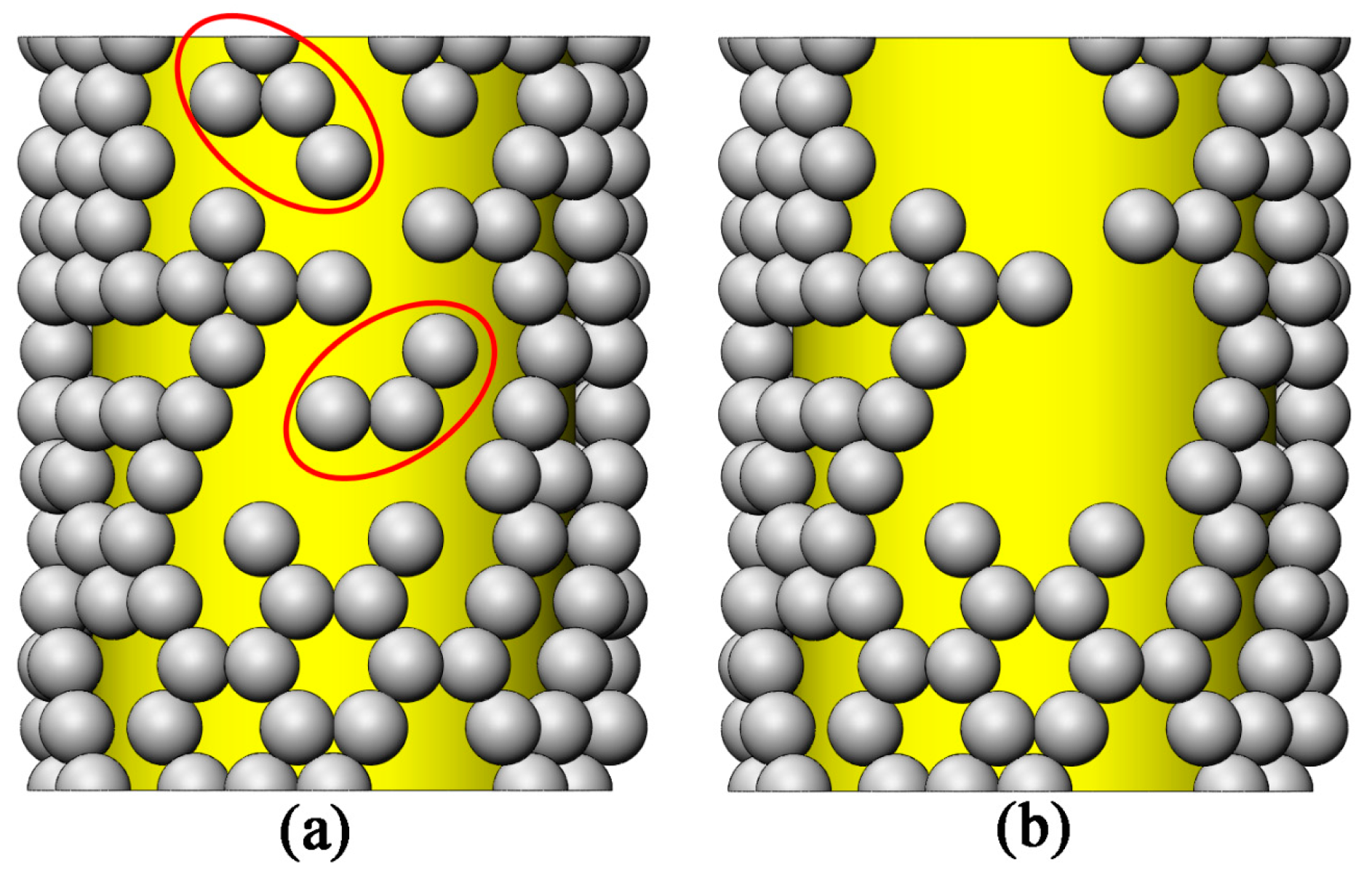
- If the outer shell is smaller than one layer, the particles will be randomly dispersed on the fiber face.
- The intersecting region between the fibers is neglected.
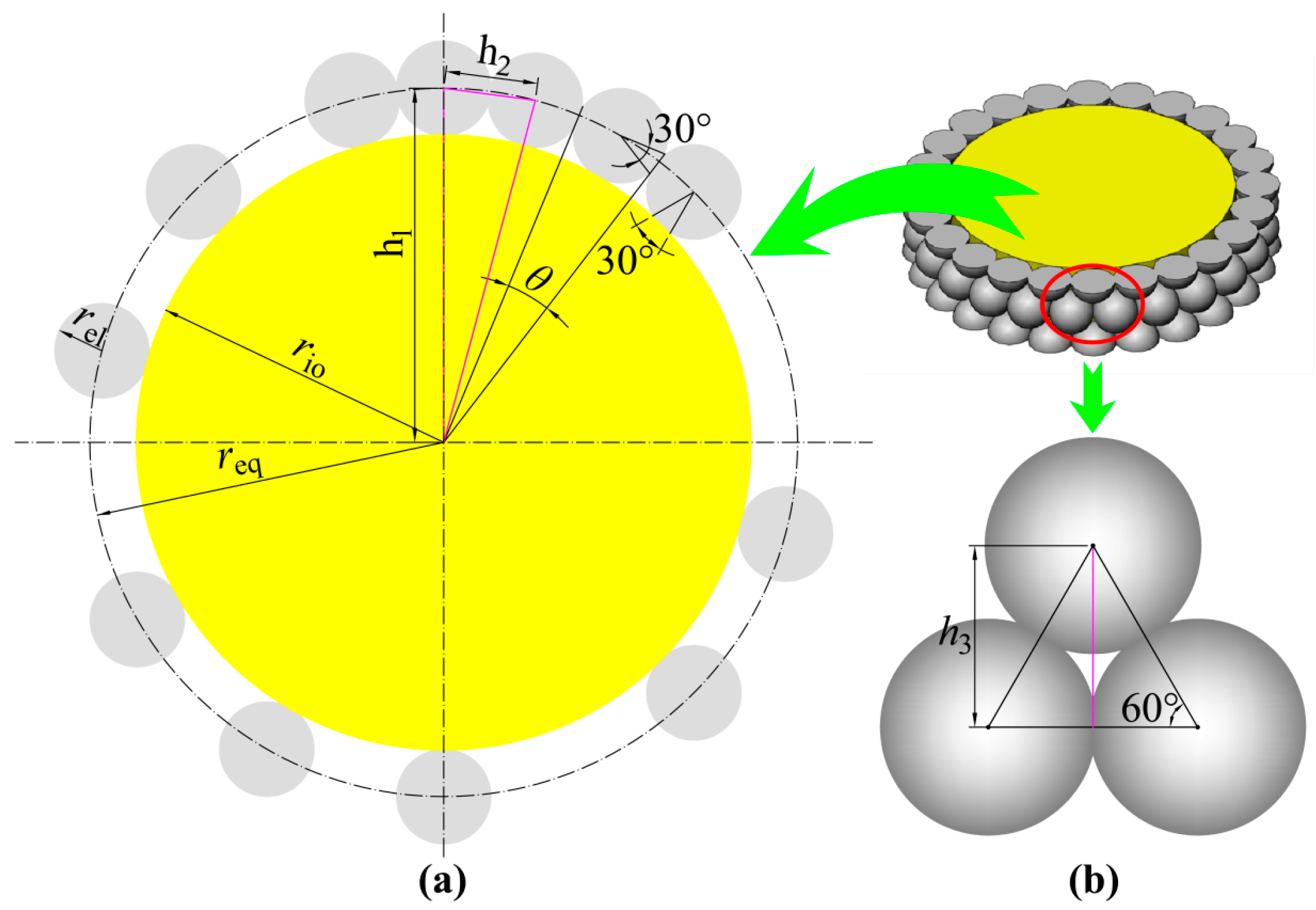
3. TPB Length Calculation
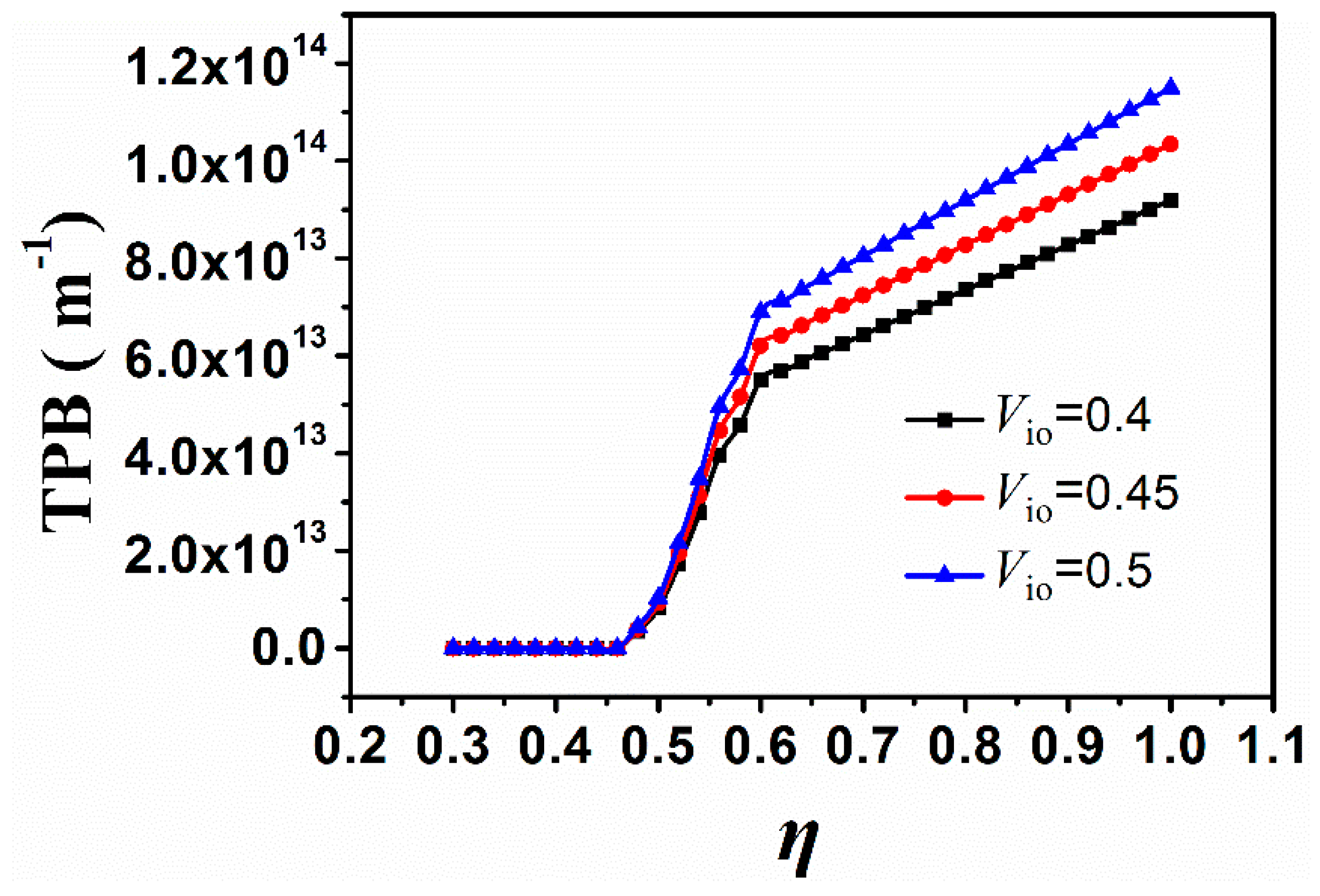
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Ullah, M.K.; Usman, A. A Brief Description of High Temperature Solid Oxide Fuel Cell’s Operation, Materials, Design, Fabrication Technologies and Performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Xia, Y.; Zou, J.; Yan, W.; Li, H. Adaptive Tracking Constrained Controller Design for Solid Oxide Fuel Cells Based on a Wiener-Type Neural Network. Appl. Sci. 2018, 8, 1758. [Google Scholar] [CrossRef]
- Chen, D.; Ding, K.; Chen, Z.; Wei, T.; Liu, K. Physics field distributions within fuel cell stacks with manifolds penetrating through the plane zone and open outlet features. Energy Convers. Manag. 2018, 178, 190–199. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, Q.; Xu, X.; Chen, D. A Simple Expression for the Tortuosity of Gas Transport Paths in Solid Oxide Fuel Cells’ Porous Electrodes. Energies 2015, 8, 13953–13959. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, J.; Kwon, O.; Lim, C.; Sengodan, S.; Shin, J.; Kim, G. Scandium Doping Effect on a Layered Perovskite Cathode for Low-Temperature Solid Oxide Fuel Cells (LT-SOFCs). Appl. Sci. 2018, 8, 2217. [Google Scholar] [CrossRef]
- Chen, D.; Hu, B.; Ding, K.; Yan, C.; Lu, L. The Geometry Effect of Cathode/Anode Areas Ratio on Electrochemical Performance of Button Fuel Cell Using Mixed Conducting Materials. Energies 2018, 11, 1875. [Google Scholar] [CrossRef]
- Lu, Y.; Gasper, P.; Pal, U.B.; Gopalan, S.; Basu, S.N. Improving intermediate temperature performance of Ni-YSZ cermet anodes for solid oxide fuel cells by liquid infiltration of nickel nanoparticles. J. Power Sources 2018, 396, 257–264. [Google Scholar] [CrossRef]
- Epting, W.K.; Mansley, Z.; Menasche, D.B.; Kenesei, P.; Suter, R.M.; Gerdes, K.; Litster, S.; Salvador, P.A. Quantifying intermediate-frequency heterogeneities of SOFC electrodes using X-ray computed tomography. J. Am. Ceram. Soc. 2017, 100, 2232–2242. [Google Scholar] [CrossRef]
- Abdullah, T.; Liu, L. Simulation-based microstructural optimization of solid oxide fuel cell for low temperature operation. Int. J. Hydrogen Energy 2016, 41, 13632–13643. [Google Scholar] [CrossRef]
- Prakash, B.S.; Kumar, S.S.; Aruna, S. Microstructure and performance of LSM/YSZ based solid oxide fuel cell cathodes fabricated from solution combustion co-synthesized powders and by solution precursor plasma spraying. Surf. Coat. Technol. 2017, 310, 25–32. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-printing inks for the fabrication of solid oxide fuel cell films: A review. Renew. Sustain. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Han, D.; Liu, J.; He, Y.-B.; Wang, H.-F.; Zhang, X.-Z.; Wang, S.-R. Fabrication of a quasi-symmetrical solid oxide fuel cell using a modified tape casting/screen-printing/infiltrating combined technique. Int. J. Hydrogen Energy 2018, 43, 960–967. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Ge, L.; Zheng, Y.; Chen, H.; Guo, L. YSZ electrolyte support with novel symmetric structure by phase inversion process for solid oxide fuel cells. Energy Convers. Manag. 2018, 177, 11–18. [Google Scholar] [CrossRef]
- Mousavi, A.; Niazmand, M.; Alizadeh, M. Preparation of aliened porous Ni-GDC nano composite by freeze-casting process. Adv. Ceram. Prog. 2017, 3, 10–15. [Google Scholar]
- Dell’Agli, G.; Spiridigliozzi, L.; Marocco, A.; Accardo, G.; Frattini, D.; Kwon, Y.; Yoon, S. Morphological and crystalline evolution of Sm-(20 mol%)–doped ceria nanopowders prepared by a combined co-precipitation/hydrothermal synthesis for solid oxide fuel cell applications. Ceram. Int. 2017, 43, 12799–12808. [Google Scholar] [CrossRef]
- Chen, J.; Wan, D.; Sun, X.; Li, B.; Lu, M. Electrochemical impedance spectroscopic characterization of impregnated La0.6Sr0.4Co0.2Fe0.8O3−δ cathode for intermediate-temperature SOFCs. Int. J. Hydrogen Energy 2018, 43, 9770–9776. [Google Scholar] [CrossRef]
- Ni, C.; Cassidy, M.; Irvine, J.T. Image analysis of the porous yttria-stabilized zirconia (YSZ) structure for a lanthanum ferrite-impregnated solid oxide fuel cell (SOFC) electrode. J. Eur. Ceram. Soc. 2018, 38, 5463–5470. [Google Scholar] [CrossRef]
- Aruna, S.; Balaji, L.; Kumar, S.S.; Prakash, B.S. Electrospinning in solid oxide fuel cells—A review. Renew. Sustain. Energy Rev. 2017, 67, 673–682. [Google Scholar] [CrossRef]
- Koo, J.Y.; Lim, Y.; Kim, Y.B.; Byun, D.; Lee, W. Electrospun yttria-stabilized zirconia nanofibers for low-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 15903–15907. [Google Scholar] [CrossRef]
- Barnett, S.; Wang, H.; Liu, Z.; Kennouche, D.; Yakal-Kremski, K. Imaging of Fuel Cell and Battery Electrodes Using Focused Ion Beam Scanning Electron Microscopy. Microsc. Microanal. 2016, 22, 1310–1311. [Google Scholar] [CrossRef]
- Terao, T.; Inoue, G.; Kawase, M.; Kubo, N.; Yamaguchi, M.; Yokoyama, K.; Tokunaga, T.; Shinohara, K.; Hara, Y.; Hara, T. Development of novel three-dimensional reconstruction method for porous media for polymer electrolyte fuel cells using focused ion beam-scanning electron microscope tomography. J. Power Sources 2017, 347, 108–113. [Google Scholar] [CrossRef]
- Johnson, T.; Bailey, J.; Iacoviello, F.; Welsh, J.; Levison, P.; Shearing, P.; Bracewell, D. Three dimensional characterisation of chromatography bead internal structure using X-ray computed tomography and focused ion beam microscopy. J. Chromatogr. A 2018, 1566, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bertei, A.; Nicolella, C. Percolation theory in SOFC composite electrodes: Effects of porosity and particle size distribution on effective properties. J. Power Sources 2011, 196, 9429–9436. [Google Scholar] [CrossRef]
- Sanyal, J.; Goldin, G.M.; Zhu, H.; Kee, R.J. A particle-based model for predicting the effective conductivities of composite electrodes. J. Power Sources 2010, 195, 6671–6679. [Google Scholar] [CrossRef]
- Zheng, K.; Ni, M. Reconstruction of solid oxide fuel cell electrode microstructure and analysis of its effective conductivity. Sci. Bull. 2016, 61, 78–85. [Google Scholar] [CrossRef]
- Chen, M.; Song, C.; Lin, Z. Property models and theoretical analysis of novel solid oxide fuel cell with triplet nano-composite electrode. Int. J. Hydrogen Energy 2014, 39, 13763–13769. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, W.; Zhang, S.; Zhang, Q.; Su, S. Residual stress analysis of a micro-tubular solid oxide fuel cell. Int. J. Hydrogen Energy 2016, 41, 16173–16180. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, W.; Huang, H.; Zhang, Y.; Wu, J.; Xu, Y. Analysis of micro-tubular SOFC stability under ambient and operating temperatures. J. Mater. Sci. Technol. 2018, 34, 1436–1440. [Google Scholar] [CrossRef]
- Shao, L.; Wang, P.; Zhang, Q.; Fan, L.; Zhang, N.; Sun, K. Nanostructured CuCo2O4 cathode for intermediate temperature solid oxide fuel cells via an impregnation technique. J. Power Sources 2017, 343, 268–274. [Google Scholar] [CrossRef]
- Vijay, P.; Tadé, M.O.; Shao, Z.; Ni, M. Modelling the triple phase boundary length in infiltrated SOFC electrodes. Int. J. Hydrogen Energy 2017, 42, 28836–28851. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Xia, C.; Ni, M. Geometric Properties of Nanostructured Solid Oxide Fuel Cell Electrodes. J. Electrochem. Soc. 2013, 160, F278–F289. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Jia, Z.; Zhao, L.; Xiong, Y.; Sun, C.; Brito, M.E. One dimensional La0.8Sr0.2Co0.2Fe0.8O3−δ/Ce0.8Gd0.2O1.9 nanocomposite cathodes for intermediate temperature solid oxide fuel cells. J. Power Sources 2012, 219, 133–139. [Google Scholar] [CrossRef]
- Li, L.; Zhang, P.; Liu, R.; Guo, S.M. Preparation of fibrous Ni-coated-YSZ anodes for solid oxide fuel cells. J. Power Sources 2011, 196, 1242–1247. [Google Scholar] [CrossRef]
- Sacanell, J.; Leyva, A.G.; Bellino, M.G.; Lamas, D.G. Nanotubes of rare earth cobalt oxides for cathodes of intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 1786–1792. [Google Scholar] [CrossRef]
- Zhao, E.; Ma, C.; Yang, W.; Xiong, Y.; Li, J.; Sun, C. Electrospinning La0.8Sr0.2Co0.2Fe0.8O3−δ tubes impregnated with Ce0.8Gd0.2O1.9 nanoparticles for an intermediate temperature solid oxide fuel cell cathode. Int. J. Hydrogen Energy 2013, 38, 6821–6829. [Google Scholar] [CrossRef]
- Lanzini, A.; Guerra, C.; Leone, P.; Santarelli, M.; Smeacetto, F.; Fiorilli, S.; Gondolini, A.; Mercadelli, E.; Sanson, A.; Brandon, N. Influence of the microstructure on the catalytic properties of SOFC anodes under dry reforming of methane. Mater. Lett. 2016, 164, 312–315. [Google Scholar] [CrossRef]
- Enrico, A.; Costamagna, P. Model of infiltrated La1−xSrxCo1−yFeyO3−δ cathodes for intermediate temperature solid oxide fuel cells. J. Power Sources 2014, 272, 1106–1121. [Google Scholar] [CrossRef]
- Chen, D.; He, H.; Zhang, D.; Wang, H.; Ni, M. Percolation Theory in Solid Oxide Fuel Cell Composite Electrodes with a Mixed Electronic and Ionic Conductor. Energies 2013, 6, 1632–1656. [Google Scholar] [CrossRef]
- Kong, W.; Gao, X.; Liu, S.; Su, S.; Chen, D. Optimization of the interconnect ribs for a cathode-supported solid oxide fuel cell. Energies 2014, 7, 295–313. [Google Scholar] [CrossRef]
- Su, S.; Zhang, Q.; Gao, X.; Periasamy, V.; Kong, W. Effects of changes in solid oxide fuel cell electrode thickness on ohmic and concentration polarizations. Int. J. Hydrogen Energy 2016, 41, 16181–16190. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Zhang, M.; Han, Z.; Zhang, Q. A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes. Appl. Sci. 2019, 9, 493. https://doi.org/10.3390/app9030493
Kong W, Zhang M, Han Z, Zhang Q. A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes. Applied Sciences. 2019; 9(3):493. https://doi.org/10.3390/app9030493
Chicago/Turabian StyleKong, Wei, Mengtong Zhang, Zhen Han, and Qiang Zhang. 2019. "A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes" Applied Sciences 9, no. 3: 493. https://doi.org/10.3390/app9030493
APA StyleKong, W., Zhang, M., Han, Z., & Zhang, Q. (2019). A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes. Applied Sciences, 9(3), 493. https://doi.org/10.3390/app9030493



