Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
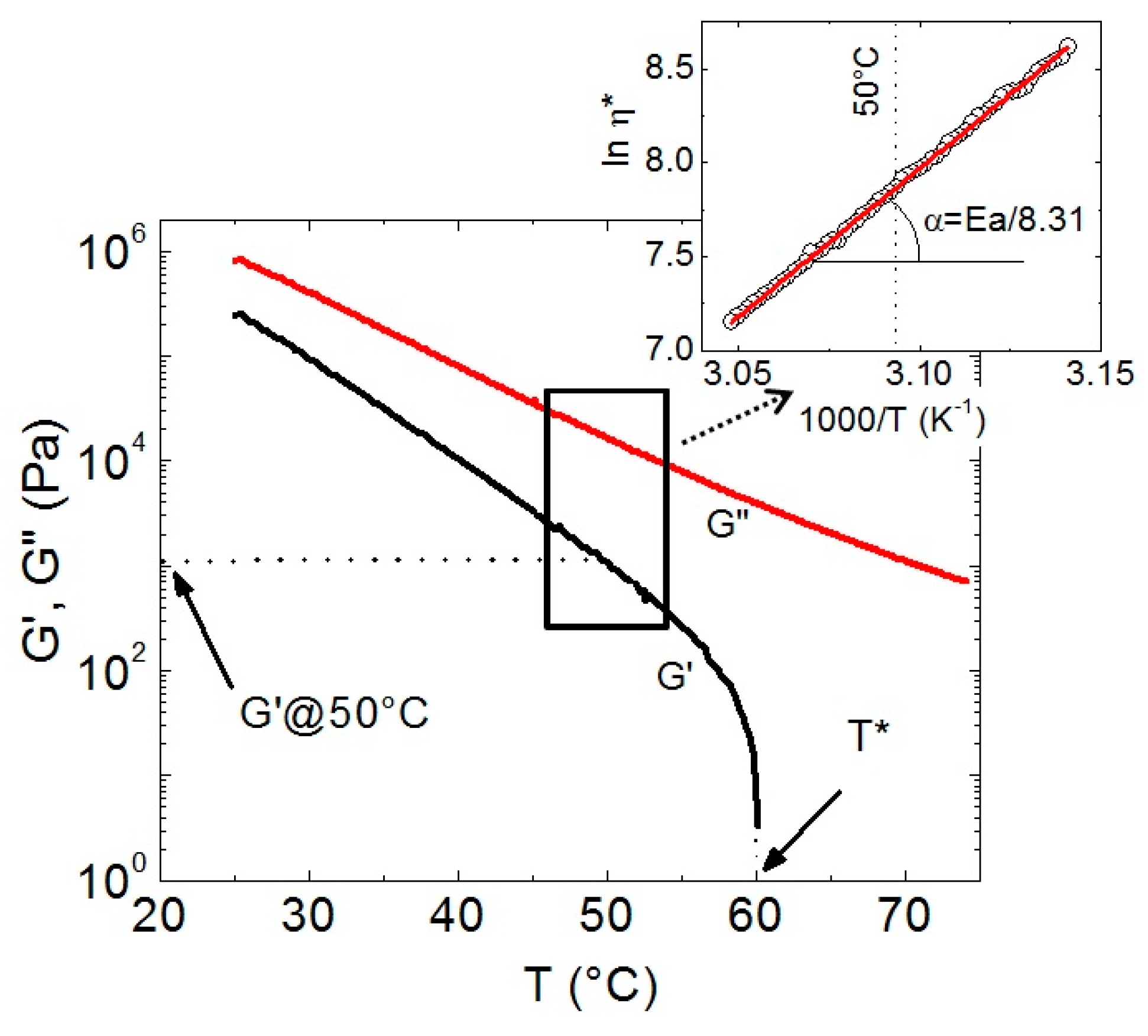
2.3. Rheological Tests
3. Results and Discussion
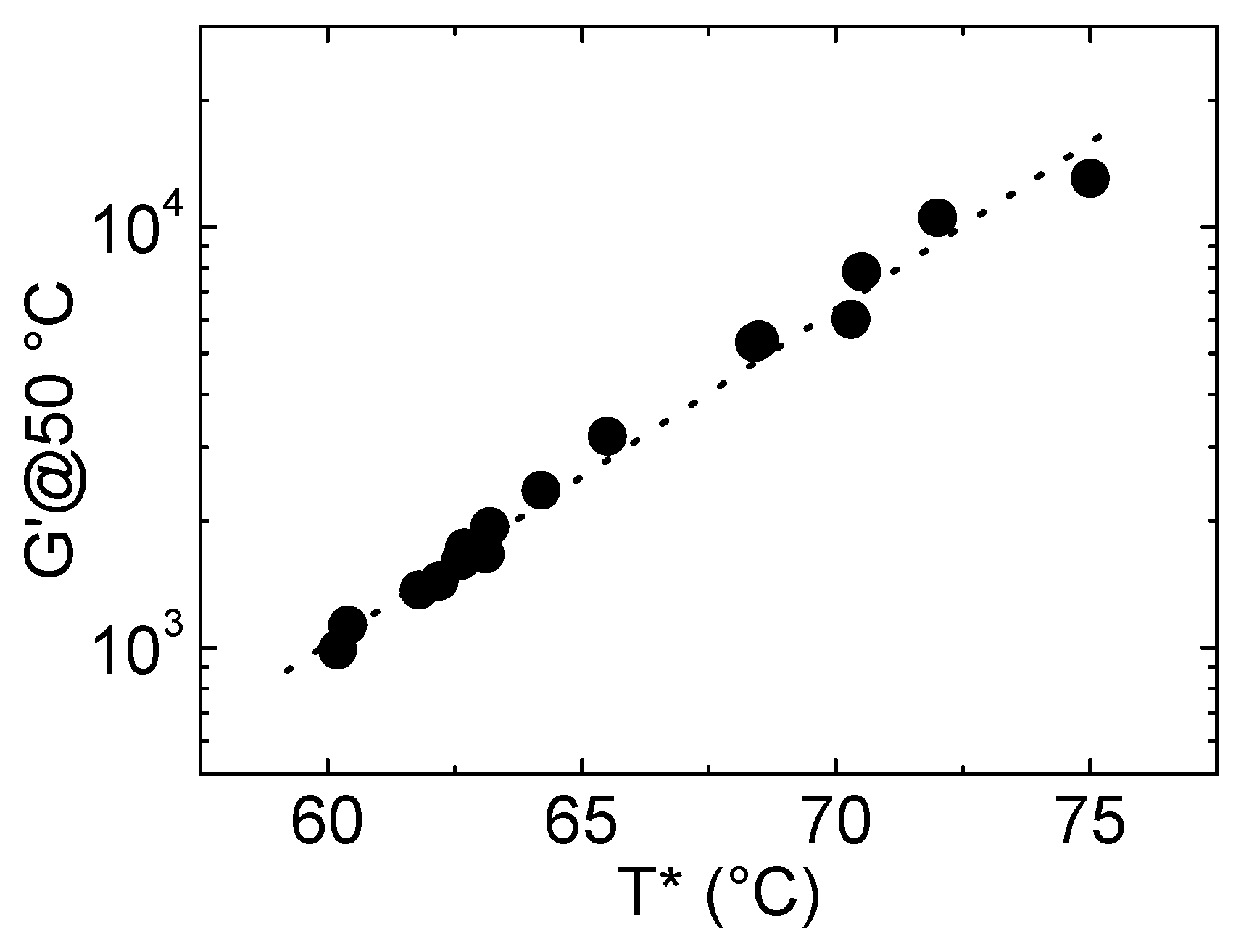
3.1. G′@50 °C and T*
3.2. Viscosity (η) as a Function of Temperature (T)—Arrhenius Model
- this behavior is observed for all the investigated samples;
- the same linear trend is observed also in a wider interval including lower temperatures;
- the effect of network breakdown at higher temperatures (G′ drop at T = T*), which represents however a strong structural modification, does not exert its effects in the temperature range considered, preserving the linear trend in the Arrhenius plot.
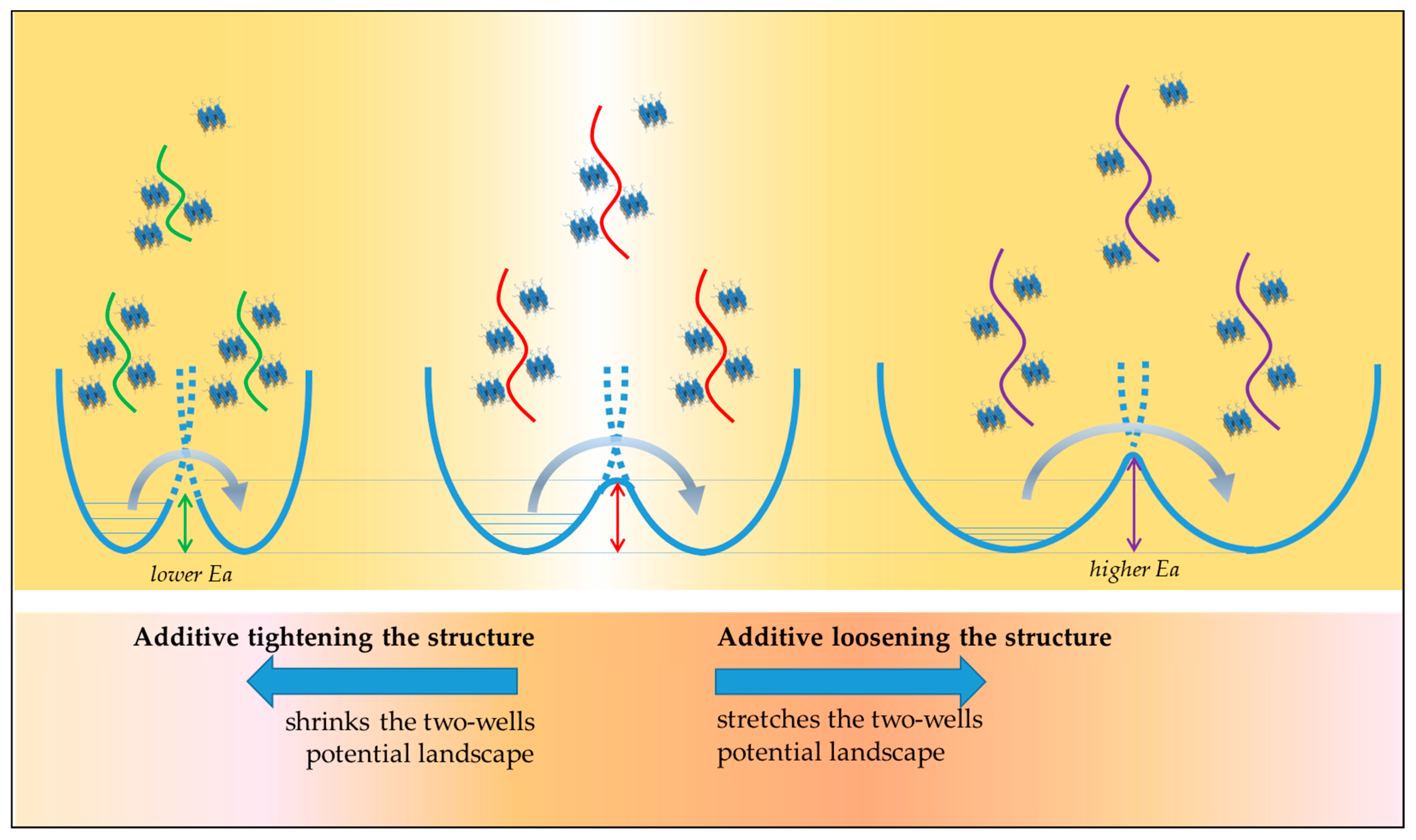
3.3. Correlation between Activation Energy (Ea) and Pre-Exponential Factor (As)
- viscosity (the viscosity of our samples is several orders of magnitudes higher);
- activation energies (Ea of our samples is from 2 to 20 times higher);
- pre-exponential factor (our samples have pre-exponential factors several orders of magnitude lower);
- structure (bitumens are highly complex materials—see introduction - contrasting the structure of simple liquids).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rozeveld, S.; Shin, E.; Bhurke, A.; France, L.; Drzal, L. Network morphology of straight and polymer modified asphalt cements. Microsc. Res. Tech. 1997, 38, 529–543. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid. Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Caputo, P.; De Santo, M.P.; Todaro, L.; Turco Liveri, V.; Oliviero Rossi, C. Effect of additives on the structural organization of asphaltene aggregates in bitumen. Constr. Build. Mater. 2019, 199, 288–297. [Google Scholar] [CrossRef]
- Jager, A.; Lackner, R.; Eisenmenger-Sittner, C.; Blab, R. Identification of Microstructural Components of Bitumen by Means of Atomic Force Microscopy (AFM). Proc. Appl. Math. Mech. 2004, 4, 400–401. [Google Scholar] [CrossRef]
- Oliviero Rossi, C.; Ashimova, S.; Calandra, P.; De Santo, M.P.; Angelico, R. Mechanical Resilience of Modified Bitumen at Different Cooling Rates: A Rheological and Atomic Force Microscopy Investigation. Appl. Sci. 2017, 7, 779. [Google Scholar] [CrossRef]
- Calandra, P.; Longo, A.; Ruggirello, A.; Turco Liveri, V. Physico-Chemical Investigation of the State of Cyanamide Confined in AOT and Lecithin Reversed Micelles. J. Phys. Chem. B 2004, 108, 8260–8268. [Google Scholar] [CrossRef]
- Calandra, P.; Giordano, C.; Ruggirello, A.; Turco Liveri, V. Physicochemical investigation of acrylamide solubilization in sodium bis(2-ethylhexyl)sulfosuccinate and lecithin reversed micelles. J. Coll. Interf. Sci. 2004, 277, 206–214. [Google Scholar] [CrossRef]
- Calandra, P.; Ruggirello, A.; Pistone, A.; Turco Liveri, V. Structural and Optical Properties of Novel Surfactant Coated TiO2-Ag Based Nanoparticles. J. Clust. Sci. 2010, 21, 767–778. [Google Scholar] [CrossRef]
- Calandra, P.; Di Marco, G.; Ruggirello, A.; Turco Liveri, V. Physico-chemical investigation of nanostructures in liquid phases: Nickel chloride ionic clusters confined in sodium bis(2-ethylhexyl) sulfosuccinate reverse micelles. J. Colloid Interface Sci. 2009, 336, 176–182. [Google Scholar] [CrossRef]
- Gentile, L.; Filippelli, L.; Oliviero Rossi, C.; Baldino, N.; Ranieri, G.A. Rheological and H-NMR spin–spin relaxation time for the evaluation of the effects of PPA addition on bitumen. Mol. Cryst. Liq. Cryst. 2012, 558, 54–63. [Google Scholar] [CrossRef]
- Radenberg, M.; Boetcher, S.; Sedaghat, N. Effect and efficiency of rejuvenators on aged asphalt binder—German experiences. In Proceedings of the 6th Eurasphalt & Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016. [Google Scholar]
- Hurley, G.C.; Prowell, B.D. Evaluation of potential processes for use in warm mix asphalt. J. Assoc. Asph. Paving Technol. 2006, 75, 41–90. [Google Scholar]
- Silva, H.M.R.D.; Oliveira, J.R.M.; Ferreira, C.I.G.; Pereira, P.A.A. Assessment of the performance of warm mix asphalts in road pavements. Int. J. Pavement Res. Technol. 2010, 3, 119–127. [Google Scholar]
- Hakseo, K.; Soon-Jae, L. Rheology of warm mix asphalt binders with aged binders. Constr. Build. Mat. 2011, 25, 183–189. [Google Scholar]
- Jamshidia, A.; Hamzaha, M.O. Performance of warm mix asphalt containing Sasobit®: State-of-the-art. Constr. Build. Mater. 2013, 38, 530–553. [Google Scholar] [CrossRef]
- Yousefi, A.A. Rubber-Modified Bitumens. Iran. Polym. J. 2002, 11, 303–309. [Google Scholar]
- Król, J.B.; Kowalski, K.J.; Niczke, L.; Radziszewski, P. Effect of bitumen fluxing using a bio-origin additive. Constr. Build. Mat. 2016, 114, 194–203. [Google Scholar] [CrossRef]
- Somé, C.; Pavoine, A.; Chailleux, E.; Andrieux, L.; DeMArco, L.; Philippe, D.-S.; Stephan, B. Rheological behaviour of vegetable oil-modified asphaltite binders and mixes. In Proceedings of the 6th Eurasphalt & Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016; p. 222. [Google Scholar]
- Caputo, P.; Porto, M.; Calandra, P.; De Santo, M.P.; Oliviero Rossi, C. Effect of epoxidized soybean oil on mechanical properties of bitumen and aged bitumen. Mol. Cryst. Liq. Cryst. 2018, 675, 68–74. [Google Scholar] [CrossRef]
- Edwards, Y.; Tasdemir, Y.; Isacssonc, U. Rheological effects of commercial waxes and polyphosphoric acid in bitumen 160/220—Low temperature performance. Fuel 2006, 85, 989–997. [Google Scholar] [CrossRef]
- Read, J.; Whiteoak, D. The Shell Bitumen Handbook, 5th ed.; Hunter, R.N., Ed.; Thomas Telford Publishing: London, UK, 2003. [Google Scholar]
- Yoon, S.; Durgashanker Bhatt, S.; Lee, W.; Lee, H.Y.; Jeong, S.Y.; Baeg, J.-O.; Wee Lee, C. Separation and characterization of bitumen from Athabasca oil sand. Korean J. Chem. Eng. 2009, 26, 64–71. [Google Scholar] [CrossRef]
- Sheu, E.Y.; Storm, D.A. Colloidal properties of asphaltenes in organic solvents. In Asphaltenes: Fundamentals and Applications; Chap.1; Sheu, E.Y., Mullins, O.C., Eds.; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Shaffie, E.; Arshad, A.K.; Alisibramulisi, A.; Ahmad, J.; Hashim, W.; Abd Rahman, Z.; Jaya, R.P. Effect of mixing variables on physical properties of modified bitumen using natural rubber latex. Int. J. Civ. Eng. Technol. 2018, 9, 1812–1821. [Google Scholar]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley Publishing: Hoboken, NJ, USA, 1994; ISBN 978-0-471-18575-8. [Google Scholar]
- Haj-Kacem, R.B.; Ouerfelli, N.; Herráez, J.; Guettari, M.; Hamda, H.; Dallel, M. Contribution to modeling the viscosity Arrhenius-type equation for some solvents by statistical correlations analysis. Fluid Phase Equilib. 2014, 383, 11–20. [Google Scholar] [CrossRef]
- Kirkwood, J.G.; Buff, F.P.; Green, M.S. The statistical mechanical theory of transport processes. III. The coefficients of shear and bulk viscosity of liquids. J. Chem. Phys. 1949, 17, 988–994. [Google Scholar] [CrossRef]
- Eyring, H. Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J. Chem. Phys. 1936, 4, 283–291. [Google Scholar] [CrossRef]
- Eyring, H.; John, M.S. Significant Liquid Structure; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Cummings, P.T.; Evans, D.J. Nonequilibrium molecular dynamics approaches to transport properties and non-Newtonian fluid rheology. Ind. Eng. Chem. Res. 1992, 31, 1237–1252. [Google Scholar] [CrossRef]
- Messaâdi, A.; Dhouibi, N.; Hamda, H.; Belgacem, F.B.M.; Adbelkader, Y.H.; Ouerfelli, N.; Hamzaoui, A.H. A New Equation Relating the Viscosity Arrhenius Temperature and the Activation Energy for Some Newtonian Classical Solvents. J. Chem. 2015, 2015, 163262. [Google Scholar] [CrossRef]
- Carvalho-Silva, V.H.; Coutinho, N.D.; Aquilanti, V. Temperature Dependence of Rate Processes Beyond Arrhenius and Eyring: Activation and Transitivity. Front. Chem. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Eyring, H. The activated complex in chemical reactions. J. Chem Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Tyrrell, H.J.V.; Harris, K.R. Diffusion in Liquids; Butterworths: London, UK, 1984. [Google Scholar]
- Byran, J.; Kantzas, A.; Bellehumer, C. Oil-viscosity predictions from low-field NMR measurements. SPE Reserv. Eval. Eng. 2005, 8, 44–52. [Google Scholar] [CrossRef]
- Rabbani, A.; Schmitt, D.R. Ultrasonic shear wave reflectometry applied to the determination of the shear moduli and viscosity of a viscoelastic bitumen. Fuel 2018, 232, 506–518. [Google Scholar] [CrossRef]


| Additive | G′@50 °C (kPa) | T* (°C) | Ea (kJ/mol) |
|---|---|---|---|
| ref | 1.0 | 60.2 | 132 |
| Algae euchemae | 13.0 | 75.0 | 130 |
| carobs | 10.5 | 72.0 | 139 |
| orange skin | 5.4 | 68.5 | 123 |
| flour 00 | 1.4 | 61.8 | 132 |
| agar | 1.9 | 63.2 | 132 |
| carragenine | 6.0 | 70.3 | 127 |
| carboxymethylcellulose | 7.8 | 70.5 | 140 |
| pectin | 1.7 | 62.7 | 139 |
| arabic gum | 1.4 | 62.2 | 131 |
| cellulose | 1.7 | 63.1 | 133 |
| Corn flour | 1.1 | 60.4 | 134 |
| starch | 1.6 | 62.6 | 131 |
| guar gum | 5.3 | 68.4 | 135 |
| xantam gum | 2.4 | 64.2 | 138 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, M.; Caputo, P.; Loise, V.; De Filpo, G.; Oliviero Rossi, C.; Calandra, P. Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior. Appl. Sci. 2019, 9, 5564. https://doi.org/10.3390/app9245564
Porto M, Caputo P, Loise V, De Filpo G, Oliviero Rossi C, Calandra P. Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior. Applied Sciences. 2019; 9(24):5564. https://doi.org/10.3390/app9245564
Chicago/Turabian StylePorto, Michele, Paolino Caputo, Valeria Loise, Giovanni De Filpo, Cesare Oliviero Rossi, and Pietro Calandra. 2019. "Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior" Applied Sciences 9, no. 24: 5564. https://doi.org/10.3390/app9245564
APA StylePorto, M., Caputo, P., Loise, V., De Filpo, G., Oliviero Rossi, C., & Calandra, P. (2019). Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior. Applied Sciences, 9(24), 5564. https://doi.org/10.3390/app9245564








