The Origins of the High Performance of Pd Catalysts Supported on Carbon Black-Embedded Carbon Nanofiber for Formic Acid Oxidation
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Effect of Heat Treatment of Carbon Black Supports on the Properties of Pd/C Catalysts
3.2. Effect of Heat Treatment of Carbon Blacks on Formic Acid Oxidation Reaction Activity
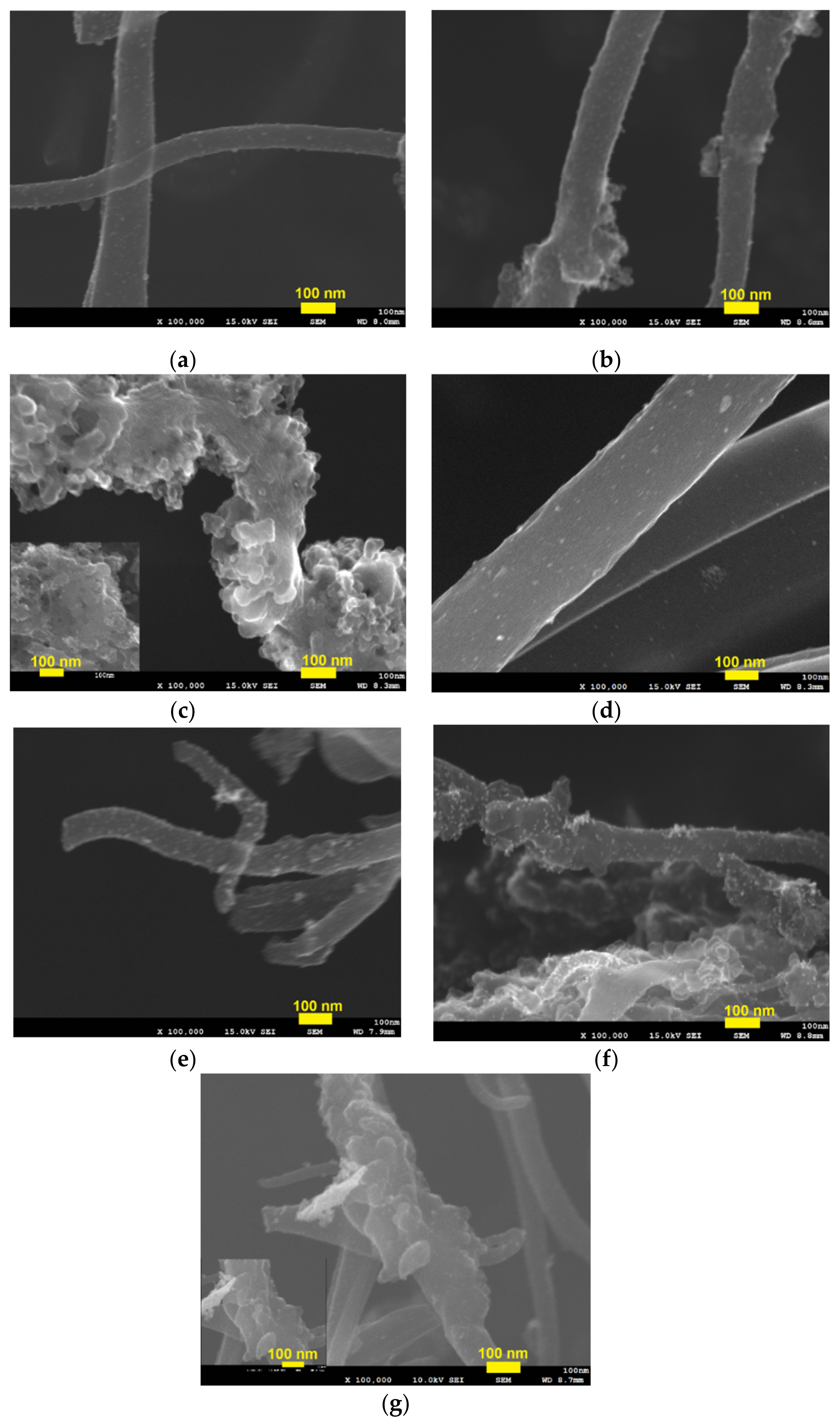
3.3. Characterization of Pd/CB Embedded in Carbon Nanofibers with Different Carbon Black Contents
3.4. Formic Acid Oxidation Reaction on Carbon Black Embedded in Carbon Nanofibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rees, N.V.; Compton, R.G. Sustainable energy: A review of formic acid electrochemical fuel cells. J. Solid State Electrochem. 2011, 15, 2095–2100. [Google Scholar] [CrossRef]
- Hong, P.; Liao, S.; Zeng, J.; Huang, X. Design, fabrication and performance evaluation of a miniature air breathing direct formic acid fuel cell based on printed circuit board technology. J. Power Sources 2010, 195, 7332–7337. [Google Scholar] [CrossRef]
- Aslam, N.M.; Masdar, M.S.; Kamarudin, S.K.; Daud, W.R.W. Overview on Direct Formic Acid Fuel Cells (DFAFCs) as an Energy Sources. APCBEE Procedia 2012, 3, 33–39. [Google Scholar] [CrossRef]
- Hong, P.; Luo, F.; Liao, S.; Zeng, J. Effects of Pt/C, Pd/C and PdPt/C anode catalysts on the performance and stability of air breathing direct formic acid fuel cells. Int. J. Hydrogen Energy 2011, 36, 8518–8524. [Google Scholar] [CrossRef]
- Haan, J.L.; Masel, R.I. The influence of solution pH on rates of an electrocatalytic reaction: Formic acid electrooxidation on platinum and palladium. Electrochim. Acta 2009, 54, 4073–4078. [Google Scholar] [CrossRef]
- Jayashree, R.; Spendelow, J.; Yeom, J.; Rastogi, C.; Shannon, M.; Kenis, P. Characterization and application of electrodeposited Pt, Pt/Pd, and Pd catalyst structures for direct formic acid micro fuel cells. Electrochim. Acta 2005, 50, 4674–4682. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, X.; Liao, J.; Liu, C.; Lu, T.; Xing, W. Preparation of Pd / C catalyst for formic acid oxidation using a novel colloid method. Electrochem. Commun. 2008, 10, 621–624. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Sources 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Holade, Y.; Morais, C.; Servat, K.; Napporn, T.W.; Kokoh, K.B. Enhancing the available specific surface area of carbon supports to boost the electroactivity of nanostructured Pt catalysts. Phys. Chem. Chem. Phys. 2014, 16, 25609–25620. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Li, S.; Feng, L.; Qin, X.; Shao, G. Effect of carbon material on Pd catalyst for formic acid electrooxidation reaction. J. Power Sources 2014, 266, 481–487. [Google Scholar] [CrossRef]
- Onishi, R.; Tsujiguchi, T.; Osaka, Y.; Kodama, A. High Formic Acid Oxidation Activity and Stability of Pd Catalyst Supported by Nanoparticle-Embedded Carbon Nanofiber. ECS Trans. 2015, 69, 663–674. [Google Scholar] [CrossRef]
- Ito, Y.; Takeuchi, T.; Tsujiguchi, T.; Abdelkareem, M.A.; Nakagawa, N. Ultrahigh methanol electro-oxidation activity of PtRu nanoparticles prepared on TiO 2 -embedded carbon nano fi ber support. J. Power Sources 2013, 242, 280–288. [Google Scholar] [CrossRef]
- Kunitomo, H.; Ishitobi, H.; Nakagawa, N. Optimized CeO2 content of the carbon nanofiber support of PtRu catalyst for direct methanol fuel cells. J. Power Sources 2015, 297, 400–407. [Google Scholar] [CrossRef]
- Inoue, H.; Hosoya, K.; Kannari, N.; Ozaki, J. Influence of heat-treatment of Ketjen Black on the oxygen reduction reaction of Pt/C catalysts. J. Power Sources 2012, 220, 173–179. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R.R. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Hassan, A.; Carreras, A.; Trincavelli, J.; Ticianelli, E.A. Effect of heat treatment on the activity and stability of carbon supported PtMo alloy electrocatalysts for hydrogen oxidation in proton exchange membrane fuel cells. J. Power Sources 2014, 247, 712–720. [Google Scholar] [CrossRef]
- Valisi, A.N.; Maiyalagan, T.; Khotseng, L.; Linkov, V.; Pasupathi, S. Effects of Heat Treatment on the Catalytic Activity and Methanol Tolerance of Carbon-Supported Platinum Alloys. Electrocatalysis 2012, 3, 108–118. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Li, H.; Li, Y.; Ye, J.; Cao, Z.; Fang, M.; Kuang, Q.; Zheng, J.; Xie, Z. Stable palladium hydride as a superior anode electrocatalyst for direct formic acid fuel cells. Nano Energy 2018, 44, 127–134. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, C.-C. Enhancement of hydrogen spillover onto carbon nanotubes with defect feature. Microporous Mesoporous Mater. 2008, 109, 549–559. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Soboleva, T.; Zhao, X.; Malek, K.; Xie, Z.; Navessin, T.; Holdcroft, S. On the micro-, meso-, and macroporous structures of polymer electrolyte membrane fuel cell catalyst layers. ACS Appl. Mater. Interfaces 2010, 2, 375–384. [Google Scholar] [CrossRef]
- Liang, J.-Z. Effects of heat treatment on electrical conductivity of HDPE/CB composites. Polym. Test. 2017, 62, 219–224. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, J.; Liang, L.; Liu, C.; Liao, J.; Xing, W. Enhanced electroactivity of Pd nanocrystals supported on H3PMo12O40/carbon for formic acid electrooxidation. J. Power Sources 2012, 210, 392–396. [Google Scholar] [CrossRef]
- Park, Y.-C.; Tokiwa, H.; Kakinuma, K.; Watanabe, M.; Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 2016, 315, 179–191. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, Z.L.; Li, C.M. Formic acid-reduced ultrasmall Pd nanocrystals on graphene to provide superior electocatalytic activity and stability toward formic acid oxidation. Nano Energy 2015, 11, 71–77. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef]
- Hu, S.; Che, F.; Khorasani, B.; Jeon, M.; Yoon, C.W.; McEwen, J.-S.; Scudiero, L.; Ha, S. Improving the electrochemical oxidation of formic acid by tuning the electronic properties of Pd-based bimetallic nanoparticles. Appl. Catal. B Environ. 2019, 254, 685–692. [Google Scholar] [CrossRef]
- Nitze, F.; Mazurkiewicz, M.; Malolepszy, A.; Mikolajczuk, A.; Kdzierzawski, P.; Tai, C.W.; Hu, G.; Kurzydłowski, K.J.; Stobinski, L.; Borodzinski, A.; et al. Synthesis of palladium nanoparticles decorated helical carbon nanofiber as highly active anodic catalyst for direct formic acid fuel cells. Electrochim. Acta 2012, 63, 323–328. [Google Scholar] [CrossRef]
- Hu, G.; Nitze, F.; Barzegar, H.R.; Sharifi, T.; Mikolajczuk, A.; Tai, C.W.; Borodzinski, A.; Wgberg, T. Palladium nanocrystals supported on helical carbon nanofibers for highly efficient electro-oxidation of formic acid, methanol and ethanol in alkaline electrolytes. J. Power Sources 2012, 209, 236–242. [Google Scholar] [CrossRef]
- Qin, Y.-H.; Jia, Y.-B.; Jiang, Y.; Niu, D.-F.; Zhang, X.-S.; Zhou, X.-G.; Niu, L.; Yuan, W.-K. Controllable synthesis of carbon nanofiber supported Pd catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2012, 37, 7373–7377. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yang, H.H.; Zhang, X.S.; Zhou, X.G.; Niu, L.; Yuan, W.K. Synthesis of highly dispersed and active palladium/carbon nanofiber catalyst for formic acid electrooxidation. J. Power Sources 2011, 196, 4609–4612. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, J.M.; Zhang, P.Y.; Li, Y.; Xiang, S.; Tang, H.G.; Fan, Y.J. Highly active carbon nanotube-supported Ru@Pd core-shell nanostructure as an efficient electrocatalyst toward ethanol and formic acid oxidation. Mol. Catal. 2017, 436, 138–144. [Google Scholar] [CrossRef]
- Kibis, L.S.; Stadnichenko, A.I.; Koscheev, S.V.; Zaikovskii, V.I.; Boronin, A.I. Highly oxidized palladium nanoparticles comprising Pd 4+ species: Spectroscopic and structural aspects, thermal stability, and reactivity. J. Phys. Chem. C 2012, 116, 19342–19348. [Google Scholar] [CrossRef]
- Bueres, R.F.; Asedegbega-Nieto, E.; Díaz, E.; Ordóñez, S.; Díez, F.V. Preparation of carbon nanofibres supported palladium catalysts for hydrodechlorination reactions. Catal. Commun. 2008, 9, 2080–2084. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Wang, X.-Z.; Fu, R.; Yang, D.-J.; Li, P.; Lv, H.; Ma, J.-X. Microstructure effect of carbon nanofibers on Pt/CNFs electrocatalyst for oxygen reduction. Int. J. Hydrogen Energy 2012, 37, 4639–4647. [Google Scholar] [CrossRef]
- Zhou, W.; Lee, J.Y. Particle size effects in Pd-catalyzed electrooxidation of formic acid. J. Phys. Chem. C 2008, 112, 3789–3793. [Google Scholar] [CrossRef]
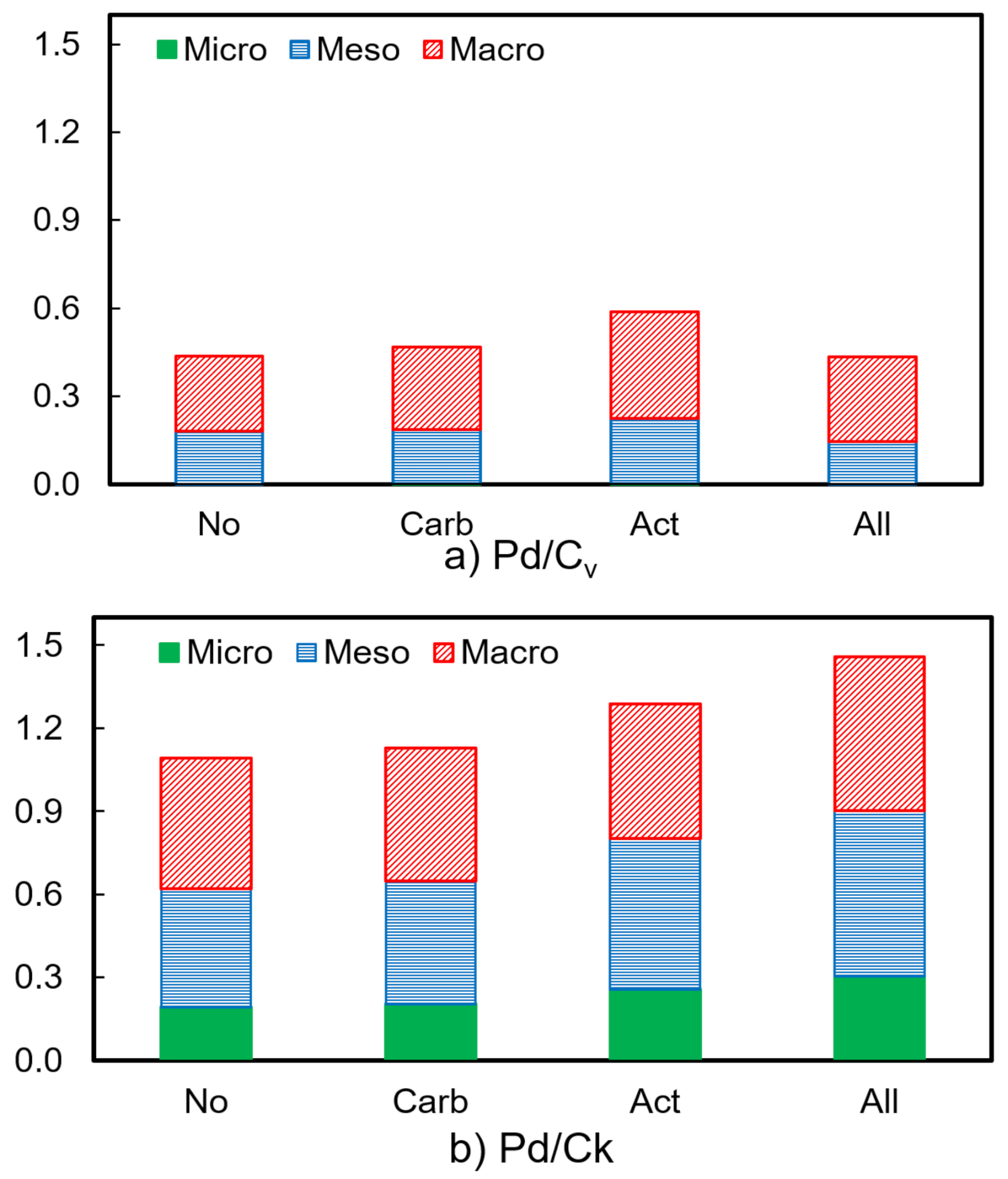
| Heat Treatment on Carbon Support | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pd Crystallite Size (nm) | |||
|---|---|---|---|---|---|---|
| Pd/Cv | Pd/Ck | Pd/Cv | Pd/Ck | Pd/Cv | Pd/Ck | |
| No | 85 | 581 | 0.44 | 1.09 | 6.6 | 3.9 |
| Carbonization (Carb) | 166 | 609 | 0.47 | 1.13 | 7.7 | 4.4 |
| Steam activation (Act) | 221 | 714 | 0.59 | 1.29 | 9.4 | 2.2 |
| Carb and act (All) | 256 | 840 | 0.53 | 1.46 | 4.6 | 4.5 |
| Catalyst | Mass Activity at Ep, ip-FAOR (mA mgPd−1) | Peak Potential, Ep (V vs. normal hydrogen electrode, NHE) | Electrochemical Active Surface Area (m2 gPd−1) |
|---|---|---|---|
| Pd Cv-carb | 1397 | 0.76 | 22.9 |
| Pd Cv-act | 1667 | 0.84 | 26.1 |
| Pd Cv-all | 810 | 0.66 | 11.1 |
| Pd Cv-no | 250 | 0.44 | 7.1 |
| Pd Ck-carb | 1083 | 0.70 | 10.3 |
| Pd Ck-act | 845 | 0.64 | 8.8 |
| Pd Ck-all | 653 | 0.50 | 6.7 |
| Pd Ck-no | 1212 | 0.64 | 17.0 |
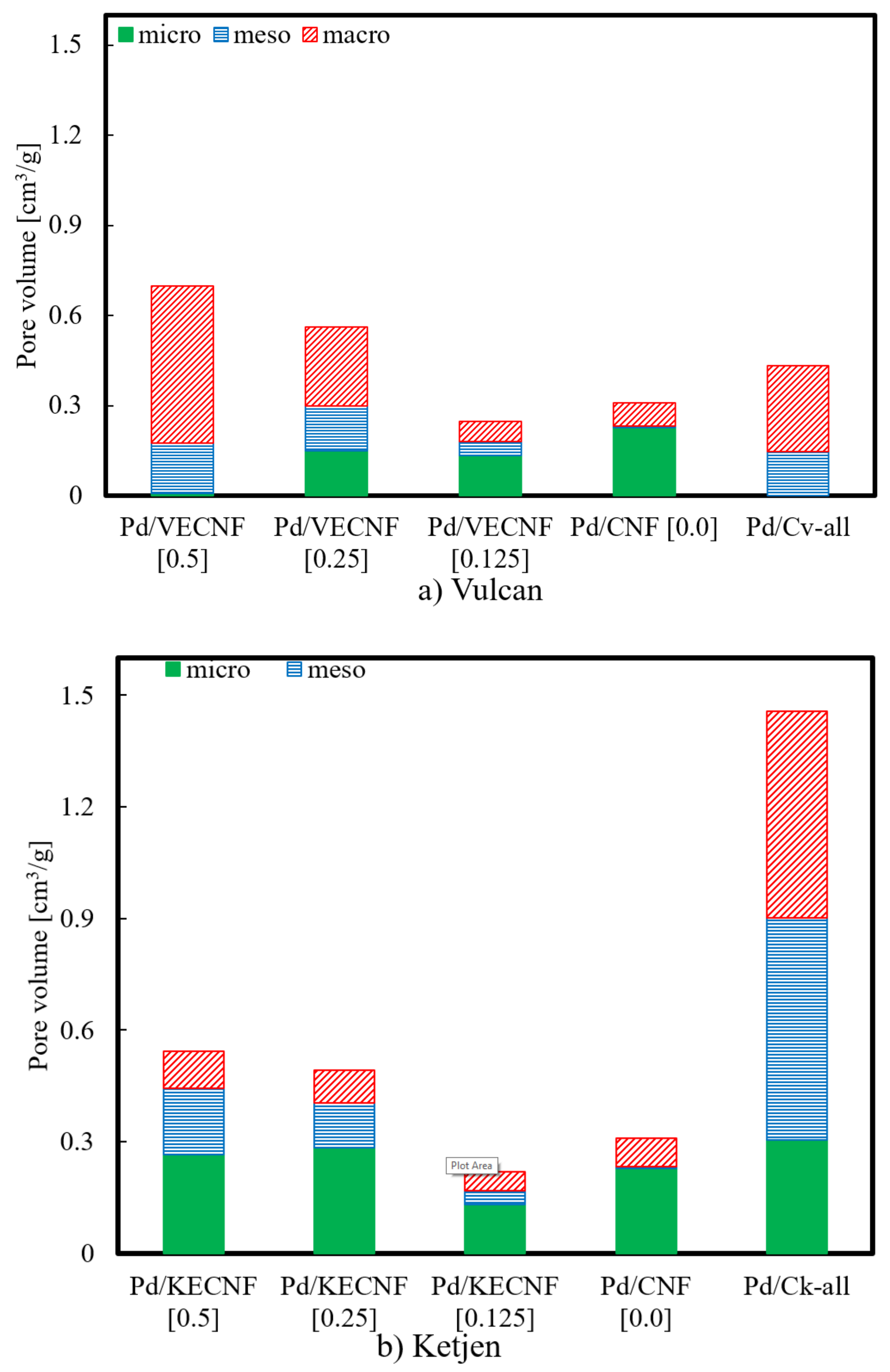
| Catalyst | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pd Crystallite Size (nm) |
|---|---|---|---|
| Pd/CNF | 544 | 0.28 | 17.0 |
| Pd/VECNF [0.125] | 321 | 0.20 | 15.8 |
| Pd/VECNF [0.25] | 391 | 0.44 | 15.8 |
| Pd/VECNF [0.50] | 250 | 0.43 | 13.4 |
| Pd/KECNF [0.125] | 405 | 0.25 | 13.7 |
| Pd/KECNF [0.25] | 573 | 0.43 | 14.1 |
| Pd/KECNF [0.50] | 558 | 0.49 | 15.9 |
| Catalyst | Mass Activity at Ep, ip-FAOR (mA mgPd−1) | Peak Potential, Ep (V vs. NHE) | Electrochemical Active Surface Area (m2 gPd−1) |
|---|---|---|---|
| Pd/VECNF [0.5] | 486 | 0.52 | 38.5 |
| Pd/VECNF [0.25] | 1477 | 0.56 | 35.5 |
| Pd/VECNF [0.125] | 481 | 0.38 | 39.9 |
| Pd/KECNF [0.5] | 945 | 0.52 | 35.8 |
| Pd/KECNF [0.25] | 2022 | 0.62 | 56.9 |
| Pd/KECNF [0.125] | 1096 | 0.60 | 25.1 |
| Pd/CNF [0.0] | 398 | 0.50 | 20.1 |
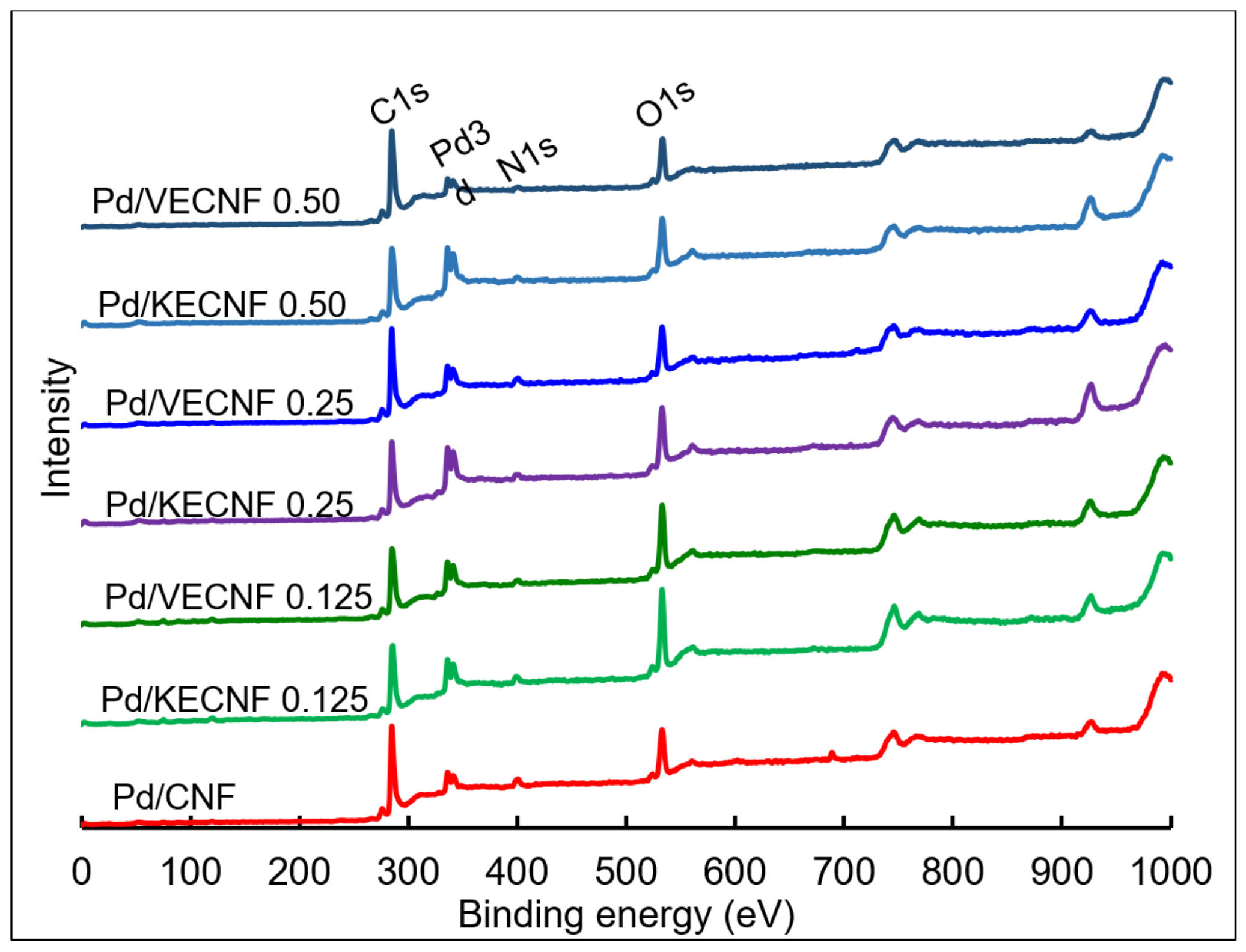
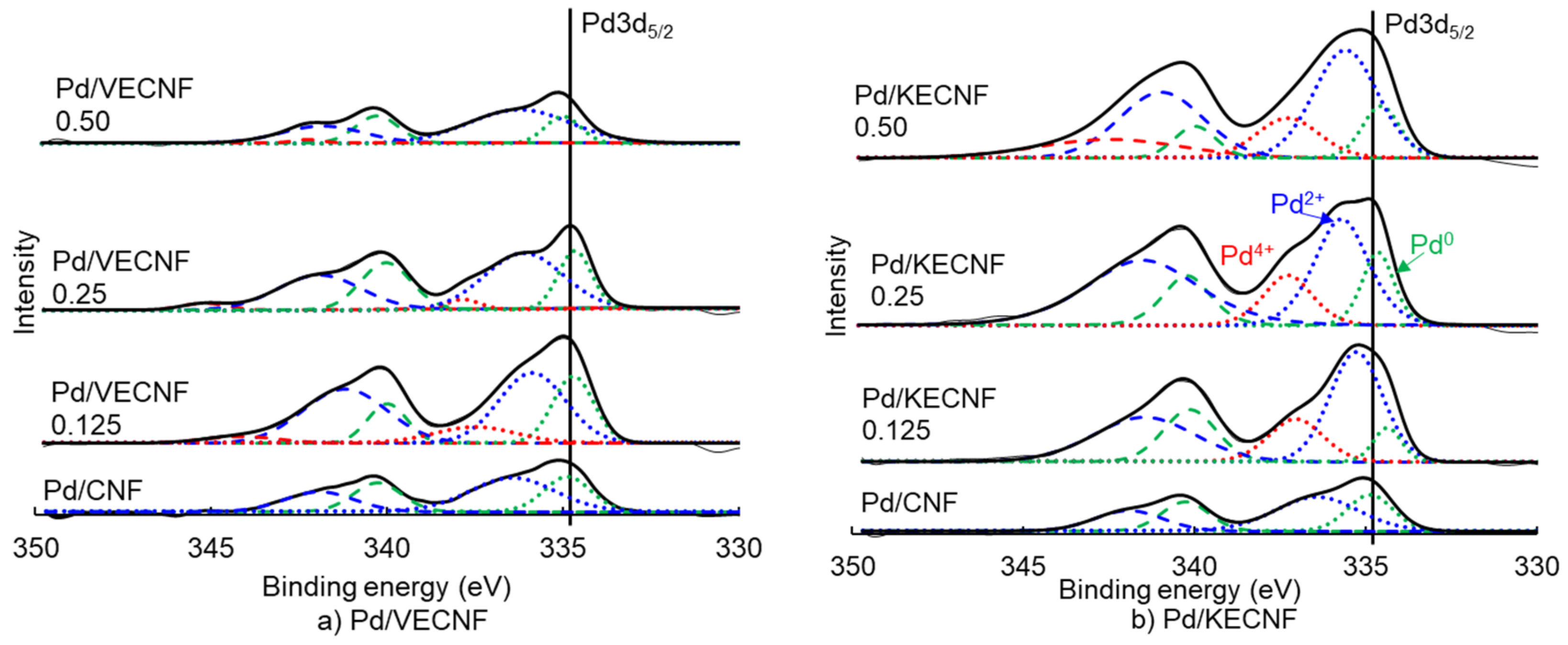
| Catalyst Samples | Pd Surface Species | Binding Energy (eV) | Relative Intensity |
|---|---|---|---|
| Pd/CNF | Pd0 | 334.88 | 0.51 |
| Pd2+ | 336.44 | 0.49 | |
| Pd4+ | 0.00 | ||
| Pd/KECNF 0.125 | Pd0 | 334.37 | 0.18 |
| Pd2+ | 335.28 | 0.59 | |
| Pd4+ | 337.05 | 0.23 | |
| Pd/KECNF 0.25 | Pd0 | 334.65 | 0.32 |
| Pd2+ | 335.71 | 0.46 | |
| Pd4+ | 337.27 | 0.22 | |
| Pd/KECNF 0.5 | Pd0 | 334.56 | 0.26 |
| Pd2+ | 335.59 | 0.54 | |
| Pd4+ | 337.28 | 0.20 | |
| Pd/VECNF 0.125 | Pd0 | 334.72 | 0.44 |
| Pd2+ | 335.89 | 0.46 | |
| Pd4+ | 337.51 | 0.10 | |
| Pd/VECNF 0.25 | Pd0 | 334.68 | 0.47 |
| Pd2+ | 336.13 | 0.45 | |
| Pd4+ | 337.86 | 0.08 | |
| Pd/VECNF0.5 | Pd0 | 335.01 | 0.44 |
| Pd2+ | 336.24 | 0.56 | |
| Pd4+ | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Aslam, N.; Tsujiguchi, T.; Osaka, Y.; Kodama, A. The Origins of the High Performance of Pd Catalysts Supported on Carbon Black-Embedded Carbon Nanofiber for Formic Acid Oxidation. Appl. Sci. 2019, 9, 5542. https://doi.org/10.3390/app9245542
Mohamed Aslam N, Tsujiguchi T, Osaka Y, Kodama A. The Origins of the High Performance of Pd Catalysts Supported on Carbon Black-Embedded Carbon Nanofiber for Formic Acid Oxidation. Applied Sciences. 2019; 9(24):5542. https://doi.org/10.3390/app9245542
Chicago/Turabian StyleMohamed Aslam, Norraihanah, Takuya Tsujiguchi, Yugo Osaka, and Akio Kodama. 2019. "The Origins of the High Performance of Pd Catalysts Supported on Carbon Black-Embedded Carbon Nanofiber for Formic Acid Oxidation" Applied Sciences 9, no. 24: 5542. https://doi.org/10.3390/app9245542
APA StyleMohamed Aslam, N., Tsujiguchi, T., Osaka, Y., & Kodama, A. (2019). The Origins of the High Performance of Pd Catalysts Supported on Carbon Black-Embedded Carbon Nanofiber for Formic Acid Oxidation. Applied Sciences, 9(24), 5542. https://doi.org/10.3390/app9245542





