Phase Space Analysis of Pig Ear Skin Temperature during Air and Road Transport
Abstract
1. Introduction
2. Materials and Methods
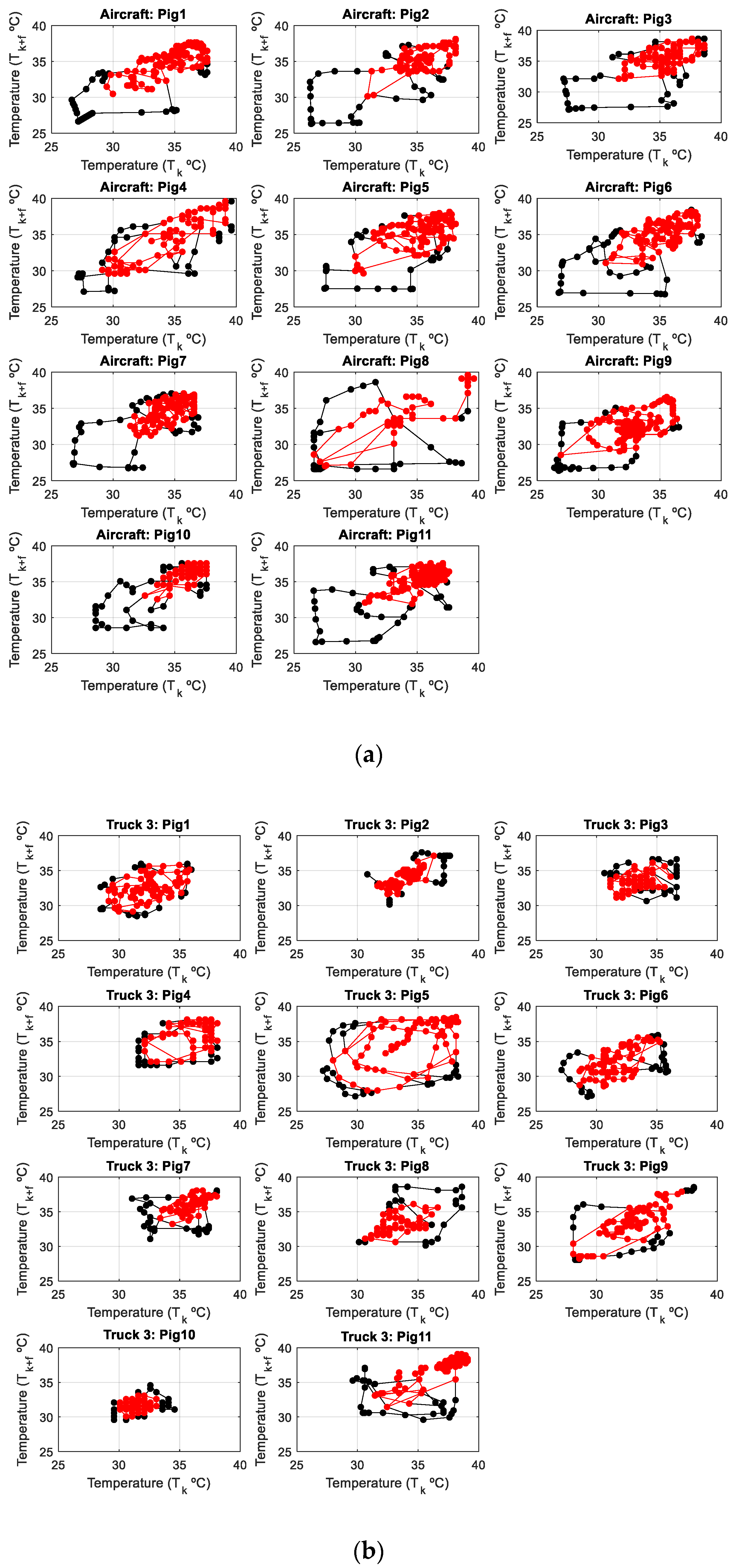
Phase Space Diagrams
3. Results and Discussion
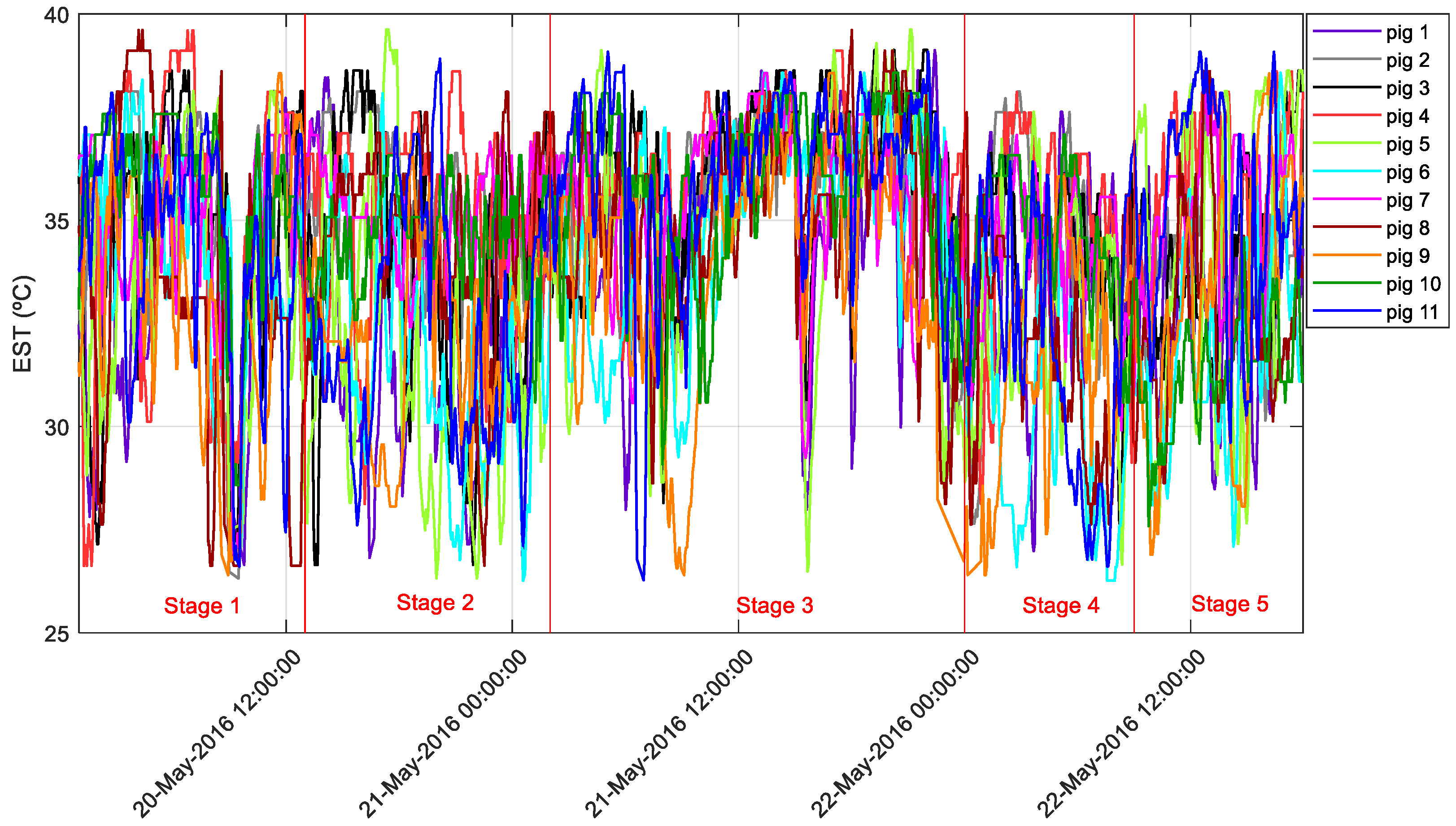
3.1. Pig Skin Temperature
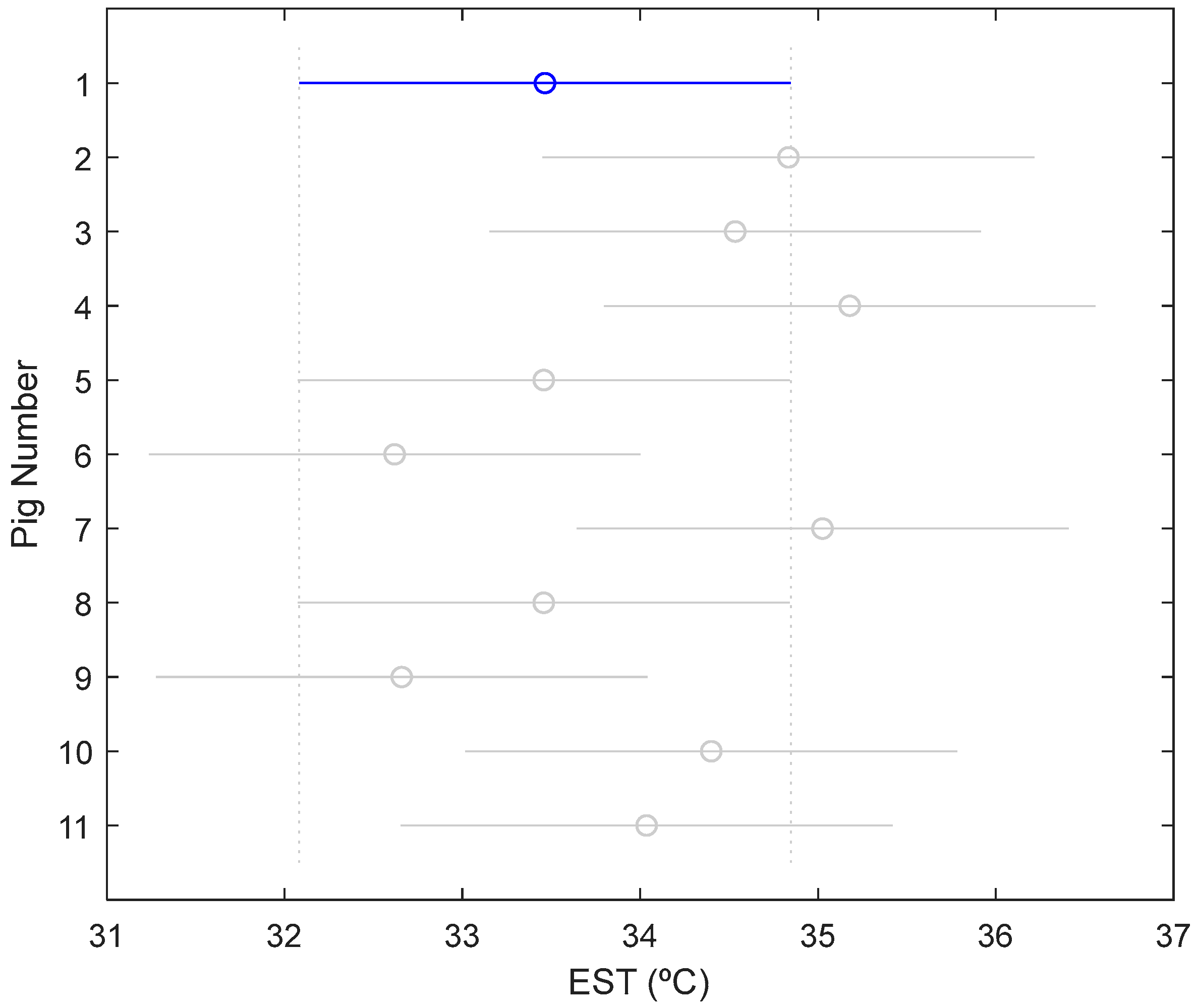
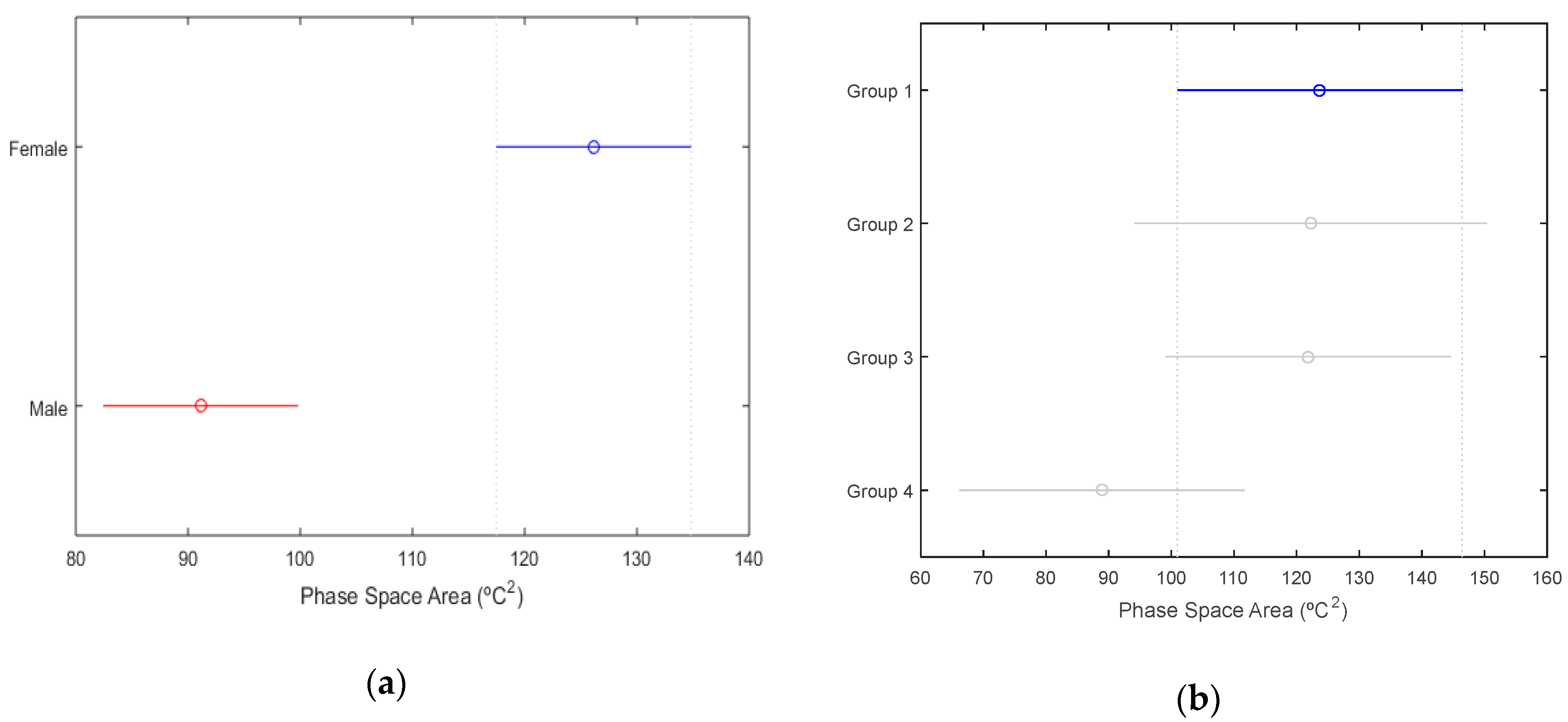
3.2. Phase Space: Variability in Skin Temperature by Animals
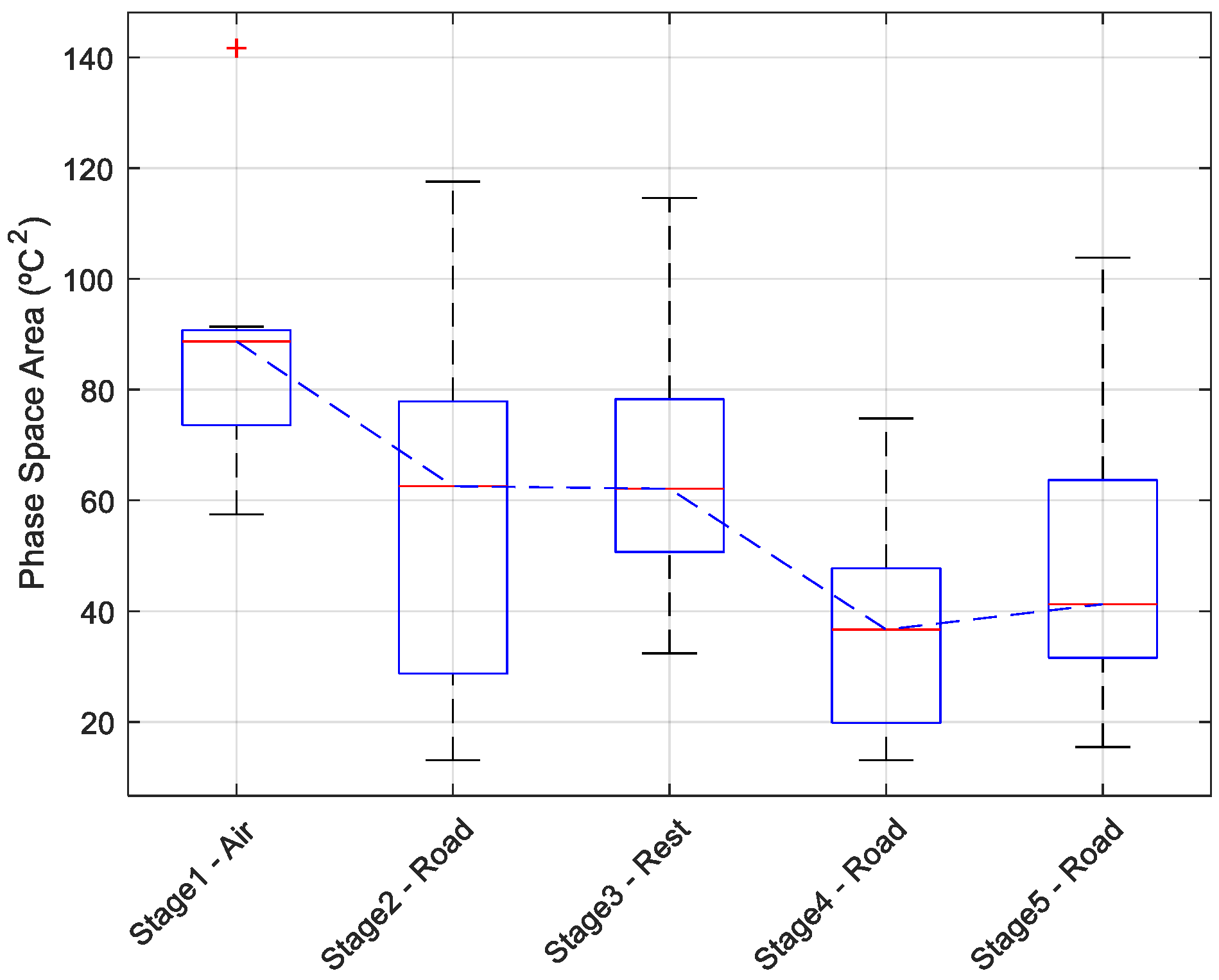
3.3. Phase Space: Variability in EST by Transport Stage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ritter, M.; Ellis, M.; Berry, N.; Curtis, S.; Anil, L.; Berg, E.; Benjamin, M.; Butler, D.; Dewey, C.; Driessen, B. Transport losses in market weight pigs: I. A review of definitions, incidence, and economic impact. Prof. Anim. Sci. 2009, 25, 404–414. [Google Scholar] [CrossRef]
- Comission, E. Guide to good practices for the transport of pigs. In Consortium of the Animal Transport Guides Project (2017-rev1); Revision May 2018; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Bench, C.; Schaefer, A.; Faucitano, L. The welfare of pigs during transport. In Welfare of Pigs: From Birth to Slaughter; Schaefer, A., Faucitano, L., Eds.; Wageningen Academic: New York, NY, USA, 2008; Volume 6, pp. 161–180. [Google Scholar]
- Faucitano, L.; Rioja-Lang, F.C.; Brown, J.A.; Brockhoff, E.J. A review of swine transportation research on priority welfare issues: A canadian perspective. Front. Vet. Sci. 2019, 6, 36. [Google Scholar]
- Lambooij, E. Transport of pigs. Livest. Handl. Transp. 2014, 1, 280–297. [Google Scholar]
- Grandin, T. Safe handling of large animals: Part II. Ir. Vet. J. 2008, 61, 758–763. [Google Scholar]
- Roldan-Santiago, P.; Martinez-Rodriguez, R.; Yanez-Pizana, A.; Trujillo-Ortega, M.E.; Sanchez-Hernandez, M.; Perez-Pedraza, E.; Mota-Rojas, D. Stressor factors in the transport of weaned piglets: A review. Vet. Med. 2013, 58, 241–251. [Google Scholar] [CrossRef]
- Villarroel, M.; Barreiro, P.; Kettlewell, P.; Farish, M.; Mitchell, M. Time derivatives in air temperature and enthalpy as non-invasive welfare indicators during long distance animal transport. Biosyst. Eng. 2011, 110, 253–260. [Google Scholar] [CrossRef]
- Lewis, N.J. Transport of early weaned piglets. Appl. Anim. Behav. Sci. 2008, 110, 128–135. [Google Scholar] [CrossRef]
- Sommavilla, R.; Faucitano, L.; Gonyou, H.; Seddon, Y.; Bergeron, R.; Widowski, T.; Crowe, T.; Connor, L.; Scheeren, M.; Goumon, S. Season, Transport Duration and Trailer Compartment Effects on Blood Stress Indicators in Pigs: Relationship to Environmental, Behavioral and Other Physiological Factors, and Pork Quality Traits. Animals 2017, 7, 8. [Google Scholar] [CrossRef]
- Lewis, M.C.; Inman, C.F.; Patel, D.; Schmidt, B.; Mulder, I.; Miller, B.; Gill Bhupinder, P.; Pluske, J.; Kelly, D.; Stokes, C.R. Direct experimental evidence that early-life farm environment influences regulation of immune responses. Pediatr. Allergy Immunol. 2012, 23, 265–269. [Google Scholar] [CrossRef]
- Brown, S.; Knowles, T.; Wilkins, L.; Chadd, S.; Warriss, P. The response of pigs to being loaded or unloaded onto commercial animal transporters using three systems. Vet. J. 2005, 170, 91–100. [Google Scholar] [CrossRef]
- Mayer, J.J.; Davis, J.D.; Purswell, J.L.; Koury, E.J.; Younan, N.H.; Larson, J.E.; Brown-Brandl, T.M. Development and characterization of a continuous tympanic temperature logging (CTTL) probe for bovine animals. Trans. ASABE 2016, 59, 703–714. [Google Scholar]
- Chen, C.-S.; Chen, W.-C. Research and Development of Automatic Monitoring System for Livestock Farms. Appl. Sci. 2019, 9, 1132. [Google Scholar] [CrossRef]
- Xiong, Y.; Green, A.; Gates, R. Characteristics of trailer thermal environment during commercial swine transport managed under US industry guidelines. Animals 2015, 5, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Stiehler, T.; Heuwieser, W.; Pfützner, A.; Burfeind, O. The course of rectal and vaginal temperature in early postpartum sows. J. Swine Health Prod. 2015, 23, 72–83. [Google Scholar]
- Wiersma, F.; Stott, G.H. A technique for securing a temperature probe adjacent to the tympanic membrane in bovine. Trans. ASAE 1983, 26, 185–0187. [Google Scholar] [CrossRef]
- Andersen, H.M.-L.; Jørgensen, E.; Dybkjær, L.; Jørgensen, B. The ear skin temperature as an indicator of the thermal comfort of pigs. Appl. Anim. Behav. Sci. 2008, 113, 43–56. [Google Scholar] [CrossRef]
- Requejo, J.M.; Garrido-Izard, M.; Correa, E.C.; Villarroel, M.; Diezma, B. Pig ear skin temperature and feed efficiency: Using the phase space to estimate thermoregulatory effort. Biosyst. Eng. 2018, 174, 80–88. [Google Scholar] [CrossRef]
- Sellier, N.; Guettier, E.; Staub, C. A review of methods to measure animal body temperature in precision farming. Am. J. Agric. Sci. Technol. 2014, 2, 74–99. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef]
- Hahn, G.; Chen, Y.; Nienaber, J.; Eigenberg, R.; Parkhurst, A. Characterizing animal stress through fractal analysis of thermoregulatory responses. J. Therm. Biol. 1992, 17, 115–120. [Google Scholar] [CrossRef]
- Korthals, R.; Eigenberg, R.; Hahn, G.; Nienaber, J. Measurements and spectral analysis of tympanic temperature regulation in swine. Trans. ASAE 1995, 38, 905–909. [Google Scholar] [CrossRef]
- IATA. International Air Transport Association Live Animals Regulations; IATA: Geneva, Switzerland, 2001. [Google Scholar]
- EC. European Council. No 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97. Off. J. Eur. Union 2005, 3, 1–44. [Google Scholar]
- Eckmann, J.-P.; Ruelle, D. Ergodic theory of chaos and strange attractors. In The Theory of Chaotic Attractors; Springer: Berlin/Heidelberg, Germany, 1985; pp. 273–312. [Google Scholar]
- Jiménez-Ariza, T.; Correa, E.; Diezma, B.; Silveira, A.C.; Zócalo, P.; Arranz, F.J.; Moya-González, A.; Garrido-Izard, M.; Barreiro, P.; Ruiz-Altisent, M. The phase space as a new representation of the dynamical behaviour of temperature and enthalpy in a reefer monitored with a multidistributed sensors network. Food Bioprocess Technol. 2014, 7, 1793–1806. [Google Scholar] [CrossRef][Green Version]
- Rocha, L.M.; Devillers, N.; Maldague, X.; Kabemba, F.Z.; Fleuret, J.; Guay, F.; Faucitano, L. Validation of Anatomical Sites for the Measurement of Infrared Body Surface Temperature Variation in Response to Handling and Transport. Animals 2019, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, D.D.; Clausen, S.; Mercer, J.B.; Pedersen, L.J. Determining the emissivity of pig skin for accurate infrared thermography. Comput. Electron. Agric. 2014, 109, 52–58. [Google Scholar] [CrossRef]
- Poczopko, P. Metabolic rate and body size relationships in adult and growing homeotherms. Acta Theriol. 1979, 24, 125–136. [Google Scholar] [CrossRef]
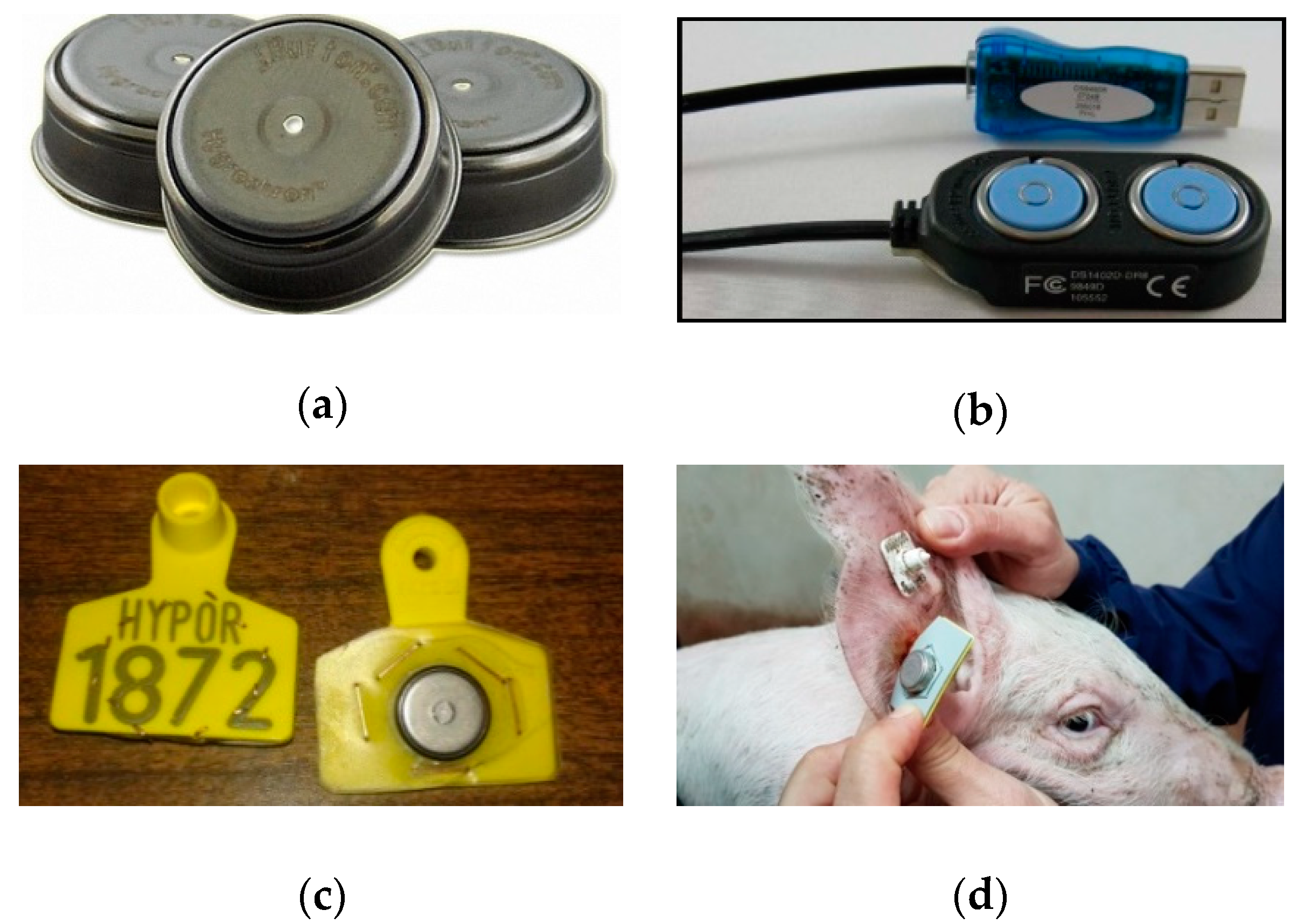
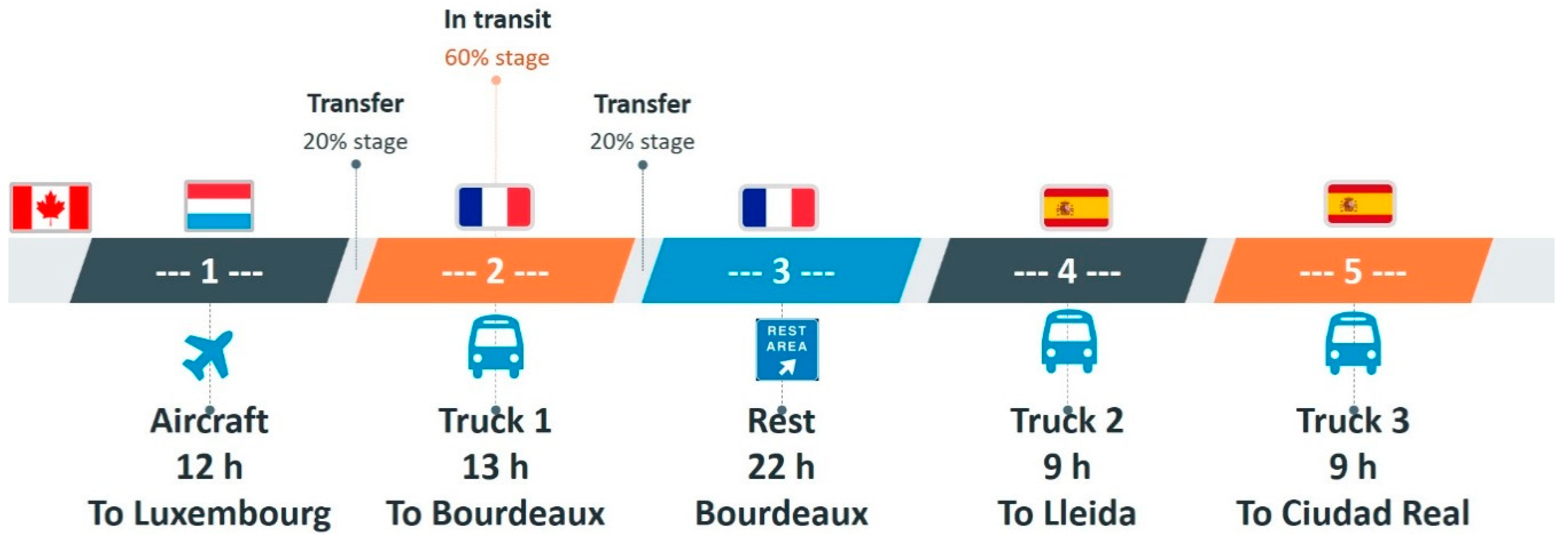


| Pig Number | Sex | Birth Date | Age Group | Weight (kg) |
|---|---|---|---|---|
| 1 | Female | 04/04/2016 | 1 | 15.38 |
| 2 | Male | 30/01/2016 | 4 | 78.47 |
| 3 | Female | 04/04/2016 | 1 | 18.19 |
| 4 | Female | 18/02/2016 | 3 | 49.48 |
| 5 | Female | 02/04/2016 | 1 | 17.41 |
| 6 | Female | 11/03/2016 | 2 | 30.65 |
| 7 | Male | 18/02/2016 | 3 | 45.22 |
| 8 | Female | 17/02/2016 | 3 | 48.5 |
| 9 | Male | 30/01/2016 | 4 | 58.51 |
| 10 | Male | 27/01/2016 | 4 | 85.64 |
| 11 | Female | 10/03/2016 | 2 | 28.93 |
| Pig Number | Area (°C2) | Centroid (°C) |
|---|---|---|
| 1 | 121.6 | 33.7 |
| 2 | 93.4 | 35.0 |
| 3 | 111.3 | 34.8 |
| 4 | 124.2 | 35.3 |
| 5 | 138.2 | 33.6 |
| 6 | 111.6 | 33.1 |
| 7 | 97.9 | 35.1 |
| 8 | 143.4 | 33.8 |
| 9 | 99.1 | 32.8 |
| 10 | 74.1 | 34.8 |
| 11 | 132.8 | 34.3 |
| Mean | 113.4 | 34.2 |
| Stage1-Air | Stage2-Road | Stage3-Rest | Stage4-Road | Stage5-Road | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area (°C2) | C (°C) | Area (°C2) | C (°C) | Area (°C2) | C (°C) | Area (°C2) | C (°C) | Area (°C2) | C (°C) | |
| Pig 1 | 74.4 | 33.4 | 48.3 | 31.0 | 80.6 | 35.2 | 74.8 | 33.7 | 41.2 | 32.4 |
| Pig 2 | 89.0 | 34.4 | 22.2 | 35.6 | 41.7 | 35.7 | 25.3 | 35.5 | 29.8 | 34.2 |
| Pig 3 | 90.5 | 34.9 | 82.4 | 33.9 | 70.3 | 36.3 | 13.7 | 34.7 | 29.8 | 33.6 |
| Pig 4 | 88.7 | 34.4 | 62.6 | 35.4 | 32.4 | 36.4 | 27.4 | 35.9 | 37.1 | 35.7 |
| Pig 5 | 78.0 | 34.1 | 117.6 | 31.6 | 96.7 | 34.9 | 41.8 | 33.3 | 103.9 | 33.9 |
| Pig 6 | 90.8 | 34.4 | 64.1 | 31.1 | 58.3 | 35.4 | 48.4 | 30.2 | 49.0 | 31.8 |
| Pig 7 | 73.3 | 33.9 | 14.5 | 34.7 | 62.1 | 35.9 | 17.9 | 34.7 | 36.9 | 35.7 |
| Pig 8 | 141.7 | 33.5 | 78.6 | 34.1 | 61.6 | 35.3 | 36.7 | 32.0 | 51.7 | 33.5 |
| Pig 9 | 67.7 | 32.2 | 56.0 | 31.9 | 71.2 | 34.1 | 45.9 | 32.4 | 68.7 | 32.8 |
| Pig 10 | 57.5 | 35.4 | 13.1 | 34.8 | 48.1 | 35.6 | 13.1 | 35.4 | 15.5 | 31.6 |
| Pig 11 | 91.4 | 34.2 | 75.8 | 32.9 | 114.6 | 35.8 | 54.9 | 31.6 | 67.6 | 35.9 |
| Mean | 85.7 | 34.1 | 57.7 | 33.4 | 67.1 | 35.5 | 36.4 | 33.6 | 48.3 | 33.7 |
| STD | 21.7 | 0.8 | 32.0 | 1.7 | 23.8 | 0.7 | 19.2 | 1.9 | 24.4 | 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Izard, M.; Correa, E.-C.; Requejo, J.-M.; Villarroel, M.; Diezma, B. Phase Space Analysis of Pig Ear Skin Temperature during Air and Road Transport. Appl. Sci. 2019, 9, 5527. https://doi.org/10.3390/app9245527
Garrido-Izard M, Correa E-C, Requejo J-M, Villarroel M, Diezma B. Phase Space Analysis of Pig Ear Skin Temperature during Air and Road Transport. Applied Sciences. 2019; 9(24):5527. https://doi.org/10.3390/app9245527
Chicago/Turabian StyleGarrido-Izard, Miguel, Eva-Cristina Correa, José-María Requejo, Morris Villarroel, and Belén Diezma. 2019. "Phase Space Analysis of Pig Ear Skin Temperature during Air and Road Transport" Applied Sciences 9, no. 24: 5527. https://doi.org/10.3390/app9245527
APA StyleGarrido-Izard, M., Correa, E.-C., Requejo, J.-M., Villarroel, M., & Diezma, B. (2019). Phase Space Analysis of Pig Ear Skin Temperature during Air and Road Transport. Applied Sciences, 9(24), 5527. https://doi.org/10.3390/app9245527






