Abstract
In this study, CuO was synthesized as a microwave absorber in the pyrolysis of a biomass model (sugarcane bagasse). CuO was synthesized for 5 min of irradiation using the following techniques: microwave (MW), ultrasound (US), combined mode (MW-US), and conduction heating (CH) as a reference material. The use of these treatments promotes changes in the morphology, as MW and US generate leaves and monolithic faceted morphologies, respectively. Changes were also generated in some textural characteristics such as crystal size, surface area, and volume-pore size. They were produced as a consequence of changes in the conditions during the crystallization stage produced by the different irradiation types. The microwave-assisted pyrolysis was performed aiming for the maximum liquid fraction (bio-oil) in the products. The reaction time, the size of the biomass, and the CuO synthesis method were also analyzed. The following particle size (ps) intervals were studied: ps < 0.5 mm, 0.5 mm < ps < 1.7 mm, 1.7 mm < ps < 3.5 mm. The best conditions at 1160 Watts in the microwave were: 4 min of reaction, particle size lower than 0.5 mm, and CuO synthesized by US. The use of CuO in the pyrolysis almost triples the amount of the obtained liquid fraction, when compared with the pyrolysis without the use of a microwave absorbent. The CuO was reduced to Cu2O and Cu after the pyrolysis. In this work, a reduction in the reaction times from hours to minutes was achieved during the synthesis of CuO and the pyrolysis biomass. The liquid fraction (bio-oil) can be raw material to obtain value-added chemical products or biofuels.
1. Introduction
Over the last 15 years, the use of biomass from agricultural waste for the production of fuels and for obtaining chemical products has become more relevant. The use of biomass generates a closed cycle of greenhouse gases (SOx, NOx, and COx) [1,2]. On the other hand, pyrolysis is an innovative technique in the production of hydrocarbons from biomass. This process is the thermal decomposition of biomass in an inert atmosphere. Pyrolysis produces biochar, non-condensable gases, and a pyrolytic liquid called bio-oil or tar with fuel oil properties. The liquid fraction is obtained from condensable gases [3,4,5]. Bio-oil obtained from pyrolysis is a complex mixture of organic compounds obtained from the decomposition of biomass (cellulose, hemicellulose, and lignin). This is a mixture of organic compounds such as acids, aldehydes, esters, alcohols, ketones, anhydrosaccharides, levoglucosan, phenolic monomers, furans; additionally, from this mixture other chemicals can be obtained by hydrocracking or hydroprocessing. Bio-oil can be used in the production of value-added chemicals or as a substitute for petroleum oils [6,7,8,9].
The microwave heating in biomass pyrolysis has created a great deal of interest due to its advantages over conduction heating: (1) heating is generated from inside the material and not from the outside as it happens with conduction, (2) there is less heat loss due to the transfer of electromagnetic energy instead of heat energy, (3) The heating is selective and without direct contact, which produces changes in the selectivity. In addition, microwave pyrolysis of biomass generates bio-oils with higher content of phenols and levoglucosan in comparison with conventional heating [10,11,12]. Microwaves are electric and magnetic fields perpendicular to each other. When these waves interact with a material they can be transmitted, reflected, or absorbed. Due to these interactions, the materials can be classified into three types (Figure 1): (I) transparent (microwaves go through the material without interactions), (II) opaque or conductive (microwaves are reflected and therefore do not penetrate the material), and (III) Absorbents (the material absorbs microwaves and it is heated) [13,14,15,16]. The biomass is a transparent material to the microwaves and it must be mixed with an absorbent material for promoting the conversion of microwaves into heat. Some materials have the advantage of absorbing microwaves and transform the electromagnetic energy into heat, some of them are oxides (CuO, Fe3O4, Ni2O3), carbon, and salts (FeS, CuS, CuCl) [17,18,19]. The CuO is a good microwave absorbent and it has shown a high selectivity for semi-volatile compounds. In the pyrolysis of pine sawdust with conduction heating, CuO has generated the highest efficiency of semi-volatile compounds compared with 31 catalysts including clays, metal oxides and zeolites. It is for this reason that CuO is proposed as a microwave absorbent [18].
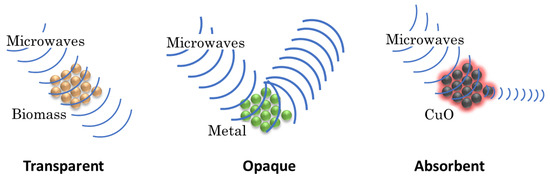
Figure 1.
Classification of materials according to their interaction with microwaves.
The sugarcane bagasse is proposed as a source of biomass, this is a waste that is produced in the order of millions of tons annually. The sugar cane occupies third place in the agricultural products in the world, and it is estimated that for each ton of sugarcane 280 kg of bagasse are generated [20,21,22].
Three different methods were employed to synthesize the copper oxide: microwave, ultrasound, and a combined mode of microwave-ultrasound irradiation. These methods generate differences in the morphological and textural properties of the synthesized materials for the analysis in the pyrolysis. On the other hand, it is expected the optimization in the production of the liquid fraction (bio-oil) taking two references: the pyrolysis without absorbent and the use of CuO synthesized with heating conduction.
2. Materials and Methods
2.1. The Biomass
Sugarcane was acquired in Mexico City, the cane was cut and crushed to extract the liquid and obtain bagasse. The bagasse was dried in a stove Memmert UN55 for 72 h at 60 °C, crushed and sieved to obtain different particle sizes: ps < 0.5 mm, 0.5 mm < ps < 1.7 mm, 1.7 mm < ps < 3.5 mm. Bagasse was characterized using the methodology reported by Adeeyo et al., 2015, which determine the content of hemicellulose (hc), cellulose (c), lignin (l), ash (a), and extractives (e). The determination of extractives was made in a Soxhlet using acetone, keeping it in reflux for four hours. The extractives were retained in the acetone and they were quantified by the difference between the dried residue and the initial bagasse.
The determination of hemicellulose was performed by boiling a fraction of the extractive free bagasse with a 0.5 M NaOH solution for three hours. The solid was filtered and washed with deionized water until pH 7 was reached, after which the residue was dried at 100 °C. To quantified the lignin content, a hydrolysis was carried out to another fraction of the extractive free bagasse with H2SO4, the dry solids were taken to an oven at 600 °C. The difference between the dry solids and the residue at 600 °C corresponds to the insoluble lignin. The soluble lignin was determined by UV-vis spectroscopy. The ash content was determined by heating the dry bagasse at 650 °C; it was obtained by weight difference with the initial bagasse. Finally, the cellulose content was calculated by applying Equation (1) [23].
%WC = 100% − %We − %Whc − %Wl − %Wa
2.2. Copper Oxide
CuO was used as a microwave absorbent material in the pyrolysis of cane bagasse. The CuO was synthesized by the precipitation method using Cu(NO3)2 as a precursor. pH 10 was maintained during the precipitation using a 0.5 M NaOH solution. The gel obtained was irradiated for 5 min with ultrasound, microwave, or combined mode. The obtained materials were identified as CuO US, Cu MW, and Cu MW US, respectively. A CW-2000a equipment was used for the three types of synthesis. In the ultrasonic mode it operates with a power of 50 W and a frequency of 40 kHz. The microwave mode works with 800 W and 2450 MHz, and for the combined mode the above conditions were used. The resulting materials were washed with distilled water. Thermogravimetric analysis was achieved in a Simultaneous Thermal Analysis STA i 1000, it was performed from 50 to 500 °C in steps of 10 °C/min in air atmosphere. The materials were heated at 350 °C as a result of the thermogravimetric analysis.
The copper oxides were characterized by X-ray diffraction in a Rigaku Miniflex 600 diffractometer using Cu Kα radiation (α = 1.54 Å). A Soller slit with a Ni filter of 0.5 mm was fitted in the incident beam, and an ultra-high speed Dtex detector was used. Measurements were performed with a sweep from 5 to 110 degrees with a step size of 0.01 and a speed of 0.9 degrees/min. Crystal size and lattice parameters were calculated using Rietveld refinement, with the help of HighScore Plus diffraction software.
The textural properties of the synthesized materials were characterized by nitrogen physisorption in a Gemini VII Surface Area Analyzer. The specific surface area was determined by BET (Brunauer, Emmet and Teller) multipoint method with a pre-treatment in temperature at 200 °C for 8 h under vacuum. The pore size was determined by the BJH (Barrett, Joyner, and Halenda) method. The pore volume was calculated at P/P0 = 0.99.
The SEM micrographs were obtained in a JEOL scanning electron microscope model JSM7800 F at 2 and 10 kV. A low electron detector (LED) or a backscatter electron detector (BED) was used. The samples were placed on conductive carbon tape.
2.3. Pyrolysis of Sugarcane Bagasse
The pyrolysis was performed using a multimodal microwave to generate the heating. Figure 2 shows the reaction system, which was designed by the group, using an LG MS1449CS microwave with variable power. For each reaction, 5 g of cane bagasse mixed with 5 wt% of CuO were placed in a template glass reactor. An inert atmosphere was generated by injecting nitrogen for 15 min before each reaction; a Dean-Stark trap at −3 °C was used to collect the condensable gases (bio-oil).
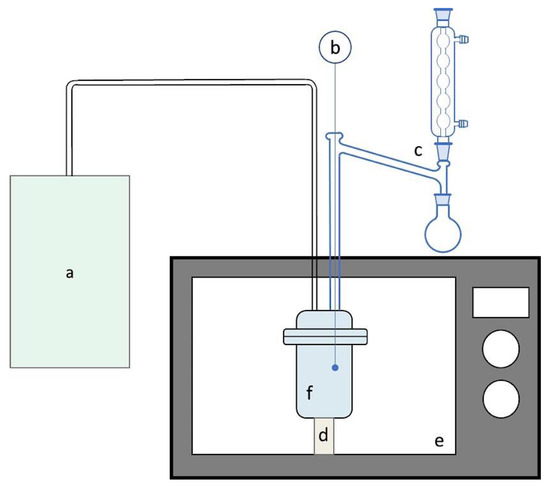
Figure 2.
Pyrolysis of sugarcane bagasse system: (a) nitrogen generator; (b) K type thermocouple; (c) Dean-Stark type trap; (d) α alumina base; (e) multimodal microwave; and (f) template glass reactor.
The solid and liquid fractions were quantified by gravimetry; the gas fraction was determined by weight difference. The remaining residue in the reactor is the solid fraction (char and CuO mixture), the condensable gases are the bio-oil and the remaining material is the non-condensable gas fraction.
3. Results and Discussion
3.1. Sugarcane Bagasse
Table 1 shows the results of the characterization of cane bagasse according to the methodology presented in Section 2.1. It is worth mentioning that the bagasse used has low ash content, which could favor the production of the liquid fraction. High content of ash favors the production of the solid and gas fractions [24]. The obtained composition has percentages similar to those reported by other authors, that is to say, higher cellulose content, followed by hemicellulose, lignin, and ash [25].

Table 1.
Composition of cane bagasse.
3.2. Copper Oxide
For the synthesis of the CuO, the coprecipitation method was used. The obtained gel was subjected to different treatments during the crystallization stage: microwave (MW), ultrasound (US), combined mode (MW-US), and also a conduction heating (CH). After the treatment, a change of color took place in the gel from blue to brown. This change is attributed to the transformation suffered by the precursors to copper oxo-hydroxide (CuxOyHz). These hydrates contain Cu-OH and Cu-O groups that are transformed into CuO [28]. Thermogravimetric analysis was made to the resulting materials (Figure 3) to know the calcination temperature at which the CuxOyHz are completely transformed into CuO. x, y, and z subscripts were calculated from the information provided from the thermographs: Cu0.31O0.44H0.26 for microwaves, and Cu0.13O0.38H0.49 for the other three methods. The thermographs show that all materials have a weight loss in the temperature interval between 170 and 260 °C and this is due to the release of water generated when transforming the CuxOyHz into CuO. The weight loss in the combined, ultrasound only, and conventional treatments was close to 30%, while the microwave heat treatment had a lower weight loss of approximately 10%. This is attributed to the fact that microwave thermal treatment favors the generation of CuO in greater measure when compared to the other treatments studied. Therefore, an adequate temperature to ensure the complete transformation into CuO is higher than 300 °C and for this reason, in all cases the samples were subjected to 350 °C for 8 h.
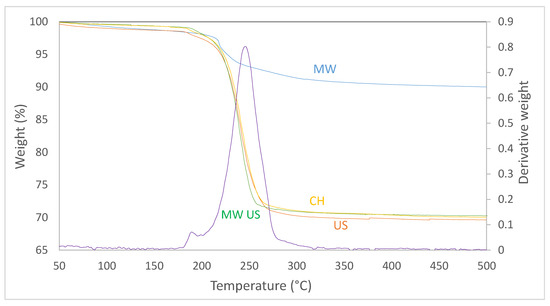
Figure 3.
Thermogravimetric analysis of CuxOyHz with different treatments.
Figure 4 shows the X-ray diffraction patterns (XRD) of materials synthesized with different treatments and Table 2 shows lattice parameters and crystal sizes for the same materials. We can observe the characteristic reflections of the monoclinic CuO in all the synthesized materials and no crystalline impurities were detected. The Inorganic Crystal Structure Database (ICSD) 00-048-1548 was used as a reference. The CuO MW presents a greater dispersion in the crystal sizes for each crystallographic direction. Lower order for the crystallographic direction (111) in comparison with the other treatments. This effect can be attributed to the vibrations generated by microwaves; it has been reported that microwave-assisted synthesis generates changes in the crystal structure (phase and degree [29,30]. According to the results shown in Table 2, it is worth mentioning that there are no significant changes in the lattice parameters for the synthesized materials. On the other hand, CuO CH has the largest crystal size while the material with microwave treatment has the smallest (3.4 times smaller than CuO CH). Smaller crystal size is due to overheating generated by microwaves. The microwaves distribute the thermal energy in the system more homogeneously compared to the conventional method where there are greater differences in temperature. Therefore, more homogeneous heating promotes a greater formation of seeds (nucleation stage) which have a lower growth due to the quick decrease in the concentration of the reactant in the surrounding space [31]. In this case, the CuO US sample has a smaller crystal size compared to the CuO CH sample. This is due to the formation of a large number of nuclei at the beginning of the crystallization stage and can be attributed to the cavitation effect [32]. The material with combined treatment (Cu MW-US) has a crystal size closer to that obtained for the CuO US. On the other hand, the growth in the different crystallographic directions for CuO MW-US is similar to that obtained by ultrasound. It can be assumed that the ultrasound treatment has a greater impact than microwave when it is applied in a combined method.
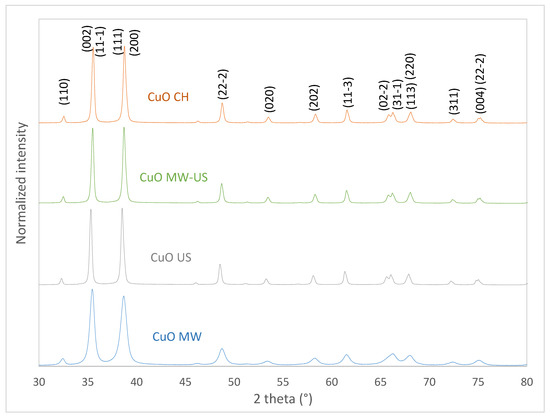
Figure 4.
X-ray diffraction patterns of CuO synthesized with microwave, ultrasound, combined mode, and conventional treatment.

Table 2.
Lattice parameters and crystal size of the CuO synthesized with different treatments.
Figure 5 shows the nitrogen adsorption-desorption isotherms of the CuO. Type IV isotherms with an H3 hysteresis loop were obtained for all the CuO samples. Type IV of isotherm is related to mesoporous solids. On the other hand, H3 hysteresis is related to materials with plate-like particles or slit-shaped pores [33,34]. The pore diameters are calculated by the BJH method and are presented in Table 3. In all cases the average pore diameters correspond to mesoporous solids. It was also found that the microwave treatment (CuO MW) promotes the largest specific area compared to the other three treatments. The second with larger surface area corresponds to the CuO CH. Using the ultrasound and combined treatment the same specific areas were obtained. For the pore diameters, the US CuO had a larger diameter and similar pore diameters were obtained for the other treatments. In the case of the pore volumes, they are similar for all treatments. Based on Table 3, it can be deduced that the type of treatment influences the formation of the porosity. The microwave treatment the one that promotes a greater specific area.
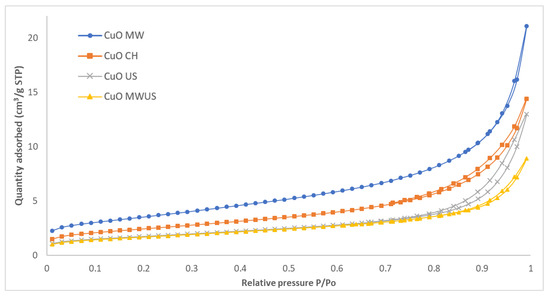
Figure 5.
Nitrogen adsorption-desorption isotherms of CuO with different treatments during the synthesis.

Table 3.
Specific area, pore diameter, and pore volume of copper oxide synthesized by different treatments.
3.3. Pyrolysis of Sugarcane Bagasse
The pyrolysis of sugarcane bagasse was performed at different reaction times (from 1 to 45 min) to determine the fraction of liquid, solid, and gas. In each reaction, 5% weight of CuO CH and 1160 Watts in a N2 atmosphere were used, the results are presented in Figure 6. At 4 min of reaction time a maximum liquid (bio-oil) yield was obtained, at higher times no significant changes were found. For this reason, this time was taken as the optimal reaction time.
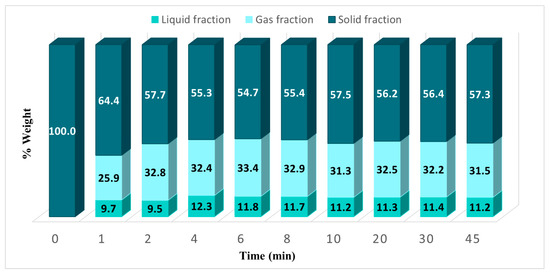
Figure 6.
Fractions obtained in the pyrolysis of cane bagasse at different reaction times using CuO as an absorbent.
Pyrolysis of cane bagasse was performed using CuO synthesized with different treatments and a test was made without CuO. The same conditions as above of power, inert atmosphere, reaction time, and weight of CuO were used in all reactions, the results are presented in Figure 7. It can be observed that there are no significant changes in the amount of liquid fraction using CuO synthesized with different treatments. There is an average of 12% weight of bio-oil, indicating that the changes in the morphological and textural properties of the absorbent do not generate significant variations in the obtained fractions during the microwave-assisted pyrolysis. The pyrolysis with CuO has a larger bio-oil product compared with the pyrolysis without absorbent, which is 12.5 and 5.2%, respectively. This is because the bagasse is not a good microwave absorbent. It should be noted that the catalysts synthesized by ultrasound and microwave techniques are performed in only 5 min of treatment.
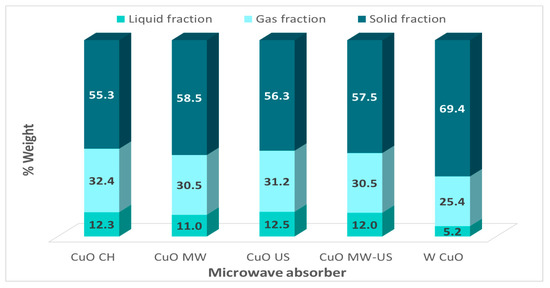
Figure 7.
Fractions obtained in the pyrolysis of cane bagasse using as absorber CuO synthesizing with microwave treatment, ultrasound, combined mode, conduction heating, and a test performed without the absorber (W CuO).
The effect of the particle size of the cane bagasse in the pyrolysis was analyzed. The reaction was achieved using different particle sizes: ps < 0.5 mm, 0.5 mm < ps < 1.7 mm, 1.7 mm < ps < 3.5 mm. The reactions were accomplished in an inert atmosphere at 1160 Watts and 10% CuO US, the results are presented in Figure 8. The smallest particle size (ps < 0.5 mm) generates the highest liquid fraction (14.5%), unlike the results reported by Varma, where the size that generated the highest liquid yield was in the interval of 1–1.7 mm when using conduction heating [35].
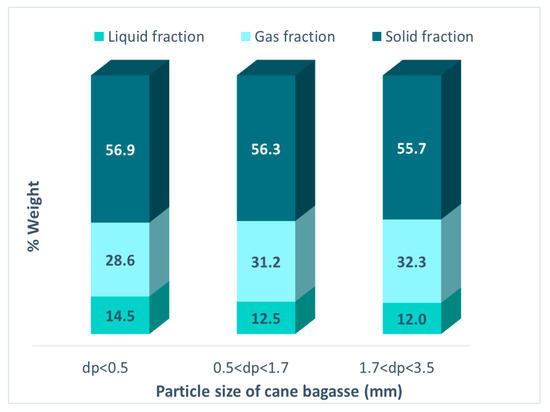
Figure 8.
Fractions obtained in the pyrolysis of cane bagasse using different particle sizes.
A smaller particle size allows a larger contact surface between the bagasse and the microwave absorbent, promoting more homogeneous heating in the bagasse-CuO mixture.
A comparison of the crystalline phases in the solid fraction was made before and after pyrolysis. Figure 9 shows the XRD patterns of cane bagasse mixed with CuO before and after the pyrolysis. The composition of the crystalline phases of the copper species present in the carbonaceous residue (after the pyrolysis) was obtained by Rietveld analysis.
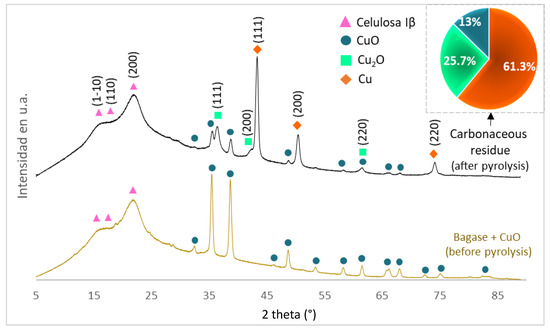
Figure 9.
XRD patterns of cane bagasse + CuO and the carbonaceous residue obtained after pyrolysis. Percentage of the crystalline phases: CuO, Cu2O, and Cu found in the carbonaceous residue.
The XRD patterns of Figure 9 show cellulose Iβ and CuO with monoclinic crystalline systems in both cases. The identification of cellulose was performed using the work published by French (A. D. French 2014) and for CuO the ICDD 01-089-5899 card. Additionally, in the carbonaceous residue, Cu2O and Cu with a cubic crystalline system were also identified; the identification was made with the ICDD 01-085-1325 and 01-077-0199 cards, respectively. Based on the information obtained from XRD patters and the Rietveld analysis (Figure 9), it can be inferred that during pyrolysis, 87% of the CuO was reduced to Cu2O and Cu. This reduction can be attributed to the presence of CO which is part of the non-condensable products during the pyrolysis [36,37,38,39,40,41]. Therefore, Cu2O and Cu have a lower capacity to transform microwaves into heat energy than the CuO compound; this explains why the reaction progress is reduced after 4 min of irradiation (Figure 6).
The morphology of CuO was analyzed before and after the pyrolysis. The scanning electron microscopy of CuO MW (Figure 10a–c), CuO US (Figure 10d–f), and the carbon-CuO US mixture (Figure 10g–i) were performed. The carbon-CuO US mixture was obtained after the pyrolysis which promoted the higher liquid fraction.
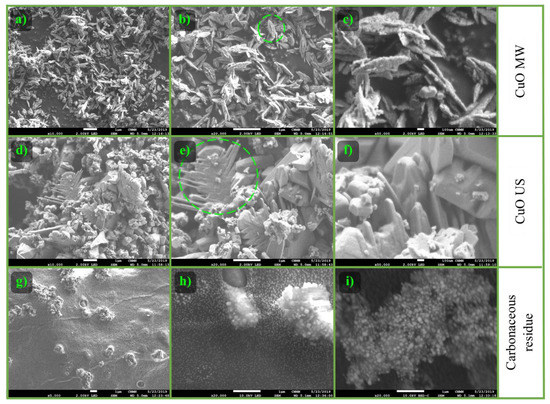
Figure 10.
SEM micrographs using LED at different magnifications: CuO MW (a) 10,000×; (b) 20,000×; (c) 50,000×; CuO US (d) 10,000×; (e) 20,000×; (f) 50,000×, and carbon-CuO US mixture (g) 10,000×; (h) 20,000×; (i) 20,000× (using backscatter electron detector (BED)).
A nanoleaves morphology with different sizes is observed in Figure 10a–c [42]. Details of the morphology are appreciated in micrograph b, where the zone marked with a circle shows the growth of this morphology through the formation of dendrites. At 50000× (c), it can be seen that these structures have porosity and the existence of a monolithic phase in a smaller proportion.
The synthesized sample by ultrasound irradiation (Figure 10d–f) has a different morphology when compared with the one obtained by microwaves. In this case, the faceted and agglomerated monoliths of different sizes and shapes (d) are presented. Micrograph (e) depicts a growth based on dendrites (circled area) similar to that found by microwaves. It should be mentioned that in general, the particle size is larger than the one obtained by microwaves. In micrograph f some porous areas and details of this morphology are identified. The difference in morphology of the CuO synthesized with ultrasound (facetted monoliths) or microwave (nanoleaves) treatment is due to the topotactic effect. This indicates that the synthesis method modifies the shape and size of CuxOyHz in the nucleation and growth during the crystal formation. This morphology is preserved in the resulting oxides after the calcination [43].
The carbon-CuO US mixture obtained after performing the pyrolysis (Figure 10g–i), shows some Cu species agglomerates on the surface of the char (f). At 20,000× (h) there is a homogeneous dispersion of Cu species on the char. It should be noted that there was a change from monolithic faceted morphology to quasispherical particles and a size reduction in CuO ((e), 20,000×) when reduced to Cu2O and Cu ((h), 20,000×). The micrograph (i), which has the same magnification but is using a backscattered electron detector, corroborates that the dispersed and agglomerated material corresponds to the Cu species.
4. Conclusions
CuO was synthesized free of crystalline impurities using ultrasound, microwave, or combined mode (microwave-ultrasound).
The crystallinity of the CuO is modified during the transformation of CuxOyHz to CuO due to the different treatments, the lattice parameters do not change. The CuO MW sample has the smallest crystal size, 3.3 times smaller than the one obtained with conduction heating (CuO CH). The combined treatment presents an intermediate crystal size between the one obtained with microwaves or ultrasound.
The treatment modified the textural characteristics (specific surface area, diameter, and pore volume) in the CuO during the synthesis. The ultrasound irradiation promotes larger pore diameter in comparison with microwave and combined treatments.
The reaction time is reduced during the synthesis of materials and reactions such as pyrolysis using microwave and ultrasound irradiation, the time could be reduced from hours to minutes.
The texture and morphology depend on the irradiation method during the nucleation stage for the synthesized materials. The synthesis method when obtaining CuO modifies the morphology due to the topotactic effect, resulting in irregular faceted monoliths using ultrasound and nanoleaves in the case of microwaves.
The best conditions to obtain bio-oil (14.5%) by the pyrolysis of sugarcane bagasse using a multimodal microwave reactor and CuO as a microwave absorber were: a power of 1160 Watts, 4 min of irradiation time, CuO US as an absorber and ps < 0.5 mm of the biomass.
Using CuO as a microwave absorber, the amount of the obtained liquid fraction is 2.7 times larger than the pyrolysis without absorber.
During the pyrolysis of cane bagasse using CuO as a microwave absorber, 87% of the CuO is reduced to Cu2O and Cu after 4 min of irradiation, slowing the reaction progress because CuO is a better microwave absorber than Cu2O and Cu.
Author Contributions
Conceptualization, E.J.P.-G., S.P.P.-C., S.O.F.-V.; methodology, E.J.P.-G., R.M.P.-G.; investigation E.J.P.-G., I.S.R.-C.; formal analysis E.J.P.-G., S.P.P.-C., S.O.F.-V.; writing—original draft E.J.P.-G., S.P.P.-C., S.O.F.-V.; writing—review and editing E.J.P.-G., S.P.P.-C., J.C.S.-O.
Funding
This research was funding by Instituto Politécnico Nacional (IPN): SIP 20196290, SIP 20196381, SIP 20181685 projects.
Acknowledgments
We want to thank Instituto Politécnico Nacional (IPN) and Laboratorio de Nanomateriales Sustentables IPN-ESIQIE. E.J.P.-G. thanks the scholarship support 565252 from CONACYT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from microwave pyrolysis of biomass: A review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Honma, S.; Hata, T.; Watanabe, T. Effect of Catalytic Pyrolysis Conditions Using Pulse Current Heating Method on Pyrolysis Products of Wood Biomass. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castells, X.E.; García, E.V. La Pirolisis: Tratamiento y Valorizacion Energética de Residuos; Ediciones Díaz de Santos: Madrid, Spain, 2012; ISBN 84-9969-131-5. [Google Scholar]
- Moldoveanu, S.C. Techniques and instrumentation in analytical chemistry. In Analytical Pyrolysis of Natural Organic Polymers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 978-0-444-82203-1. [Google Scholar]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Diebold, J.P. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils; Technical Report NREL/SR-570-27613, 753818; National Renewable Energy Lab.: Golden, CO, USA, 1999.
- Dobele, G.; Rossinskaja, G.; Dizhbite, T.; Telysheva, G.; Meier, D.; Faix, O. Application of catalysts for obtaining 1,6-anhydrosaccharides from cellulose and wood by fast pyrolysis. J. Anal. Appl. Pyrolysis 2005, 74, 401–405. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Robinson, J.; Dodds, C.; Stavrinides, A.; Kingman, S.; Katrib, J.; Wu, Z.; Medrano, J.; Overend, R. Microwave Pyrolysis of Biomass: Control of Process Parameters for High Pyrolysis Oil Yields and Enhanced Oil Quality. Energy Fuels 2015, 29, 1701–1709. [Google Scholar] [CrossRef]
- Haque, K.E. Microwave energy for mineral treatment processes—A brief review. Int. J. Miner. Proc. 1999, 57, 1–24. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Díaz, J.A.M.; Moreno, Á.S.H. Aplicaciones Industriales del Calentamiento con Energía Microondas; Universidad Técnica de Cotopaxi: Latacunga, Ecuador, 2017; ISBN 9978395342. [Google Scholar]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Rao, K.J.; Ramesh, P.D. Use of microwaves for the synthesis and processing of materials. Bull. Mater. Sci. 1995, 18, 447–465. [Google Scholar] [CrossRef]
- Henkel, C.; Muley, P.D.; Abdollahi, K.K.; Marculescu, C.; Boldor, D. Pyrolysis of energy cane bagasse and invasive Chinese tallow tree (Triadica sebifera L.) biomass in an inductively heated reactor. Energy Convers. Manag. 2016, 109, 175–183. [Google Scholar] [CrossRef]
- Dominguez, A.; Menéndez, J.A.; Fernandez, Y.; Pis, J.J.; Nabais, J.M.V.; Carrott, P.J.M.; Carrott, M.M.L.R. Conventional and microwave induced pyrolysis of coffee hulls for the production of a hydrogen rich fuel gas. J. Anal. Appl. Pyrolysis 2007, 79, 128–135. [Google Scholar] [CrossRef]
- Torri, C.; Reinikainen, M.; Lindfors, C.; Fabbri, D.; Oasmaa, A.; Kuoppala, E. Investigation on catalytic pyrolysis of pine sawdust: Catalyst screening by Py-GC-MIP-AED. J. Anal. Appl. Pyrolysis 2010, 88, 7–13. [Google Scholar] [CrossRef]
- Martinez, E.A.; Villarreal, M.L.M.; Almeida e Silva, J.B.; Solenzal, A.I.N.; Canilha, L.; Mussatto, S.I. Uso De Diferentes Materias Primas Para La Producción Biotecnológica De Xilitol Use of Different Raw Materials for Biotechnological Xylitol Production Uso De Diferentes Materias Primas Para Á Producción Biotecnolóxica De Xilitol. Cienc. Tecnol. Aliment. 2002, 3, 295–301. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Carrere, H.; Maciel Filho, R.; Costa, A.C. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour. Technol. 2011, 102, 7887–7895. [Google Scholar] [CrossRef]
- Bezerra, T.L.; Ragauskas, A.J. A review of sugarcane bagasse for second-generation bioethanol and biopower production: Biorefining Sugar Bagasse. Biofuels Bioprod. Biorefin. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Dantas, G.A.; Legey, L.F.L.; Mazzone, A. Energy from sugarcane bagasse in Brazil: An assessment of the productivity and cost of different technological routes. Renew. Sustain. Energy Rev. 2013, 21, 356–364. [Google Scholar] [CrossRef]
- Ayeni, A.O.; Adeeyo, O.A.; Oresegun, O.M.; Oladimeji, T.E. Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass. Am. J. Eng. Res. 2015, 4, 2320–2847. [Google Scholar]
- Pattiya, A.; Sukkasi, S.; Goodwin, V. Fast pyrolysis of sugarcane and cassava residues in a free-fall reactor. Energy 2012, 44, 1067–1077. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Martín, C.; Klinke, H.B.; Thomsen, A.B. Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb. Technol. 2007, 40, 426–432. [Google Scholar] [CrossRef]
- Lin, B.-J.; Chen, W.-H. Sugarcane Bagasse Pyrolysis in a Carbon Dioxide Atmosphere with Conventional and Microwave-Assisted Heating. Front. Energy Res. 2015, 3, 4. [Google Scholar] [CrossRef]
- Moreno-Valencia, E.I.; Paredes-Carrera, S.P.; Sánchez-Ochoa, J.C.; Flores-Valle, S.O.; Avendaño-Gómez, J.R. Diclofenac degradation by heterogeneous photocatalysis with Fe3O4/TixOy/activated carbon fiber composite synthesized by ultrasound irradiation. Mater. Res. Express 2017, 4, 115026. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Rodríguez-Clavel, I.S.; Paredes-Carrera, S.P.; Flores-Valle, S.O.; Paz-García, E.J.; Sánchez-Ochoa, J.C.; Pérez-Gutiérrez, R.M. Effect of Microwave or Ultrasound Irradiation in the Extraction from Feather Keratin. Available online: https://www.hindawi.com/journals/jchem/2019/1326063/cta/ (accessed on 6 December 2019).
- Burgaz, E.; Erciyes, A.; Andac, M.; Andac, O. Synthesis and characterization of nano-sized metal organic framework-5 (MOF-5) by using consecutive combination of ultrasound and microwave irradiation methods. Inorg. Chim. Acta 2019, 485, 118–124. [Google Scholar] [CrossRef]
- Enomoto, N.; Okitsu, K. Application of Ultrasound in Inorganic Synthesis. In Sonochemistry and the Acoustic Bubble; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–206. ISBN 978-0-12-801530-8. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Boyce, A.L.; Graville, S.R.; Sermon, P.A.; Vong, M.S.W. Reduction of CuO-containing catalysts, CuO: I: TPR and TGA. React. Kinet. Catal. Lett. 1991, 44, 1–11. [Google Scholar] [CrossRef]
- Boyce, A.L.; Graville, S.R.; Sermon, P.A.; Vong, M.S.W. Reduction of CuO-containing catalysts, CuO: II: XRD and XPS. React. Kinet. Catal. Lett. 1991, 44, 13–18. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Pérez, M.; Frenkel, A.I. Reduction of CuO in H2: In Situ Time-Resolved XRD Studies. Catal. Lett. 2003, 85, 247–254. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Fu, B.; Hu, G. Chemical structure and gas products of different rank coals during pyrolysis: Based on in-situ FTIR and TG/MS analysis techniques. J. Therm. Anal. Calorim. 2019, 136, 2017–2031. [Google Scholar] [CrossRef]
- Wang, X.; Kersten, S.R.A.; Prins, W.; van Swaaij, W.P.M. Biomass Pyrolysis in a Fluidized Bed Reactor. Part 2: Experimental Validation of Model Results. Ind. Eng. Chem. Res. 2005, 44, 8786–8795. [Google Scholar] [CrossRef]
- Park, Y.-K.; Yoo, M.L.; Heo, H.S.; Lee, H.W.; Park, S.H.; Jung, S.-C.; Park, S.-S.; Seo, S.-G. Wild reed of Suncheon Bay: Potential bio-energy source. Renew. Energy 2012, 42, 168–172. [Google Scholar] [CrossRef]
- Kaur, G.; Saini, K.; Tripathi, A.K.; Jain, V.; Deva, D.; Lahiri, I. Room temperature growth and field emission characteristics of CuO nanostructures. Vacuum 2017, 139, 136–142. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-811631-9. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).