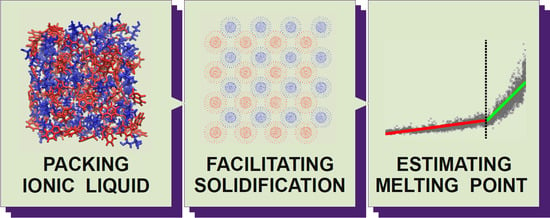
Predicting Melting Points of Biofriendly Choline-Based Ionic Liquids with Molecular Dynamics
Abstract
Featured Application
Abstract
1. Introduction
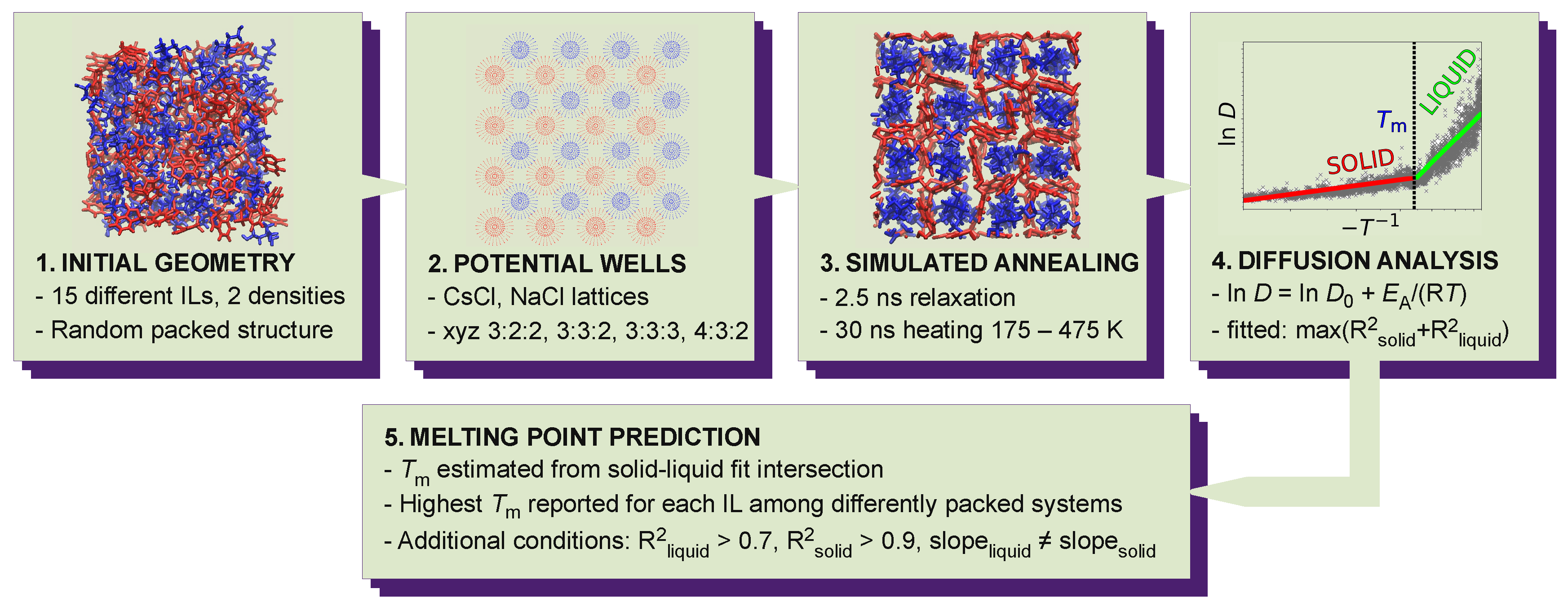
2. Materials and Methods
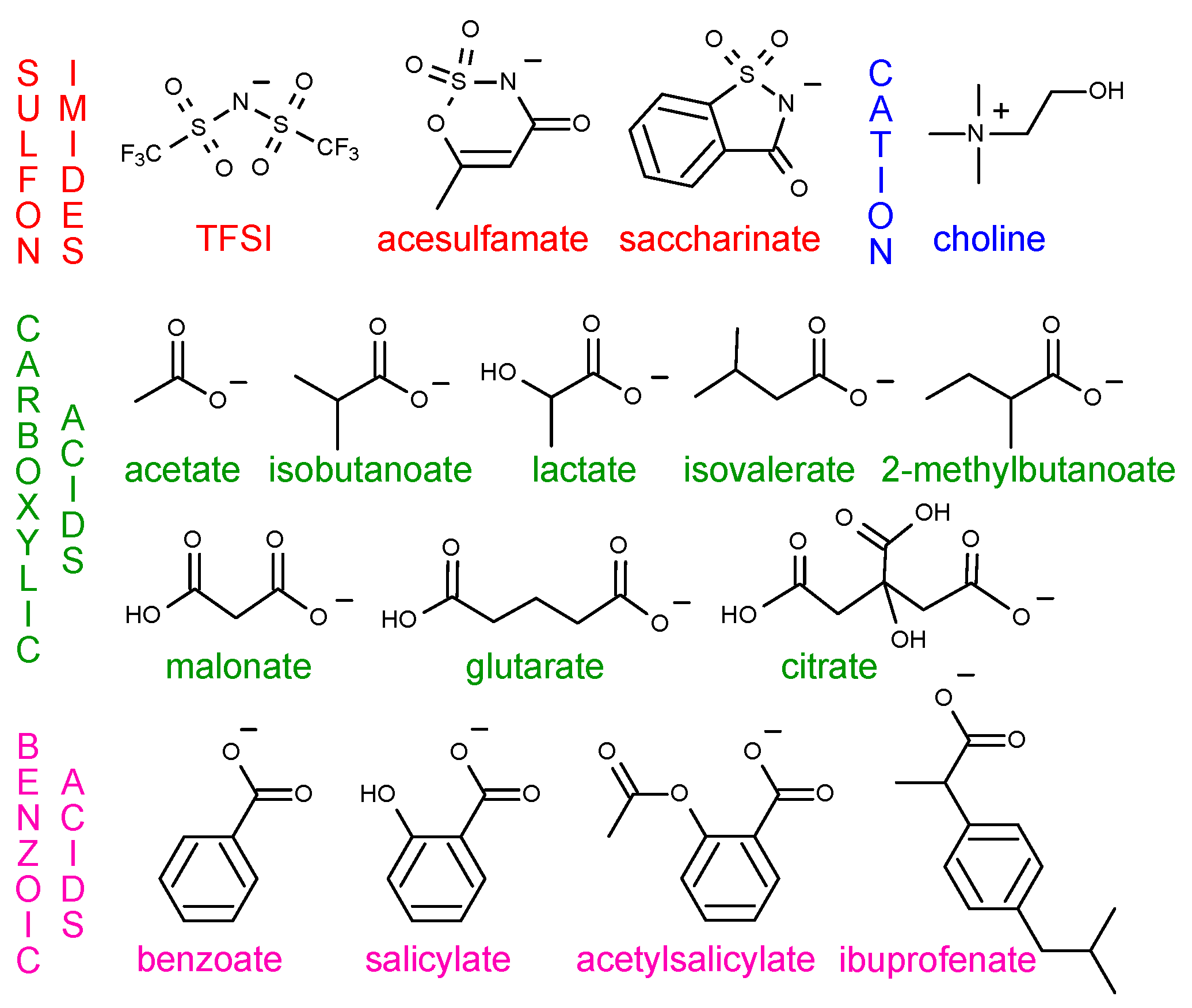
2.1. Models
2.2. Simulations Details
2.3. Solid Phase Structures
2.4. Annealing Simulations
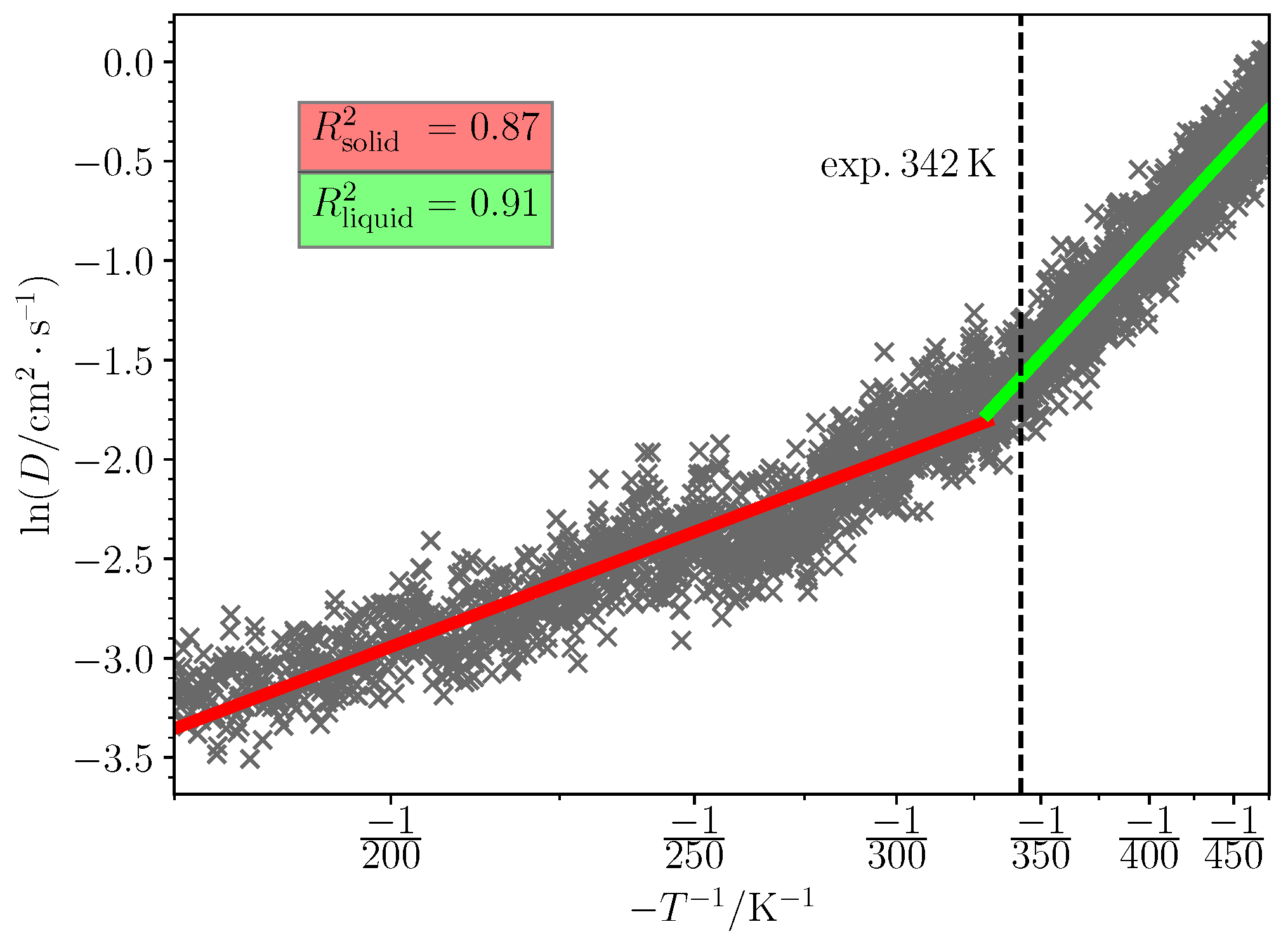
2.5. Melting Point Prediction
2.6. Force Fields and Partial Charges
2.7. Experimental
3. Results
3.1. Experimental Results
3.2. Predicted Melting Points
3.3. Annealing Temperature Rate Effect
3.4. The Choice of Partial Charges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IL | ionic liquid |
| MD | molecular dynamics |
| QSAR | quantitative strucutre–activity relationship |
| PSCP | pseudo supercritical path model |
| TFSI | bis[(trifluoromethyl)sulfonyl]imide |
| OPLS-AA | optimized potentials for liquid simulations for all atoms, a molecular dynamics force field |
| NVT | a molecular dynamics ensemble where the amount of particles, volume and temperature are constant |
| NPT | a molecular dynamics ensemble where the amount of particles, pressure and temperature are constant |
| DFT | density functional theory |
| CHELPG | charges from electrostatic potentials using a grid based method |
| GFN2-xTB | Grimme’s semi empirical tight binding method |
| QSPR | quantitative structure–property relationship |
References
- Fedorov, M.V.; Kornyshev, A.A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- De Vos, N.; Maton, C.; Stevens, C.V. Electrochemical Stability of Ionic Liquids: General Influences and Degradation Mechanisms. ChemElectroChem 2014, 1, 1258–1270. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.F.; Pang, L. Environmental application, fate, effects, and concerns of ionic liquids: A review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef]
- Bubak, G.; Gendron, D.; Ceseracciu, L.; Ansaldo, A.; Ricci, D. Parylene-Coated Ionic Liquid-Carbon Nanotube Actuators for User-Safe Haptic Devices. ACS Appl. Mater. Interfaces 2015, 7, 15542–15550. [Google Scholar] [CrossRef]
- Rinne, P.; Põldsalu, I.; Johanson, U.; Tamm, T.; Põhako-Esko, K.; Punning, A.; van den Ende, D.; Aabloo, A. Encapsulation of ionic electromechanically active polymer actuators. Smart Mater. Struct. 2019, 28, 074002. [Google Scholar] [CrossRef]
- Shoa, T.; Madden, J.D.; Munce, N.R.; Yang, V.X.D. Steerable Catheters. In Biomedical Applications of Electroactive Polymer Actuators; Carpi, F., Smela, E., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 229–248. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.N.; Ferreira, R.; Leitao, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids—Toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Does It Really Matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar] [CrossRef]
- Muhammad, N.; Hossain, M.I.; Man, Z.; El-Harbawi, M.; Bustam, M.A.; Noaman, Y.A.; Mohamed Alitheen, N.B.; Ng, M.K.; Hefter, G.; Yin, C.Y. Synthesis and Physical Properties of Choline Carboxylate Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2191–2196. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Mourao, T.; Tome, L.C.; Florindo, C.; Rebelo, L.P.N.; Marrucho, I.M. Understanding the Role of Cholinium Carboxylate Ionic Liquids in PEG-Based Aqueous Biphasic Systems. ACS Sustain. Chem. Eng. 2014, 2, 2426–2434. [Google Scholar] [CrossRef]
- Zhang, Y.; Maginn, E.J. A comparison of methods for melting point calculation using molecular dynamics simulations. J. Chem. Phys. 2012, 136, 144116. [Google Scholar] [CrossRef]
- Eike, D.M.; Maginn, E.J. Atomistic simulation of solid-liquid coexistence for molecular systems: Application to triazole and benzene. J. Chem. Phys. 2006, 124, 164503. [Google Scholar] [CrossRef]
- Eike, D.M.; Brennecke, J.F.; Maginn, E.J. Toward a robust and general molecular simulation method for computing solid-liquid coexistence. J. Chem. Phys. 2005, 122, 014115. [Google Scholar] [CrossRef]
- Pereira, J.F.; Barber, P.S.; Kelley, S.P.; Berton, P.; Rogers, R.D. Double salt ionic liquids based on 1-ethyl-3-methylimidazolium acetate and hydroxyl-functionalized ammonium acetates: Strong effects of weak interactions. Phys. Chem. Chem. Phys. 2017, 19, 26934–26943. [Google Scholar] [CrossRef]
- Brünig, T.; Krekić, K.; Bruhn, C.; Pietschnig, R. Calorimetric Studies and Structural Aspects of Ionic Liquids in Designing Sorption Materials for Thermal Energy Storage. Chem. Eur. J. 2016, 22, 16200–16212. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Driesen, K.; Janssen, C.R.; Van Hecke, K.; Van Meervelt, L.; Kossmann, S.; Kirchner, B.; Binnemans, K. Choline Saccharinate and Choline Acesulfamate: Ionic Liquids with Low Toxicities. J. Phys. Chem. B 2007, 111, 5254–5263. [Google Scholar] [CrossRef]
- Villanueva, M.; Parajo, J.; Sanchez, P.B.; Garcia, J.; Salgado, J. Liquid range temperature of ionic liquids as potential working fluids for absorption heat pumps. J. Chem. Thermodyn. 2015, 91, 127–135. [Google Scholar] [CrossRef]
- Karu, K. GitHub Repository of the Force Fields and Workflow Used in: Predicting Melting Points of Biofriendly Choline-Based Ionic Liquids with Molecular Dynamics. Available online: https://github.com/vilab-tartu/MP-FFs (accessed on 12 October 2019).
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Sambasivarao, S.V.; Acevedo, O. Development of OPLS-AA Force Field Parameters for 68 Unique Ionic Liquids. J. Chem. Theory Comput. 2009, 5, 1038–1050. [Google Scholar] [CrossRef]
- Canongia Lopes, J.N.; Deschamps, J.; Padua, A.A.H. Modeling Ionic Liquids Using a Systematic All-Atom Force Field. J. Phys. Chem. B 2004, 108, 2038–2047. [Google Scholar] [CrossRef]
- Domanski, J.; Beckstein, O.; Iorga, B.I. Ligandbook—An online repository for small and drug-like molecule force field parameters. Bioinformatics 2017, 33, 1747–1749. [Google Scholar] [CrossRef]
- Rigby, J.; Izgorodina, E.I. Assessment of atomic partial charge schemes for polarisation and charge transfer effects in ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 1632–1646. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Zahn, S.; MacFarlane, D.; Izgorodina, E.I. Assessment of Kohn-Sham Density Functional Theory and Moller-Plesset Perturbation Theory for Ionic Liquids. Phys. Chem. Chem. Phys. 2013, 15, 13664–13675. [Google Scholar] [CrossRef] [PubMed]
- Karu, K.; Mišin, M.; Ers, H.; Sun, J.; Ivaništšev, V. Performance of SCAN density functional for a set of ionic liquid ion pairs. Int. J. Quantum Chem. 2018, 118, e25582. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Rengstl, D.; Fischer, V.; Kunz, W. Low-melting mixtures based on choline ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 22815–22822. [Google Scholar] [CrossRef]
- Diogo, H.P.; Moura Ramos, J.J. Study of the thermal behavior of choline ibuprofenate using differential scanning calorimetry and hot-stage microscopy. J. Mol. Struct. 2014, 1078, 174–180. [Google Scholar] [CrossRef]
- Wolf, J.; Aboody, R. Choline salicylate: A new and more rapidly absorbed durg for salicylate therapy. Int. Record. Med. 1960, 173, 234–241. [Google Scholar]
- Schröder, C. Comparing reduced partial charge models with polarizable simulations of ionic liquids. Phys. Chem. Chem. Phys. 2012, 14, 3089–3102. [Google Scholar] [CrossRef]
- Lage-Estebanez, I.; Ruzanov, A.; de la Vega, J.M.G.; Fedorov, M.V.; Ivaništšev, V.B. Self-interaction error in DFT-based modelling of ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 2175–2182. [Google Scholar] [CrossRef]
- Karu, K.; Ruzanov, A.; Ers, H.; Ivaništšev, V.; Lage-Estebanez, I.; de la Vega, J.M.G. Predictions of Physicochemical Properties of Ionic Liquids with DFT. Computation 2016, 4, 25. [Google Scholar] [CrossRef]
- Dommert, F.; Wendler, K.; Berger, R.; Delle Site, L.; Holm, C. Force Fields for Studying the Structure and Dynamics of Ionic Liquids: A Critical Review of Recent Developments. ChemPhysChem 2012, 13, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, B.; Malberg, F.; Firaha, D.S.; Hollóczki, O. Ion pairing in ionic liquids. J. Phys. Condens. Matter. 2015, 27, 463002. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, J.S.; Rodriguez, F.; Bravo, J.L.; Rothenberg, G.; Seddon, K.R.; Lopez-Martin, I. Optimising an artificial neural network for predicting the melting point of ionic liquids. Phys. Chem. Chem. Phys. 2008, 10, 5826–5831. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Jain, R.; Lomaka, A.; Petrukhin, R.; Karelson, M.; Visser, A.E.; Rogers, R.D. Correlation of the Melting Points of Potential Ionic Liquids (Imidazolium Bromides and Benzimidazolium Bromides) Using the CODESSA Program. J. Chem. Inf. Comput. Sci. 2002, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Lomaka, A.; Petrukhin, R.; Jain, R.; Karelson, M.; Visser, A.E.; Rogers, R.D. QSPR Correlation of the Melting Point for Pyridinium Bromides, Potential Ionic Liquids. J. Chem. Inf. Comput. Sci. 2002, 42, 71–74. [Google Scholar] [CrossRef]
- Varnek, A.; Kireeva, N.; Tetko, I.V.; Baskin, I.I.; Solovev, V.P. Exhaustive QSPR Studies of a Large Diverse Set of Ionic Liquids: How Accurately Can We Predict Melting Points? J. Chem. Inf. Model. 2007, 47, 1111–1122. [Google Scholar] [CrossRef]
- Trohalaki, S.; Pachter, R. Prediction of Melting Points for Ionic Liquids. QSAR Comb. Sci. 2005, 24, 485–490. [Google Scholar] [CrossRef]
- Venkatraman, V.; Evjen, S.; Knuutila, H.K.; Fiksdahl, A.; Alsberg, B.K. Predicting ionic liquid melting points using machine learning. J. Mol. Liq. 2018, 264, 318–326. [Google Scholar] [CrossRef]
- Eike, D.M.; Brennecke, J.F.; Maginn, E.J. Predicting melting points of quaternary ammonium ionic liquids. Green Chem. 2003, 5, 323–328. [Google Scholar] [CrossRef]
- Preiss, U.; Bulut, S.; Krossing, I. In Silico Prediction of the Melting Points of Ionic Liquids from Thermodynamic Considerations: A Case Study on 67 Salts with a Melting Point Range of 337 C. J. Phys. Chem. B 2010, 114, 11133–11140. [Google Scholar] [CrossRef] [PubMed]
- Lazzus, J.A. A group contribution method to predict the melting point of ionic liquids. Fluid Phase Equilib. 2012, 313, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Maginn, E.J. The effect of C2 substitution on melting point and liquid phase dynamics of imidazolium based-ionic liquids: Insights from molecular dynamics simulations. Phys. Chem. Chem. Phys. 2012, 14, 12157–12164. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.N.; Ahrens, T.J. Superheating systematics of crystalline solids. Appl. Phys. Lett. 2003, 82, 1836–1838. [Google Scholar] [CrossRef]
- Phillpot, S.R.; Lutsko, J.F.; Wolf, D.; Yip, S. Molecular-dynamics study of lattice-defect-nucleated melting in silicon. Phys. Rev. B 1989, 40, 2831–2840. [Google Scholar] [CrossRef]
- Lutsko, J.F.; Wolf, D.; Phillpot, S.R.; Yip, S. Molecular-dynamics study of lattice-defect-nucleated melting in metals using an embedded-atom-method potential. Phys. Rev. B 1989, 40, 2841–2855. [Google Scholar] [CrossRef]
- Morris, J.R.; Wang, C.Z.; Ho, K.M.; Chan, C.T. Melting line of aluminum from simulations of coexisting phases. Phys. Rev. B 1994, 49, 3109–3115. [Google Scholar] [CrossRef]
- Mondal, A.; Balasubramanian, S. Quantitative Prediction of Physical Properties of Imidazolium Based Room Temperature Ionic Liquids through Determination of Condensed Phase Site Charges: A Refined Force Field. J. Phys. Chem. B 2014, 118, 3409–3422. [Google Scholar] [CrossRef]
- Gieldon, A.; Bobrowski, M.; Bielicka-Gieldon, A.; Czaplewski, C. Theoretical calculation of the physico-chemical properties of 1-butyl-4-methylpyridinium based ionic liquids. J. Mol. Liq. 2017, 225, 467–474. [Google Scholar] [CrossRef]
- Zhang, Y.; Maginn, E.J. A Simple AIMD Approach to Derive Atomic Charges for Condensed Phase Simulation of Ionic Liquids. J. Phys. Chem. B 2012, 116, 10036–10048. [Google Scholar] [CrossRef]
- Schroder, C.; Rudas, T.; Neumayr, G.; Benkner, S.; Steinhauser, O. On the collective network of ionic liquid/water mixtures. I. Orientational structure. J. Chem. Phys. 2007, 127, 234503. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, P.; Tobias, D.J. Ions at the Air/Water Interface. J. Phys. Chem. B 2002, 106, 6361–6373. [Google Scholar] [CrossRef]
- Jungwirth, P.; Winter, B. Ions at Aqueous Interfaces: From Water Surface to Hydrated Proteins. Annu. Rev. Phys. Chem. 2008, 59, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Minofar, B.; Vácha, R.; Wahab, A.; Mahiuddin, S.; Kunz, W.; Jungwirth, P. Propensity for the Air/Water Interface and Ion Pairing in Magnesium Acetate vs Magnesium Nitrate Solutions: Molecular Dynamics Simulations and Surface Tension Measurements. J. Phys. Chem. B 2006, 110, 15939–15944. [Google Scholar] [CrossRef]
- Petersen, P.B.; Saykally, R.J.; Mucha, M.; Jungwirth, P. Enhanced Concentration of Polarizable Anions at the Liquid Water Surface: SHG Spectroscopy and MD Simulations of Sodium Thiocyanide. J. Phys. Chem. B 2005, 109, 10915–10921. [Google Scholar] [CrossRef]
- Picálek, J.; Minofar, B.; Kolafa, J.; Jungwirth, P. Aqueous solutions of ionic liquids: Study of the solution/vapor interface using molecular dynamics simulations. Phys. Chem. Chem. Phys. 2008, 10, 5765. [Google Scholar] [CrossRef]
- Bedrov, D.; Piquemal, J.P.; Borodin, O.; MacKerell, A.D.; Roux, B.; Schröder, C. Molecular Dynamics Simulations of Ionic Liquids and Electrolytes Using Polarizable Force Fields. Chem. Rev. 2019, 119, 7940–7995. [Google Scholar] [CrossRef]
- Salanne, M. Simulations of room temperature ionic liquids: From polarizable to coarse-grained force fields. Phys. Chem. Chem. Phys. 2015, 17, 14270–14279. [Google Scholar] [CrossRef]
- Pádua, A.A.H. Resolving dispersion and induction components for polarisable molecular simulations of ionic liquids. J. Chem. Phys. 2017, 146, 204501. [Google Scholar] [CrossRef]
- Bernardes, C.E.S.; Shimizu, K.; Lopes, J.N.C.; Marquetand, P.; Heid, E.; Steinhauser, O.; Schröder, C. Additive polarizabilities in ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 1665–1670. [Google Scholar] [CrossRef]
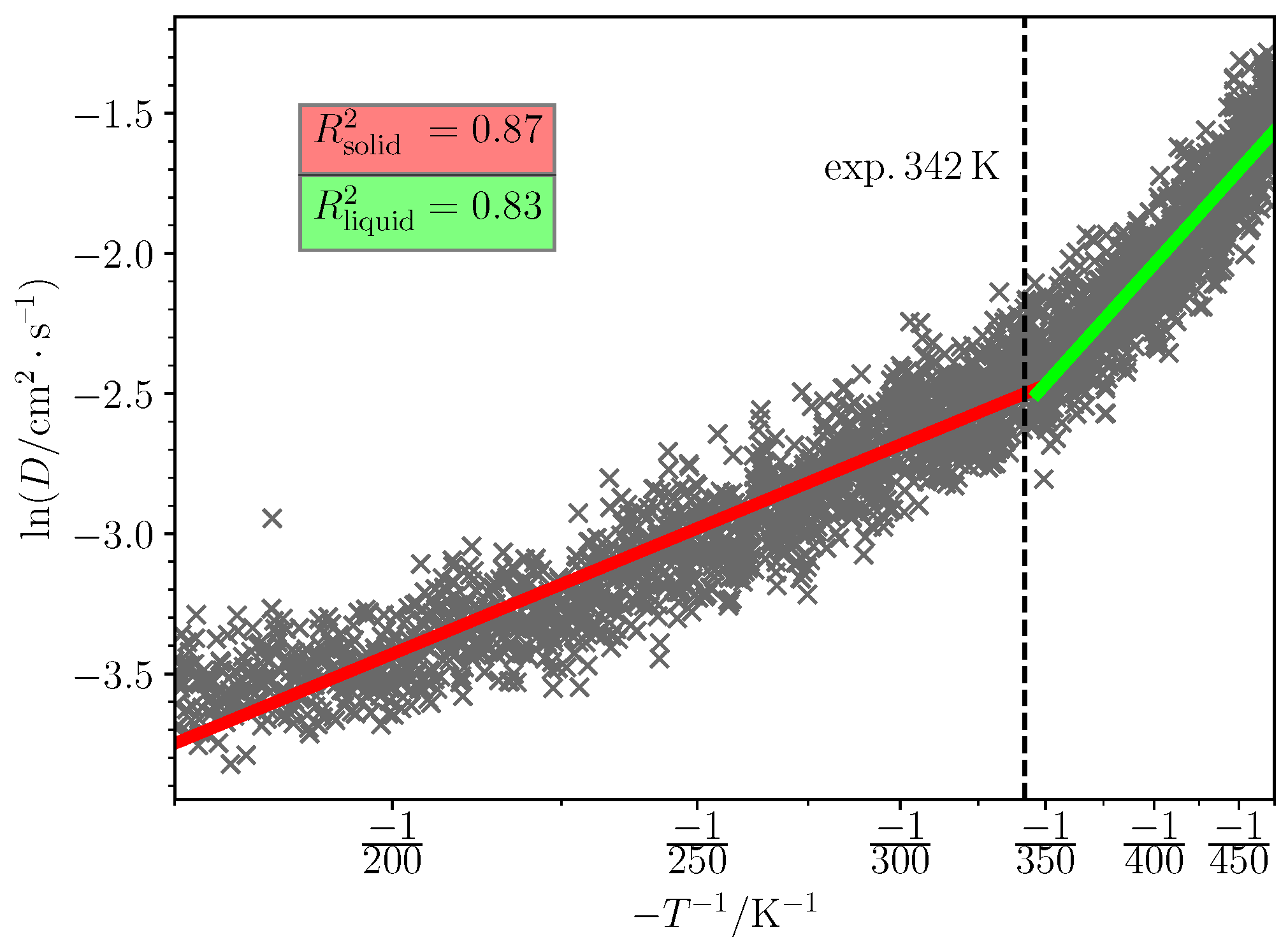
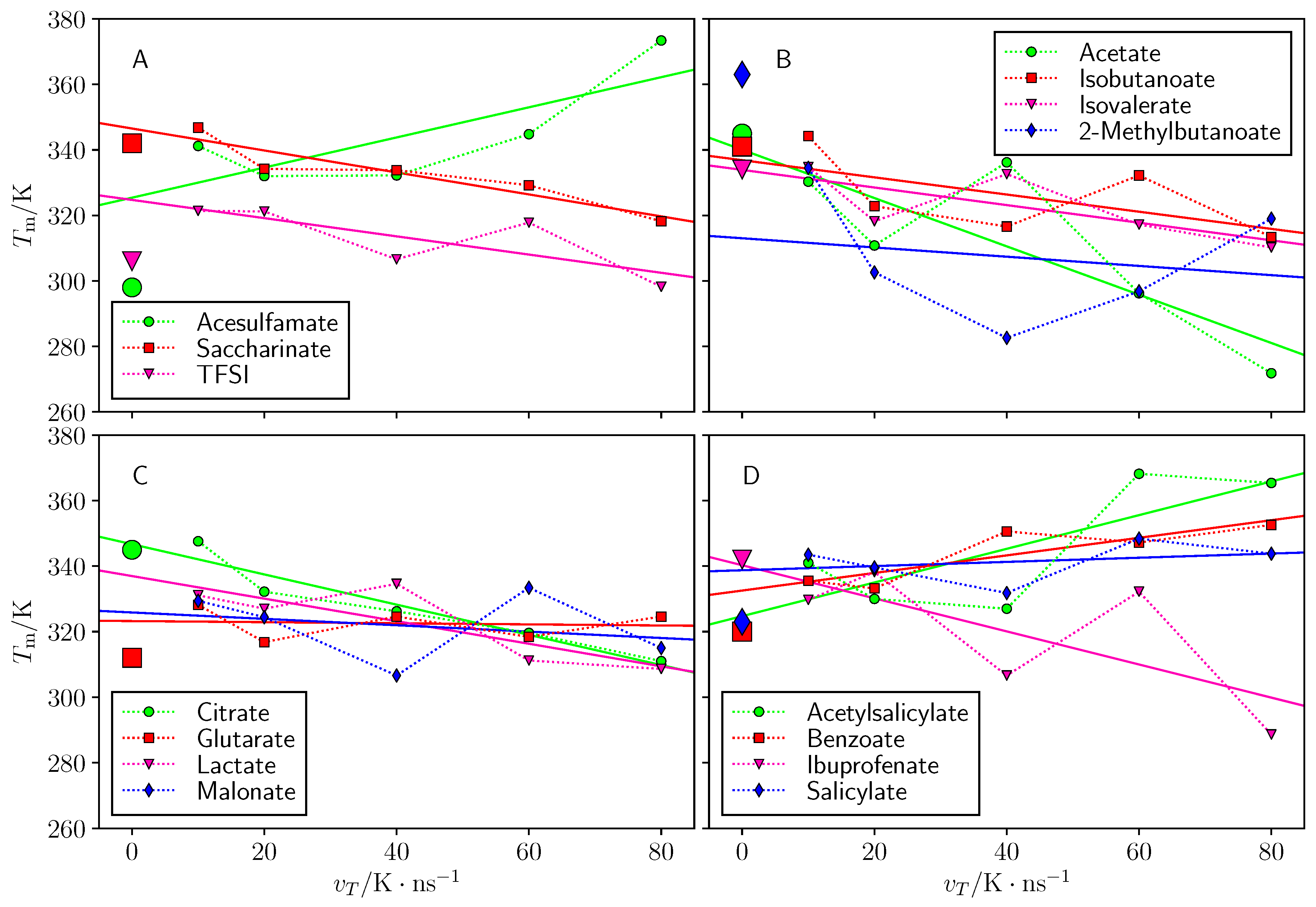
| Cation: Choline Anion | (K) | (K) | T Range (K) | Water Content (%) |
|---|---|---|---|---|
| Acetate | 208 | 354 | 173–373 | 2.66 |
| Citrate | n/d | 378 | 323–423 | <0.01 |
| Glutarate | 215 | n/d | 173–373 | 0.02 |
| Isobutanoate | 207 | 341 | 173–373 | 0.14 |
| Lactate | 211 | n/d | 173–373 | 0.77 |
| Isovalerate | n/d | 334 | 323–423 | 0.02 |
| Malonate | 206 | n/d | 173–373 | 0.24 |
| 2-Methylbutanoate | 212 | 363 | 173–373 | 0.25 |
| Cation: Choline Anion | Experimental (K) | Predicted (K) | ΔTM (K) |
|---|---|---|---|
| Acesulfamate | 298 [23] | 341 | 43 |
| Acetate | 353 [11], 324 [14], 345 [15], | 330 | |
| Benzoate | 320 [14] | 335 | 15 |
| Citrate | 345 [16], 376 [17], 378 | 348 | |
| Glutarate | 312 [38] | 328 | 16 |
| Ibuprofenate | 342 [39] | 330 | |
| Isobutanoate | 308 [11], 341 | 344 | |
| Isovalerate | 334 | 335 | 1 |
| 2-Methylbutanoate | 363 | 335 | |
| Saccharinate | 342 [23] | 347 | 5 |
| Salicylate | 323 [40] | 343 | 20 |
| TFSI | 306 [24] | 321 | 15 |
| Acetylsalicylate | n/a | 341 | n/a |
| Lactate | n/a | 331 | n/a |
| Malonate | n/a | 329 | n/a |
| Cation: Choline Anion | Experimental (K) | DFT (K) | DFT 0.8 (K) | GFN2-xTB (K) |
|---|---|---|---|---|
| Acetate | 359 | 354 | 330 | |
| Benzoate | 320 [14] | 381 | 365 | 335 |
| Salicylate | 323 [40] | 377 | 364 | 343 |
| TFSI | 306 [24] | 348 | 345 | 321 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karu, K.; Elhi, F.; Põhako-Esko, K.; Ivaništšev, V. Predicting Melting Points of Biofriendly Choline-Based Ionic Liquids with Molecular Dynamics. Appl. Sci. 2019, 9, 5367. https://doi.org/10.3390/app9245367
Karu K, Elhi F, Põhako-Esko K, Ivaništšev V. Predicting Melting Points of Biofriendly Choline-Based Ionic Liquids with Molecular Dynamics. Applied Sciences. 2019; 9(24):5367. https://doi.org/10.3390/app9245367
Chicago/Turabian StyleKaru, Karl, Fred Elhi, Kaija Põhako-Esko, and Vladislav Ivaništšev. 2019. "Predicting Melting Points of Biofriendly Choline-Based Ionic Liquids with Molecular Dynamics" Applied Sciences 9, no. 24: 5367. https://doi.org/10.3390/app9245367
APA StyleKaru, K., Elhi, F., Põhako-Esko, K., & Ivaništšev, V. (2019). Predicting Melting Points of Biofriendly Choline-Based Ionic Liquids with Molecular Dynamics. Applied Sciences, 9(24), 5367. https://doi.org/10.3390/app9245367