Cultivation of hMSCs in Human Plasma Prevents the Cytotoxic and Genotoxic Potential of ZnO-NP In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media Preparation for Cell Culture
2.1.1. Plasma Isolation from Human Peripheral Blood
2.1.2. Isolation and Culture of Human Mesenchymal Stem Cells
2.2. Chemicals
2.3. Characterization of Nanoparticles
3. Cell Treatment
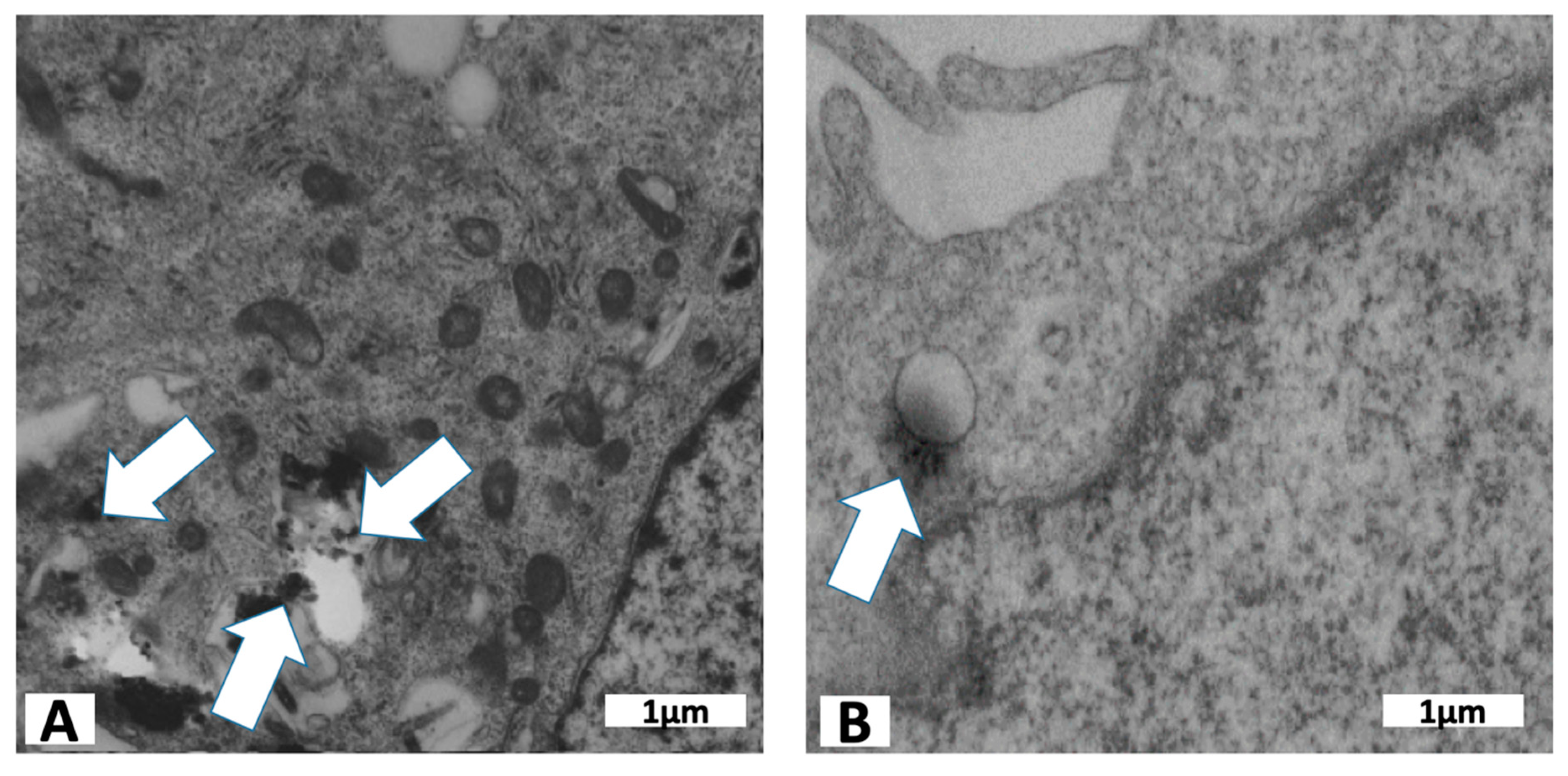
3.1. Cell Preparation for TEM
3.2. Measurement of Cell Death
3.3. Alkaline Single-Cell Microgel Electrophoresis (Comet) Assay
3.4. Measurement of Protein and Zn2+ Concentrations in Plasma and DMEM-EM
3.5. Statistical Analysis
4. Results
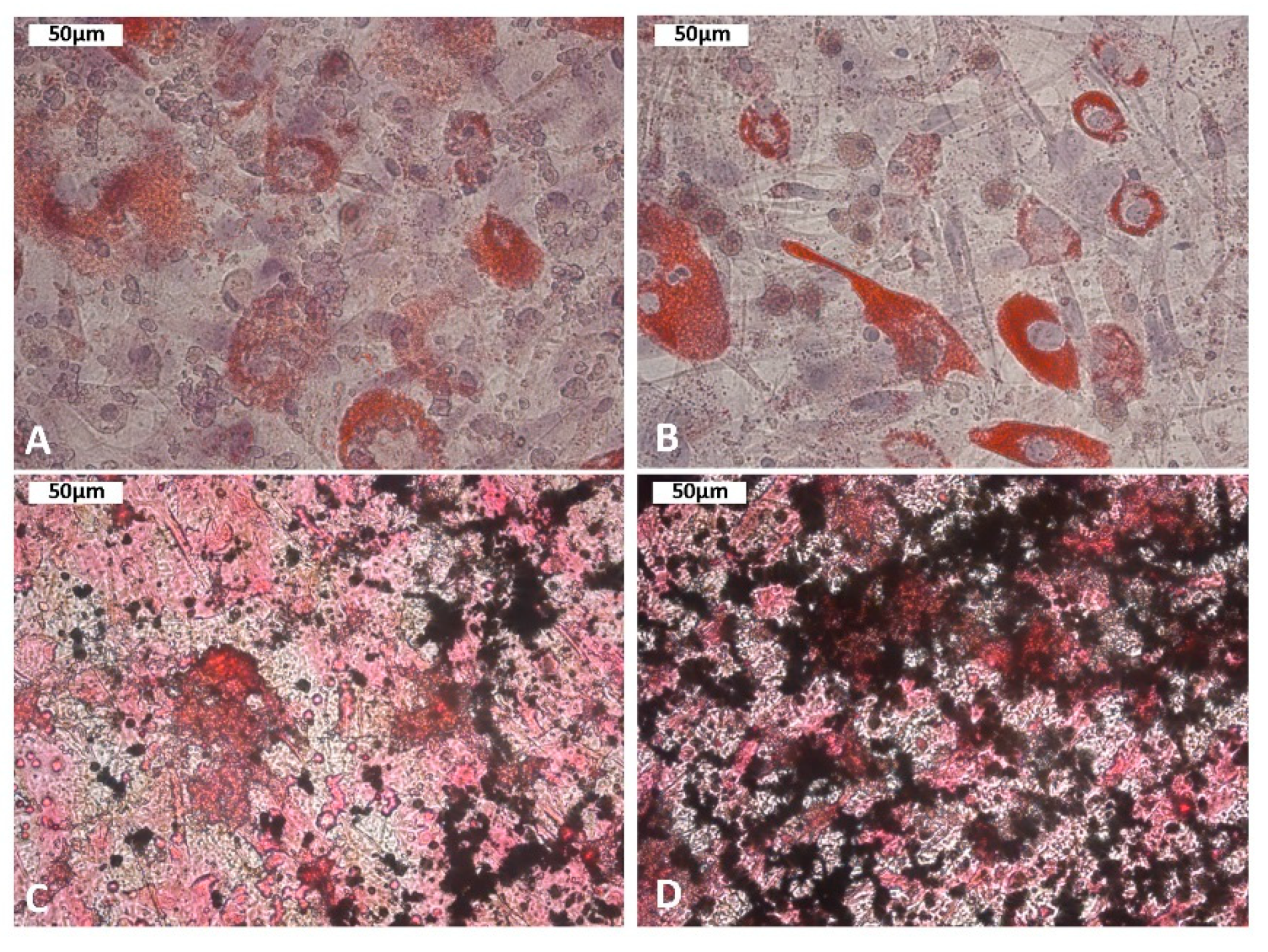
4.1. Isolation and Characterization of MSC
4.2. Analysis of MSC Proliferation
4.3. Particle Characterization
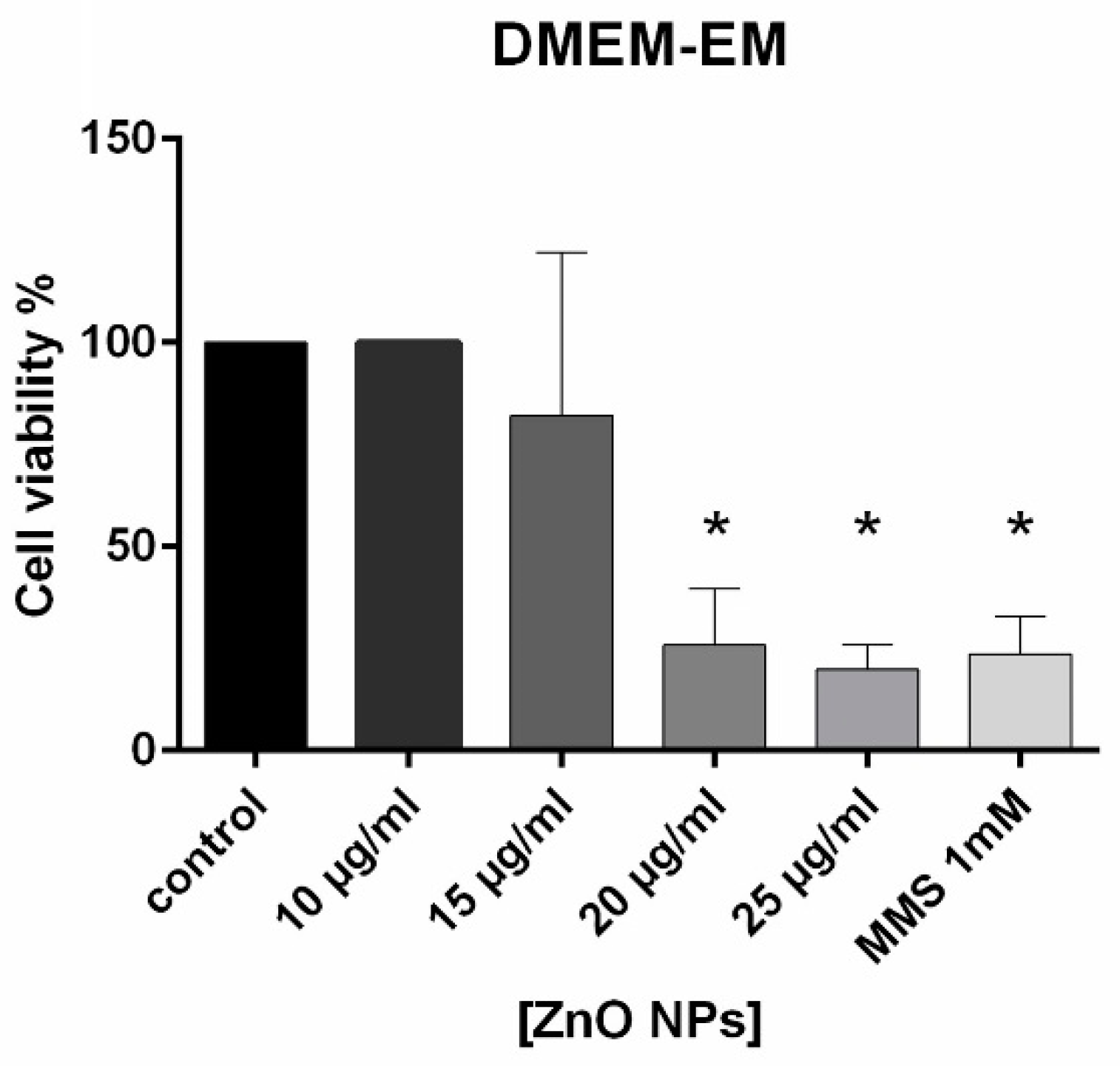
4.4. Cell Viability
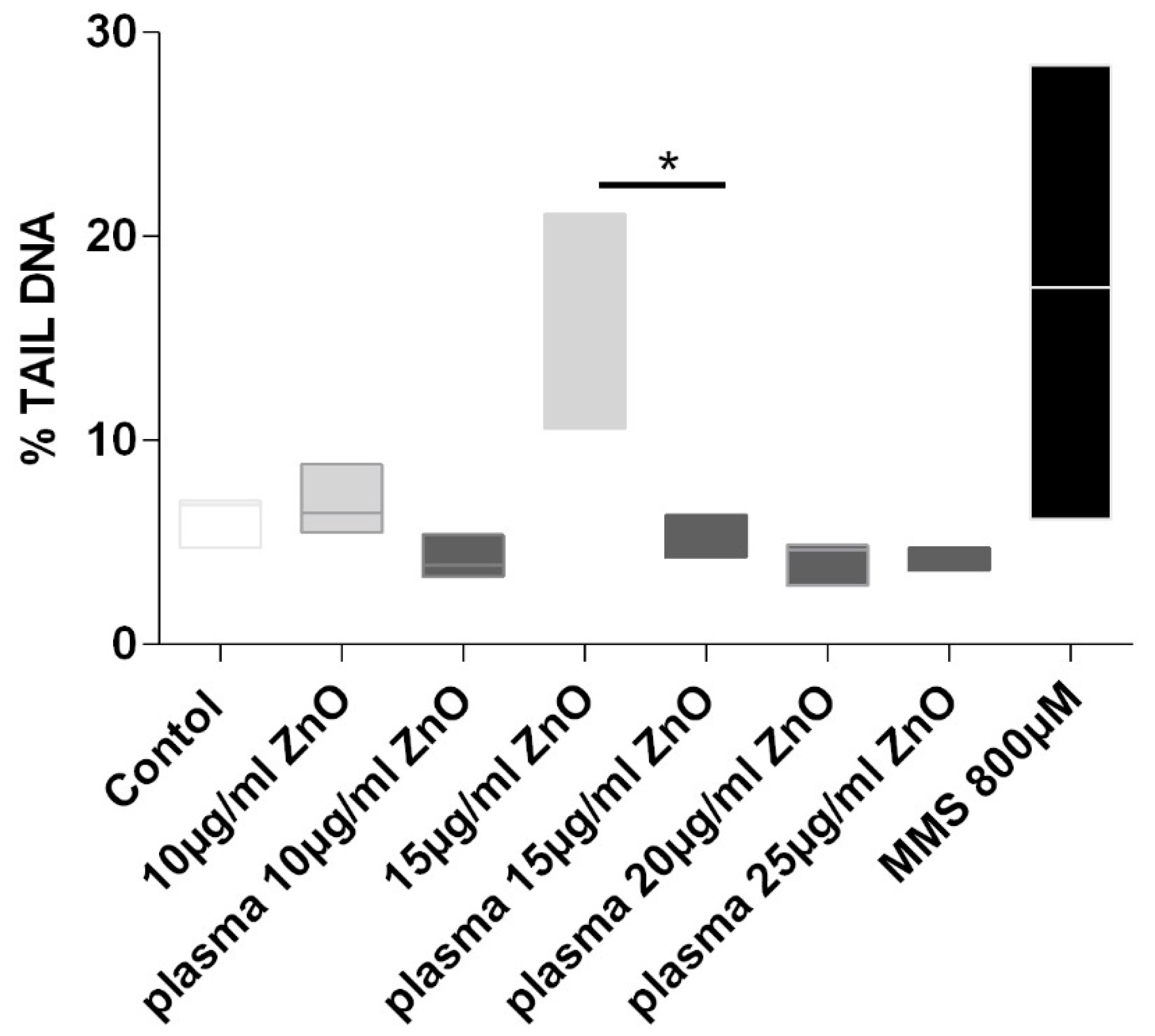
4.5. Genotoxicity
4.6. Measurement of Protein and Zn2+ Concentration in Plasma and DMEM-EM
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Englert, B.C. Nanomaterials and the environment: Uses, methods and measurement. J. Environ. Monit. JEM 2007, 9, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Magaye, R.; Castranova, V.; Zhao, J.S. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Klingshirn, C. ZnO: Material, physics and applications. Chemphyschem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Vermylen, J.; Nemmar, A.; Nemery, B.; Hoylaerts, M.F. Ambient air pollution and acute myocardial infarction. J. Thromb. Haemost. JTH 2005, 3, 1955–1961. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. In Vitro 2011, 25, 657–663. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Huang, Y.J. Effects of serum on cytotoxicity of nano- and micro-sized ZnO particles. J. Nanopart Res. 2013, 15, 1829. [Google Scholar] [CrossRef]
- Frolich, K.; Scherzed, A.; Mlynski, R.; Technau, A.; Hagen, R.; Kleinsasser, N.; Radeloff, A. Multipotent stromal cells for autologous cell therapy approaches in the guinea pig model. ORL J. Oto Rhino Laryngol. Its Relat. Spec. 2011, 73, 9–16. [Google Scholar] [CrossRef]
- Scherzad, A.; Hackenberg, S.; Froelich, K.; Rak, K.; Hagen, R.; Taeger, J.; Bregenzer, M.; Kleinsasser, N. Chronic exposure of low dose salinomycin inhibits MSC migration capability in vitro. Biomed. Rep. 2016, 4, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Scherzad, A.; Ickrath, P.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N.; Hackenberg, S. Zinc oxide nanoparticles antagonize the effect of Cetuximab on head and neck squamous cell carcinoma in vitro. Cancer Biol. Ther. 2017, 18, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Ickrath, P.; Wagner, M.; Scherzad, A.; Gehrke, T.; Burghartz, M.; Hagen, R.; Radeloff, K.; Kleinsasser, N.; Hackenberg, S. Time-Dependent Toxic and Genotoxic Effects of Zinc Oxide Nanoparticles after Long-Term and Repetitive Exposure to Human Mesenchymal Stem Cells. Int. J. Environ. Res. Public Health 2017, 14, 1590. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Lee, S.B.; Cho, S.G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 761. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Visser, W.H.; Bakowsky, U.; Engberts, J.B.; Hoekstra, D. Interference of serum with lipoplex-cell interaction: Modulation of intracellular processing. Biochim. Biophys. Acta 2002, 1560, 25–36. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Huang, Y.J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO nanoparticles toward human lung epithelial cells. Sci. Total Environ. 2011, 409, 1219–1228. [Google Scholar] [CrossRef]
- Du, X.J.; Wang, J.L.; Iqbal, S.; Li, H.J.; Cao, Z.T.; Wang, Y.C.; Du, J.Z.; Wang, J. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater. Sci. 2018, 6, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Nakanishi, T.; Shanmugam, S.; Takahama, S.; Zhang, H. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf. B Biointerfaces 2009, 71, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Massignani, M.; LoPresti, C.; Blanazs, A.; Madsen, J.; Armes, S.P.; Lewis, A.L.; Battaglia, G. Controlling cellular uptake by surface chemistry, size, and surface topology at the nanoscale. Small 2009, 5, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Froelich, K.; Hagen, R.; Kleinsasser, N. Functional responses of human adipose tissue-derived mesenchymal stem cells to metal oxide nanoparticles in vitro. J. Biomed. Nanotechnol. 2013, 9, 86–95. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Bartczak, D.; Nitti, S.; Millar, T.M.; Kanaras, A.G. Exocytosis of peptide functionalized gold nanoparticles in endothelial cells. Nanoscale 2012, 4, 4470–4472. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm. Res. 2003, 20, 212–220. [Google Scholar] [CrossRef]



| Protein and Zn2+ Concentration in Plasma and DMEM-EM | ||
|---|---|---|
| ZnO (µg/mL) | DMEM-EM ng/mL | Plasma ng/mL |
| 0 | 0 | 9.9 |
| 10 | 121.9 | 122.2 |
| 15 | 130.7 | 170.4 |
| 20 | 150.5 | 242.4 |
| 25 | 171.1 | 396.5 |
| Protein µg/µL | DMEM-EM | Plasma |
| 10.6 | 54.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherzad, A.; Meyer, T.; Ickrath, P.; Gehrke, T.E.; Bregenzer, M.; Hagen, R.; Dembski, S.; Hackenberg, S. Cultivation of hMSCs in Human Plasma Prevents the Cytotoxic and Genotoxic Potential of ZnO-NP In Vitro. Appl. Sci. 2019, 9, 4994. https://doi.org/10.3390/app9234994
Scherzad A, Meyer T, Ickrath P, Gehrke TE, Bregenzer M, Hagen R, Dembski S, Hackenberg S. Cultivation of hMSCs in Human Plasma Prevents the Cytotoxic and Genotoxic Potential of ZnO-NP In Vitro. Applied Sciences. 2019; 9(23):4994. https://doi.org/10.3390/app9234994
Chicago/Turabian StyleScherzad, Agmal, Till Meyer, Pascal Ickrath, Thomas Eckhart Gehrke, Maximillian Bregenzer, Rudolf Hagen, Sofia Dembski, and Stephan Hackenberg. 2019. "Cultivation of hMSCs in Human Plasma Prevents the Cytotoxic and Genotoxic Potential of ZnO-NP In Vitro" Applied Sciences 9, no. 23: 4994. https://doi.org/10.3390/app9234994
APA StyleScherzad, A., Meyer, T., Ickrath, P., Gehrke, T. E., Bregenzer, M., Hagen, R., Dembski, S., & Hackenberg, S. (2019). Cultivation of hMSCs in Human Plasma Prevents the Cytotoxic and Genotoxic Potential of ZnO-NP In Vitro. Applied Sciences, 9(23), 4994. https://doi.org/10.3390/app9234994






