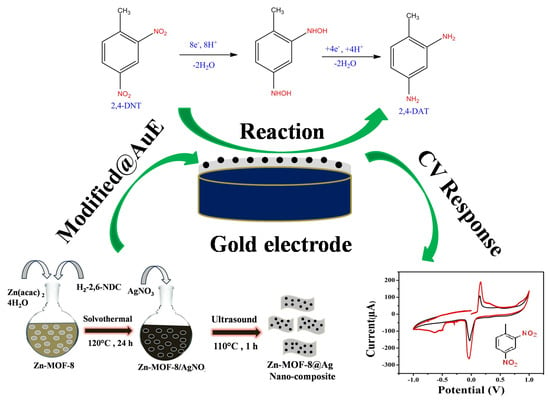
Construction of Silver Quantum Dot Immobilized Zn-MOF-8 Composite for Electrochemical Sensing of 2,4-Dinitrotoluene
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
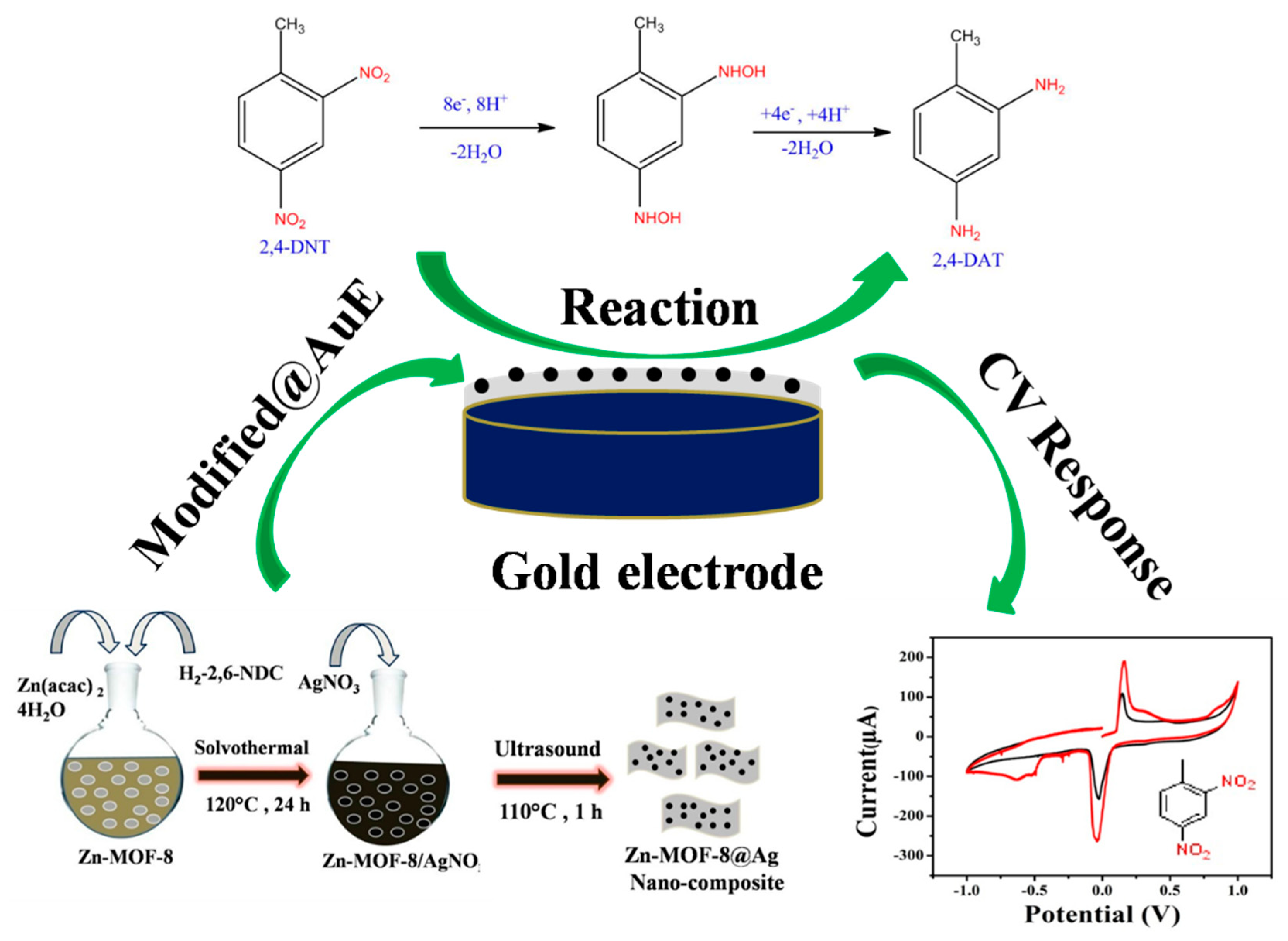
2.3. Synthesis of Nanostructure Zn-MOF-8 and Their Composites with Silver Quantum Dot Particles
2.4. Fabrication of Au/Zn-MOF-8@AgQDs Composite Modified Electrode
3. Results and Discussion
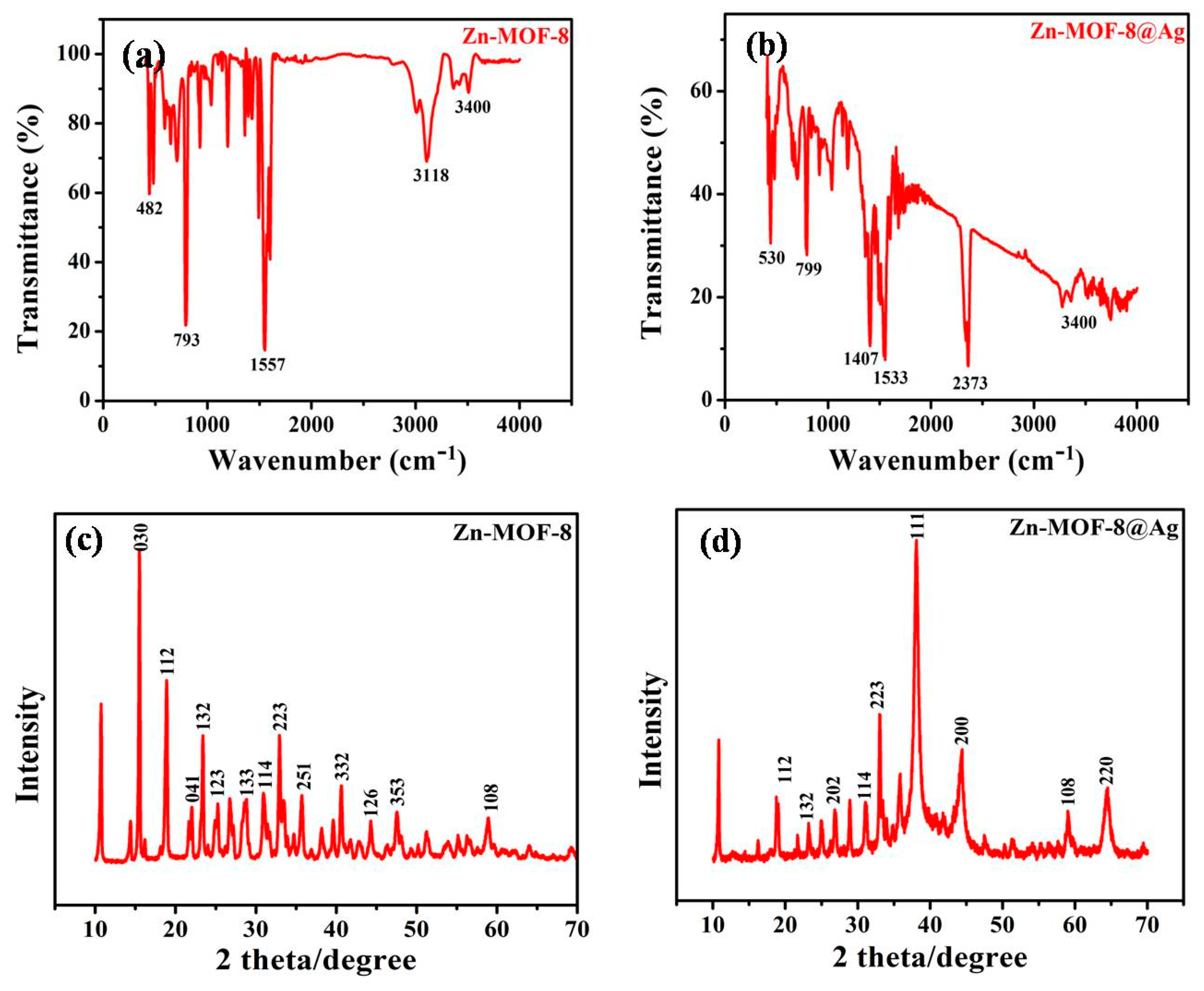
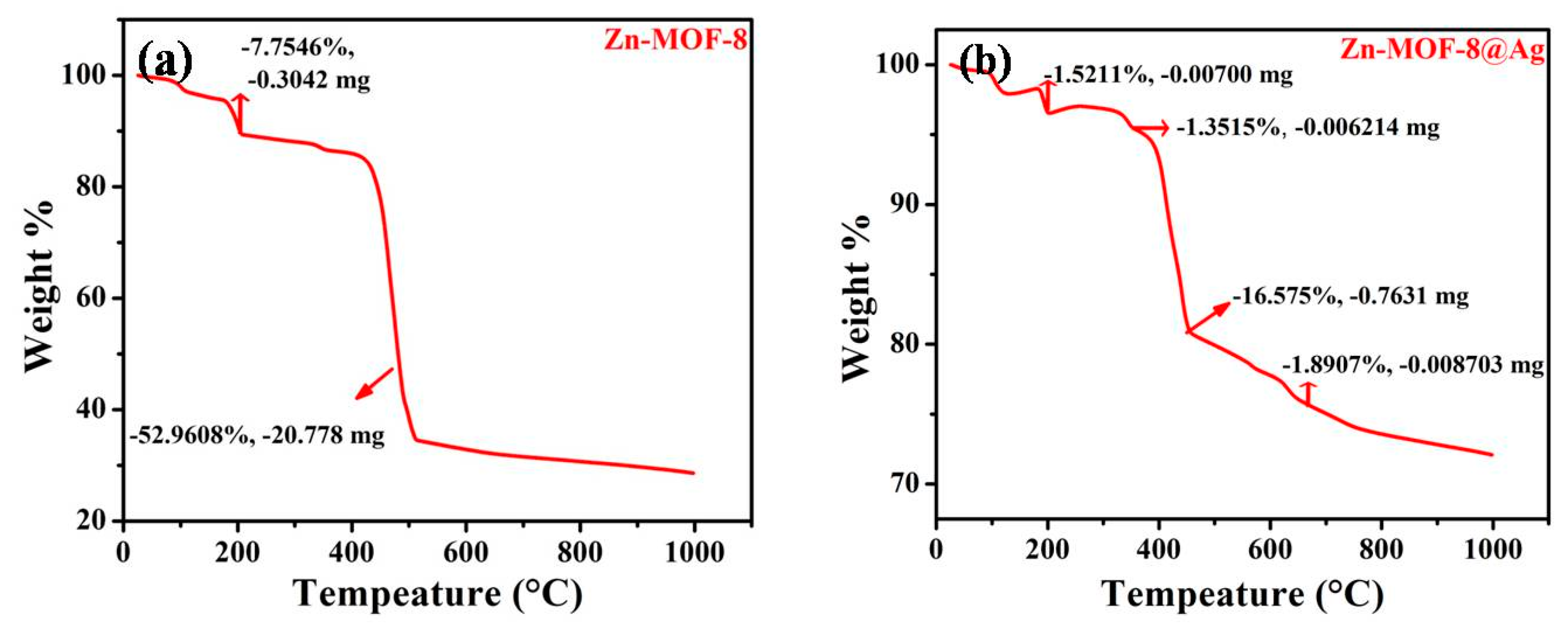
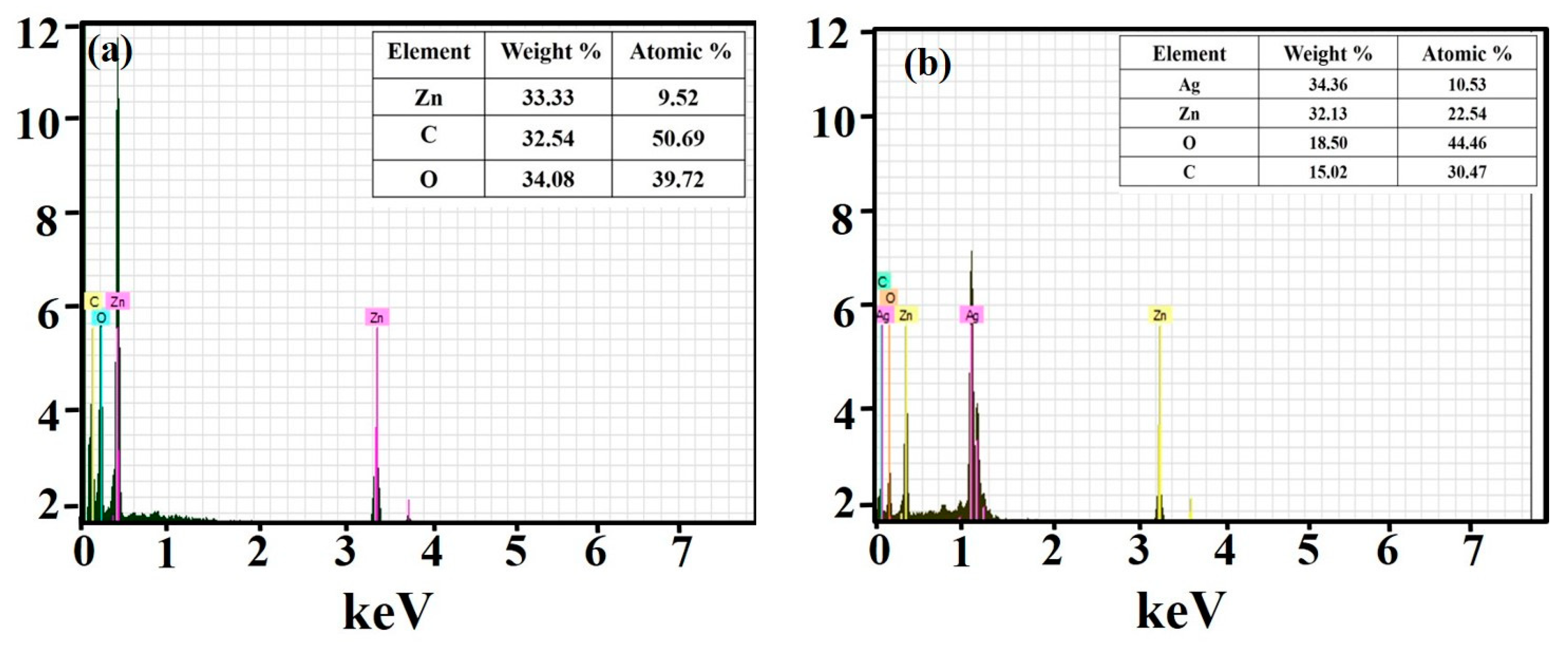
3.1. Characterization of Zn-MOF-8@AgQDs Composite
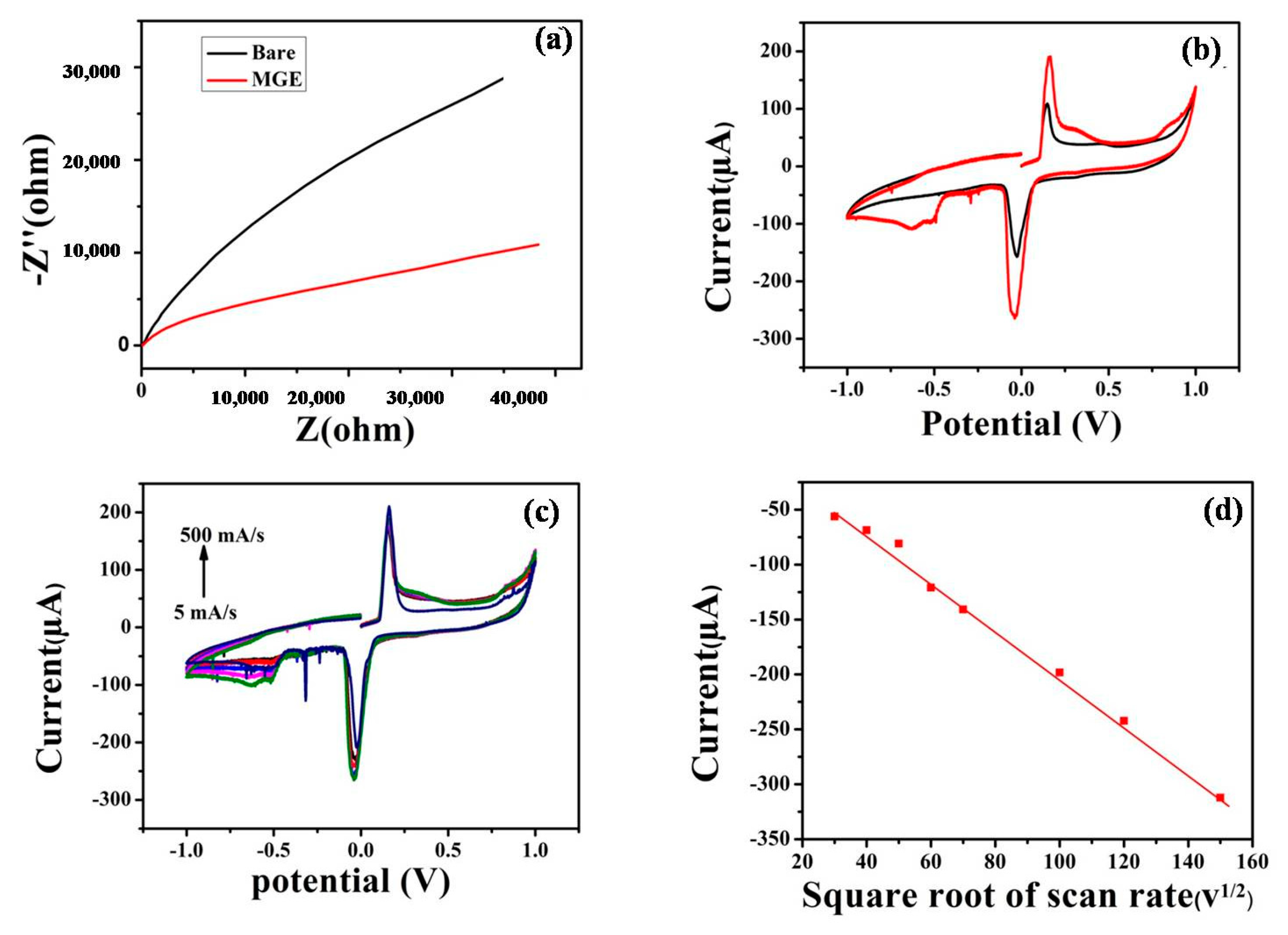
3.2. Electrochemical Impedance Spectroscopy (EIS) Study
3.3. The Electrocatalytic Reduction and Detection of 2,4-Dinitrotoluene
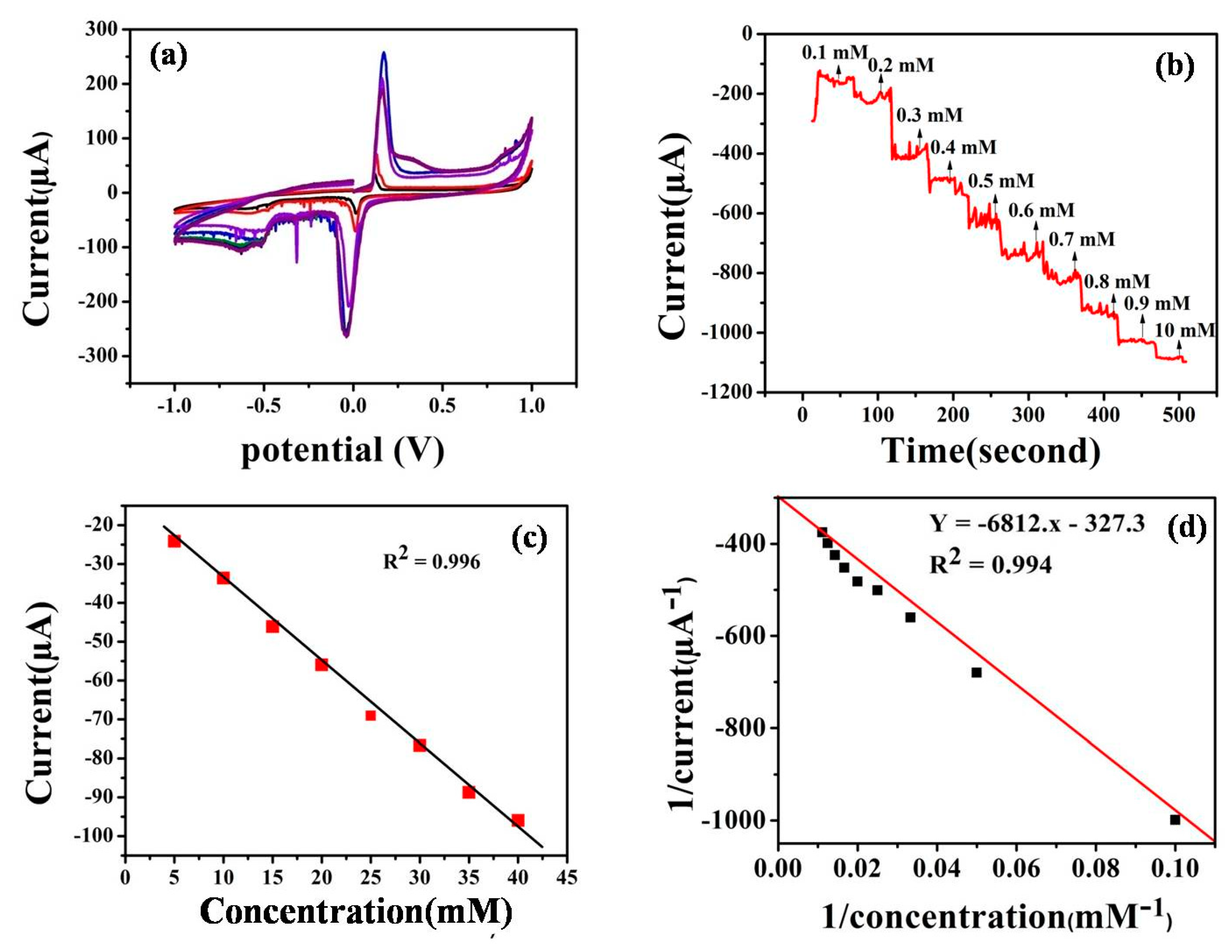
3.3.1. Cyclic Voltammetry
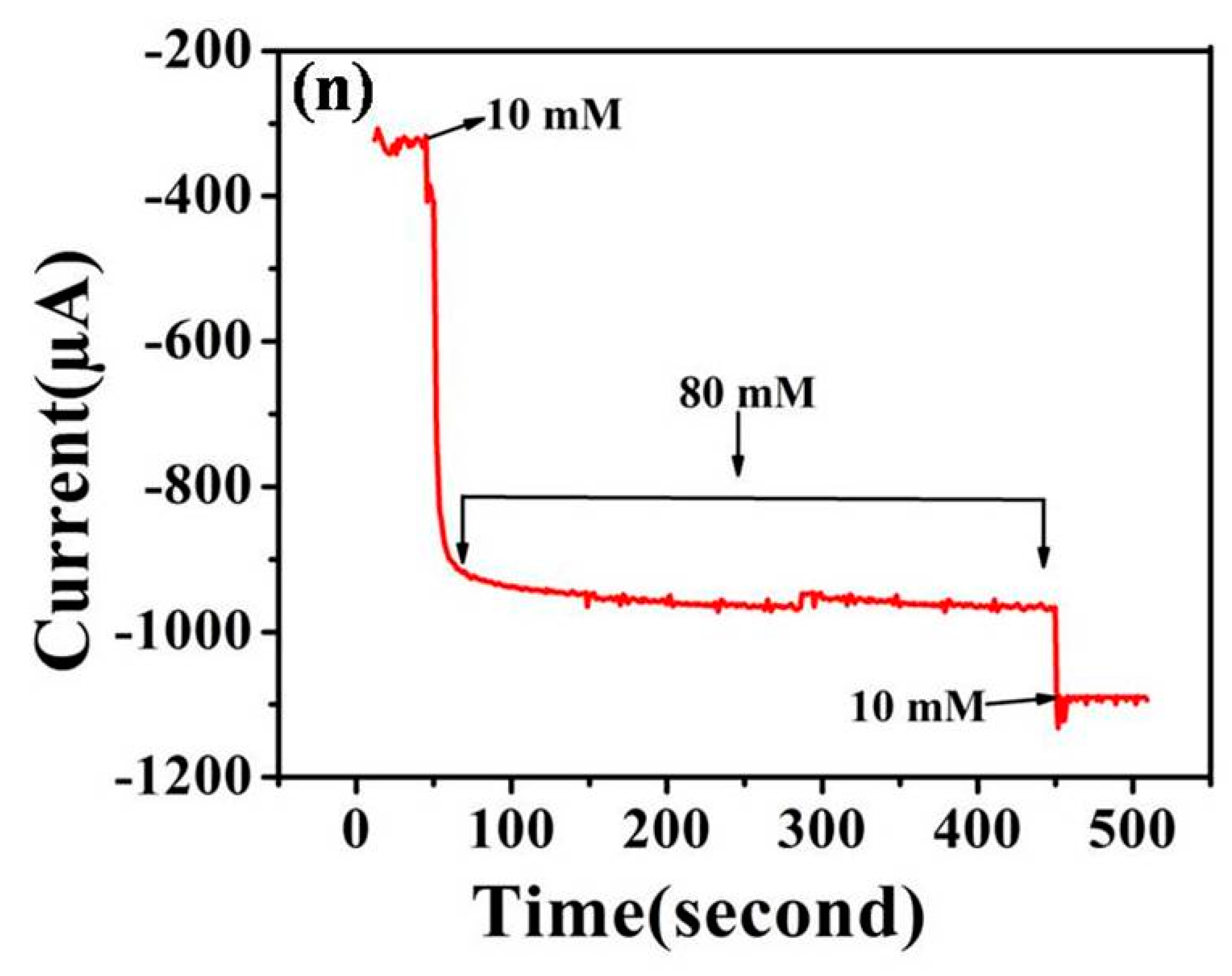
3.3.2. Amperometric Determination of 2,4-DNT
3.3.3. Comparative Study of the Previously Reported Literature
3.3.4. Interference Study
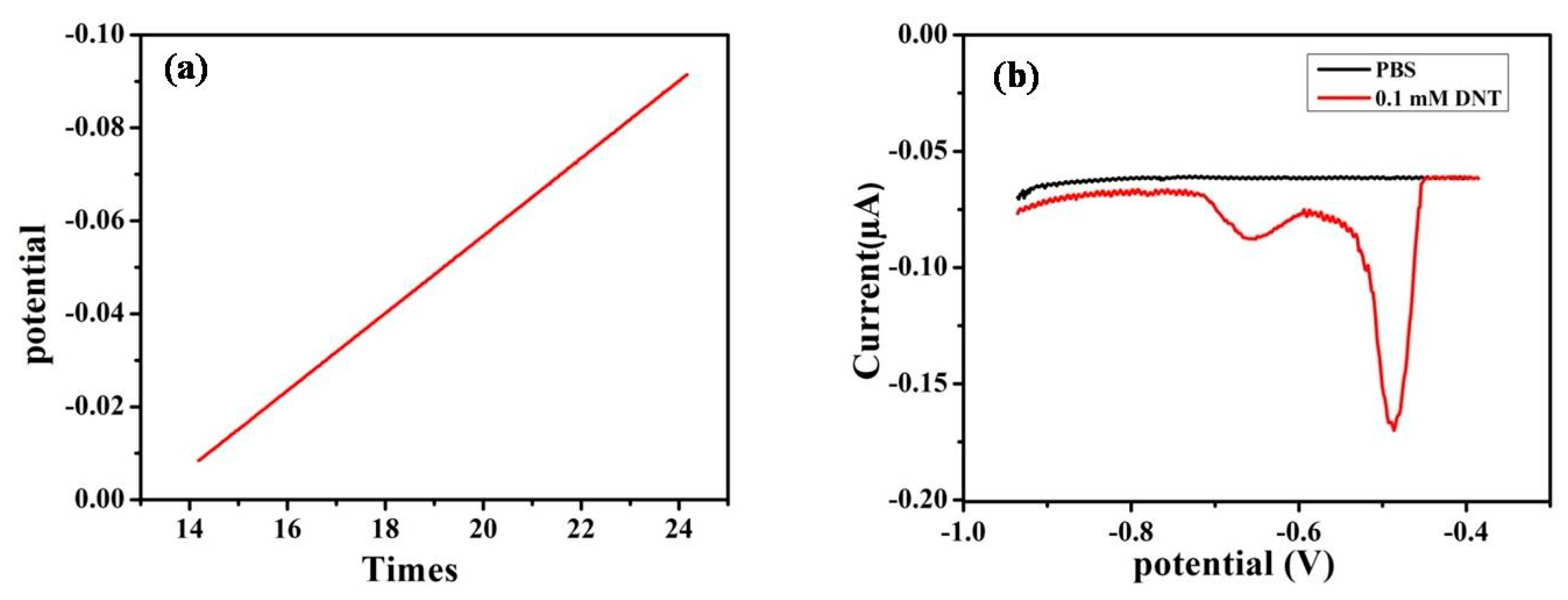
3.3.5. Linear Sweep Voltammetry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caygill, J.S.; Collyer, S.D.; Holmes, J.L.; Davis, F.; Higson, S.P. Disposable screen-printed sensors for the electrochemical detection of TNT and DNT. Analyst 2013, 138, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jha, P.; Chouksey, A.; Tandon, R.P.; Chaudhury, P.K.; Rawat, J.S. Flexible single walled nanotube based chemical sensor for 2, 4-dinitrotoluene sensing. J. Mater. Sci. Mater. Electron. 2018, 29, 6200–6205. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed]
- Kannan, O.K.; Bhalla, R.; Kapoor, J.C.; Nimal, A.T.; Mittal, U.; Yadava, R.D.S. Detection of landmine signature using SAW-based polymer-coated chemical sensor. Def. Sci. J. 2004, 54, 309–315. [Google Scholar] [CrossRef]
- Krausa, M.; Massong, H.; Rabenecker, P.; Ziegler, H. Chemical methods for the detection of mines and explosives, Chap 1. In Detection of Explosives and Landmines: Methods and Field Experience; Schubert, H., Kuznetsov, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 66, pp. 1–19. [Google Scholar]
- Marshall, M.; Oxley, J.C. (Eds.) Chap. 2 Explosives: The threats and the materials. In Aspects of Explosives Detection; Elsevier: Amsterdam, The Netherlands, 2011; pp. 11–26. [Google Scholar]
- Kovalev, I.S.; Taniya, O.S.; Slovesnova, N.V.; Kim, G.A.; Santra, S.; Zyryanov, G.V.; Chupakhin, O.N. Fluorescent detection of 2, 4-DNT and 2, 4, 6-TNT in aqueous media by using simple water-soluble pyrene derivatives. Chem. Asian J. 2016, 11, 775–781. [Google Scholar] [CrossRef]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer. Anal. Chem. 2010, 82, 4015–4019. [Google Scholar] [CrossRef]
- Bratin, K.; Kissinger, P.T.; Briner, R.C.; Bruntlett, C.S. Determination of nitro aromatic, nitramine, and nitrate ester explosive compounds in explosive mixtures and gunshot residue by liquid chromatography and reductive electrochemical detection. Anal. Chim. Acta 1981, 130, 295–311. [Google Scholar] [CrossRef]
- Li, X.J.; Ye, C.W.; Huo, X.L.; Zeng, Z. Solid-phase microextraction using a diglycidyloxycalix[4]arene coated fiber combined with gas chromatography: Very simple, rapid and sensitive method for the determination of chlorobenzenes in water. Microchim. Acta 2010, 168, 161–167. [Google Scholar] [CrossRef]
- Forbes, T.P.; Sisco, E. Mass spectrometry detection and imaging of inorganic and organic explosive device signatures using desorption electro-flow focusing ionization. Anal. Chem. 2014, 86, 7788–7797. [Google Scholar] [CrossRef]
- Peng, L.; Hua, L.; Wang, W.; Zhou, Q.; Li, H. On-site rapid detection of trace non-volatile inorganic explosives by stand-alone ion mobility spectrometry via acid-enhanced evaporization. Sci. Rep. 2014, 4, 6631. [Google Scholar] [CrossRef]
- Zhou, Q.; Peng, L.; Jiang, D.; Wang, X.; Wang, H.; Li, H. Detection of nitro-based and peroxide-based explosives by fast polarity-switchable ion mobility spectrometer with ion focusing in vicinity of Faraday detector. Sci. Rep. 2015, 5, 10659. [Google Scholar] [CrossRef] [PubMed]
- Roscioli, K.M.; Davis, E.; Siems, W.F.; Mariano, A.; Su, W.; Guharay, S.K.; Hill, H.H. Modular ion mobility spectrometer for explosives detection using corona ionization. Anal. Chem. 2011, 83, 5965–5971. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Deng, Y.; Veksler, D.B.; Shur, M.S.; Zhang, X.C.; Schauki, D.; Fitch, M.J.; Osiander, R.; Dodson, C.; et al. Spectroscopic characterization of explosives in the far-infrared region. In Proceedings of the Terahertz for Military and Security Applications II, Orlando, FL, USA, 12–16 April 2004; Volume 5411, pp. 1–9. [Google Scholar]
- Collin, O.L.; Niegel, C.; DeRhodes, K.E.; McCord, B.R.; Jackson, G.P. Fast Gas Chromatography of Explosive Compounds Using a Pulsed-Discharge Electron Capture Detector. J. Forensic Sci. 2006, 51, 815–818. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Wang, J. Nanomaterial-based electrochemical detection of explosives: A review of recent developments. Anal. Methods 2013, 5, 4296–4309. [Google Scholar] [CrossRef]
- Gardner, J.W. Review of conventional electronic noses and their possible application to the detection of explosives, Chap.1. In Electronic Noses & Sensors for the Detection of Explosives; Gardner, J.W., Yinon, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–28. [Google Scholar]
- Chua, C.K.; Pumera, M.; Rulisek, L. Reduction pathways of 2, 4, 6-trinitrotoluene: An electrochemical and theoretical study. J. Phys. Chem. C 2012, 116, 4243–4251. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Influence of Methyl Substituent Position on Redox Properties of Nitroaromatics Related to 2, 4, 6-Trinitrotoluene. Electroanalysis 2011, 23, 2350–2356. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Marashianpour, Z.; Karimi, M.S.; Mohammad-Zadeh, M. Electrochemical synthesis and characterization of zinc carbonate and zinc oxide Nanoparticles. J. Mol. Struct. 2015, 1099, 232–238. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Nasrabadi, M.R. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta A 2015, 136, 1249–1254. [Google Scholar] [CrossRef]
- Shahidzadeh, M.; Shabihi, P.; Pourmortazavi, S.M. Sonochemical preparation of copper (II) chromite nanocatalysts and particle size optimization via taguchi method. J. Inorg. Organomet. Polym. Mater. 2015, 25, 986–994. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Roushani, M.; Hajimirsadeghi, S.S. Electrochemical preparation and thermal characterization of copper sulfide Nanoparticles. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2014, 44, 951–958. [Google Scholar] [CrossRef]
- Choi, H.H.; Lee, J.; Dong, K.Y.; Ju, B.K.; Lee, W. Gas sensing performance of composite materials using conducting polymer/single-walled carbon nanotubes. Macromol. Res. 2012, 20, 143–146. [Google Scholar] [CrossRef]
- Sagar, M.; Mudhalwadkar, R.; Sonar, G. Metal oxide semiconductor based thin film sensor for nitro aromatic explosive detection. In Proceedings of the International Conference for convergence for Technology, Pune, India, 6–8 April 2014; pp. 1–4. [Google Scholar]
- Chen, T.W.; Sheng, Z.H.; Wang, K.; Wang, F.B.; Xia, X.H. Determination of explosives using electrochemically reduced graphene. Chem. Asian J. 2011, 6, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.T.; Ambrosi, A.; Pumera, M. Nitroaromatic explosives detection using electrochemically exfoliated graphene. Sci. Rep. 2016, 6, 33276. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Mohammad, A.; Mathur, P.; Mobin, S.M. Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics. Electrochim. Acta 2016, 215, 435–446. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Pourmortazavi, S.M. Synthesis and application of carbowax/polypyrrole nanocomposite for fabrication of electrochemical sensor to detect 2, 4-DNT vapor. Mater. Res. Express 2017, 4, 086303–086312. [Google Scholar] [CrossRef]
- Yaghi, O.M. Metal–organic frameworks: A tale of two entanglements. Nat. Mater. 2007, 6, 92–93. [Google Scholar] [CrossRef]
- Pan, L.; Parker, B.; Huang, X.; Olson, D.H.; Lee, J.; Li, J. Zn (tbip)(H2tbip= 5-tert-butyl isophthalic acid): A highly stable guest-free microporous metal organic framework with unique gas separation capability. J. Am. Chem. Soc. 2006, 128, 4180–4181. [Google Scholar] [CrossRef]
- Pan, L.; Sander, M.B.; Huang, X.; Li, J.; Smith, M.; Bittner, E.; Bockrath, B.; Johnson, J.K. Microporous metal organic materials: Promising candidates as sorbents for hydrogen storage. J. Am. Chem. Soc. 2004, 126, 1308–1309. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Ji, G.; Cui, R.; Liu, Z. One-pot synthesis of hierarchical-pore metal–organic frameworks for drug delivery and fluorescent imaging. CrystaEngComm 2018, 20, 1087–1093. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, L.; Han, L.; Zhang, Z.; Zhao, H. Synthesis, structure and characterization of two solvatochromic metal–organic frameworks for chemical-sensing applications. Cryst. Eng. Comm. 2018, 20, 2237–2240. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Garcia-Garcia, P.; Corma, A. Catalytic Transfer Hydrogenation of Biomass-Derived Carbonyls over Hafnium-Based Metal–Organic Frameworks. ChemSusChem 2018, 11, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zong, B.; Mao, S. Metal–organic framework-based sensors for environmental contaminant sensing. Nano-Micro Lett. 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic catalysis of Au@Ag core−shell nanoparticles stabilized on metal−organic framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.N.; Edgar, M.; Gonzalez, J.; Slawin, A.M.; Tunstall, D.P.; Grewal, P.; Wright, P.A. Structural studies and computer simulation of the inclusion of aromatic hydrocarbons in a zinc 2, 6-naphthalene dicarboxylate framework compound. J. Phys. Chem. B 2004, 108, 535–543. [Google Scholar] [CrossRef]
- Pilloni, M.; Kumar, V.B.; Ennas, G.; Porat, Z.E.; Scano, A.; Cabras, V.; Gedanken, A. Formation of metallic silver and copper in non-aqueous media by ultrasonic radiation. Ultrason. Sonochem. 2018, 47, 108–113. [Google Scholar] [CrossRef]
- Arul, P.; John, S.A. Silver nanoparticles built-in zinc metal organic framework modified electrode for the selective non-enzymatic determination of H2O2. Electrochim. Acta 2017, 235, 680–689. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kulkarni, R.M. An electrochemical sensor for clozapine at ruthenium doped TiO2 nanoparticles modified electrode. Sens. Actuators B: Chem. 2017, 247, 858–867. [Google Scholar] [CrossRef]

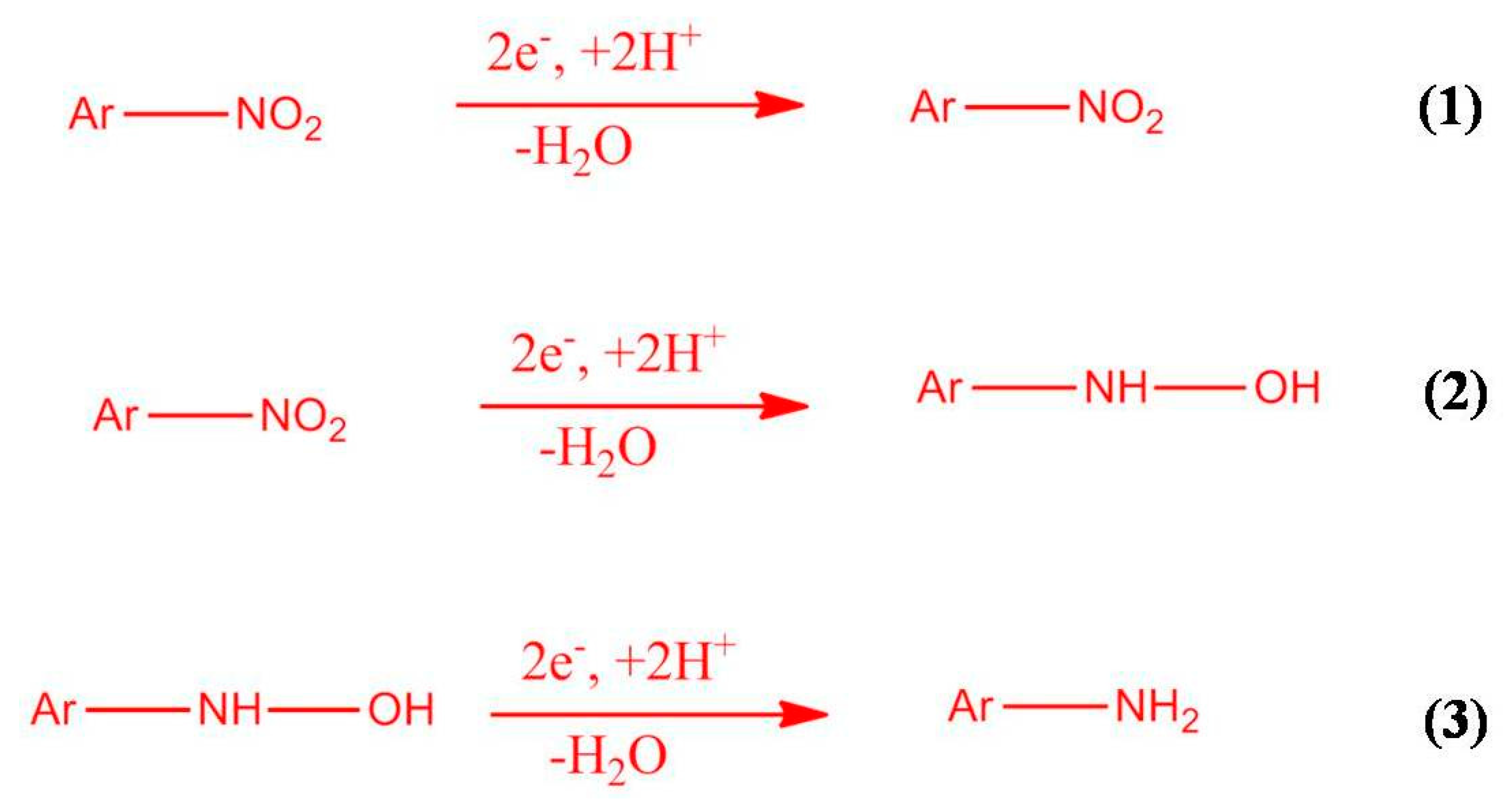
| Type of Electrode | Linear Range (µM) | Sensitivity (µA−1 µM−1 cm−2) | Detection Limit (µM) | References |
|---|---|---|---|---|
| GC/G-Li-CO3 | 0–0.20 | −0.552 | 0.035 | [28] |
| GCE/rGO/SrTiO3 | 0.5–0.8 | 71.66 | 0.128 | [29] |
| GE/Zn-MOF-8@Ag | 0.002–0.9 | −0.238 | 0.041 | Our work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, S.; Sharma, B.; Kapoor, S.; Malhotra, R.; Varma, R.S.; Dilbaghi, N. Construction of Silver Quantum Dot Immobilized Zn-MOF-8 Composite for Electrochemical Sensing of 2,4-Dinitrotoluene. Appl. Sci. 2019, 9, 4952. https://doi.org/10.3390/app9224952
Rani S, Sharma B, Kapoor S, Malhotra R, Varma RS, Dilbaghi N. Construction of Silver Quantum Dot Immobilized Zn-MOF-8 Composite for Electrochemical Sensing of 2,4-Dinitrotoluene. Applied Sciences. 2019; 9(22):4952. https://doi.org/10.3390/app9224952
Chicago/Turabian StyleRani, Sushma, Bharti Sharma, Shivani Kapoor, Rajesh Malhotra, Rajender S. Varma, and Neeraj Dilbaghi. 2019. "Construction of Silver Quantum Dot Immobilized Zn-MOF-8 Composite for Electrochemical Sensing of 2,4-Dinitrotoluene" Applied Sciences 9, no. 22: 4952. https://doi.org/10.3390/app9224952
APA StyleRani, S., Sharma, B., Kapoor, S., Malhotra, R., Varma, R. S., & Dilbaghi, N. (2019). Construction of Silver Quantum Dot Immobilized Zn-MOF-8 Composite for Electrochemical Sensing of 2,4-Dinitrotoluene. Applied Sciences, 9(22), 4952. https://doi.org/10.3390/app9224952