Abstract
Thioredoxins (Trxs) are proteins that act as antioxidants by facilitating the reduction of other proteins and are highly conserved in all organisms. Plant H-type Trx isoforms have different structures and perform multiple functions. Previous studies have reported that the low molecular weight AtTrx-H2 acts as a disulfide reductase and the high molecular weight AtTrx-H3 functions as an oxidoreductase and a molecular chaperone. In this study, we compared the antifungal activities of Arabidopsis Trx-H2 and -H3 with engineered proteins 2N3C and 3N2C via domain-swapping between the N- and C-terminal regions of Trx-H2 and -H3. All AtTrx-H variant proteins inhibited cell growth of various pathogenic fungal strains at pH 5.2 and pH 7.2 and showed significant intracellular accumulation in the fungal cells. Interestingly, only two engineered proteins penetrated the fungal cell wall and membrane, indicating their ability to destabilize the fungal cell membrane before internalization into the cytosol. To our knowledge, this is the first study that demonstrates novel functions of plant antioxidants AtTrx-H2 and -H3 as antifungal proteins and shows their enhanced activity using the domain swapping technique.
1. Introduction
Thioredoxins (Trxs) are small ubiquitous proteins that participate in a wide variety of biological functions [1]. Several subgroups of Trxs, including Trx-m, -f, -x, -y, -z, -o, and -h are found in Arabidopsis thaliana [2]. Trx H-type proteins are the most abundant Trx proteins in A. thaliana and can perform multiple functions, such as redox regulation, antifungal activity, and chaperone functions, in various organisms [3,4,5,6,7]. In particular, the immune response of A. thaliana Trx-H3 and Trx-H5 against pathogenic microorganisms is well known [6,8,9]. Trx proteins contain a common motif ‘Trx-fold’ with a conserved active site sequence Trp-Cys-Gly-Pro-Cys (WCGPC) or Trp-Cys-Pro-Pro-Cys (WCPPC) [10]. As AtTrx-H2 and AtTrx-H3 contain two different active site motifs—WCGPC and WCPPC, respectively—their functions and structures are also substantially different [11].
Chimeric proteins prepared by swapping of two or more functional domains may exhibit newly evolved functions [12]. For example, several chimeric proteins exhibit enhanced or reduced antimicrobial activity [13,14,15], chaperone activity [11,16], protein solubility [17], and self-assembly properties [18]. Previous studies have demonstrated that AtTrx-H2, AtTrx-H3, and two chimeric proteins (AtTrx-H-2N3C and AtTrx-H-3N2C designed by switching the N- and C-terminal regions of AtTrx H2 and AtTrx H3, respectively) undergo functional switches due to changes in their hydrophobicity and structure [11]. A. thaliana Trx isoform proteins have distinct structural and functional specificities. While AtTrx-H2 formed as low-molecular-mass structures showed only disulfide reductase activity, AtTrx-H3 with high-molecular-mass complexes functions as both a disulfide reductase and as a chaperone.
Here, we hypothesized that a change of the unique structural feature of each AtTrx H-type protein might result in variation in its functional properties. Interestingly, the Trx-like proteins Trx-H5 and OsTDX, are used as antimicrobial agents against pathogenic fungus [6,19]. In this study, we sought to determine the effects of swapping the N- or C-terminal domains of AtTrx-H2 and AtTrx-H3 on their antifungal activities. To monitor the structure–function relationship, we first constructed two proteins domain-swapped between the N- and C-terminal regions of AtTrx-H2 and AtTrx-H3. Then, we investigated changes in the antifungal activity and compared the action mechanism of four recombinant proteins—AtTrx-H2, AtTrx-H3, AtTrx-H-2N3C, and AtTrx-H-3N2C—against pathogenic mold and yeast species.
2. Materials and Methods
2.1. Materials
SYTOX Green and 5-Carboxyfluorescein N-hydroxysuccinimide (FAM NHS) ester were purchased from Molecular Probes Inc. (Eugene, OR) and BioActs (Incheon, Korea), respectively. 2′,7′-dichlorofluorescein diacetate (DCF-DA) was supplied by Molecular Probes Inc., (Eugene, OR, USA).
Aspergillus flavus (Korea Collection for Type Cultures (KCTC) 6905), A. fumigatus (KCTC 6145), Colletotrichum gloeosporioides (KCTC 6169), Fusarium graminearum (KCTC 16656) F. moniliforme (KCTC 6149), F. oxysporum (KCTC 16909), F. solani (KCTC 6326), Penicillium verrucosum (KCTC 6265), Trichoderma harzianum (KCTC 6043), T. viride (KCTC 6047), Trichophyton rubrum (KCTC 6345), Candida albicans (KCTC 7270), C. krusei (Culture Collection of Antimicrobial Resistant Microbes (CCARM) 14017), C. parapsilosis (CCARM 14016), Cryptpcoccus sp. (KCTC 17072), and Hannaella zeae (KCTC 17076) were obtained from the Korea Collection for Type Cultures (KCTC, Jeongup-si, Korea) and Culture Collection of Antimicrobial Resistant Microbes (CCARM, Seoul Women’s University, Seoul, Korea).
2.2. Purification of Recombinant AtTrx-H2, -H3, -2N3C and -3N2C Proteins
The AtTrx-H2, -H3, -2N3C and -3N2C genes were cloned from the Arabidopsis cDNA library using polymerase chain reaction [11], and the amplified products were ligated into the BamHI/XhoI sites of the pET28(a) expression vector. The resulting DNA constructs were transformed into E. coli BL21 (DE3) cells and cultured in Luria-Bertani (LB) broth containing 30 µg/mL kanamycin at 37 °C. The His-tagged H2, H3, 2N3C, and 3N2C fusion proteins were applied to Ni-NTA resin (Thermo Fisher Scientific, Waltham, MA, USA) [11], and purified by BioLogic DuoFlow™ Medium-Pressure Chromatography Systems (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using an Enrich size-exclusion chromatography (SEC) 650 column (Bio-Rad Laboratories, Inc., 10 × 300 mm) at a flow rate of 0.6 mL/min at Room Temperature (RT) with phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4). The purity of H2, H3, 2N3C, and 3N2C proteins was examined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
2.3. Antifungal Assay
Spores from 4-day-old fungi grown on potato dextrose (PD; Becton Dickinson, Sparks, MD, USA) agar were collected using 0.08% Triton X-100. The yeast cells were subcultured overnight in yeast extract peptone dextrose medium (YPD; Becton Dickinson). The fungal cells were diluted to a concentration of 104 spores/mL in PBS and 2-(N-morpholino)ethanesulfonic acid (MES) buffer containing 20% PD for mold or YPD for yeast and added to two-fold serially diluted proteins in 96-well plates. After 24 h (for yeast) to 36 h (for mold) incubation at 28 °C, mycelium growth was monitored using an inverted light microscope. The minimum inhibitory concentration (MIC) value for each fungal strain was determined as the lowest concentration that completely inhibited visible fungal growth. All assays were performed in triplicate.
2.4. Confocal Laser Scanning Microscopy (CLSM) Analysis
The cellular localization of FAM-labeled proteins in fungal cells was analyzed using confocal laser scanning microscopy (CLSM). C. gloeosporioides cells were incubated with FAM-labeled proteins for 2 h at 28 °C and washed three times with PBS by centrifugation. The washed cells were spotted onto a coverglass treated with mounting solution (50% glycerol and 0.1% n-propyl-gallate) [20] and observed under a CLSM (LSM 510 META, Carl Zeiss, Gottingen, Germany). Zeiss LSM image software was used for image acquisition and analysis.
2.5. Reactive Oxygen Species (ROS) Generation
DCF-DA was used to examine the oxidative damage in C. albicans. C. albicans was treated with AtTrx proteins at a MIC concentration for 8 h and the cells were stained with 100 μM DCF-DA for 1 h. The fluorescence was captured by fluorescence microscopy.
2.6. SYTOX Green Uptake
C. albicans or C. gloeosporioides cells pre-incubated with 0.5 μM SYTOX Green for 15 min in the dark were incubated with the proteins at their MICs for 1 h and analyzed using fluorescence microscopy and flow cytometry (CytoFLEX, Beckman Coulter Inc., CA, USA).
2.7. Scanning Electron Microscopy (SEM)
Following incubation of the proteins with pre-cultivated C. albicans cells (5 × 105 cells/mL) for 4 h at their MIC, cells were fixed overnight with 2% glutaraldehyde at 4 °C. The fixed cells were dehydrated in graded ethanol and dried in a critical point dryer under CO2. The gold-coated specimens were observed under a field emission scanning electron microscope (SEM) (JSM-7100F, JEOL Ltd., Tokyo, Japan) [19].
3. Results and Discussion
3.1. Purification and Identification of AtTrx-H2, -H3, -2N3C, and -3N2C Proteins
Antimicrobial peptides and proteins (AMPs) including host defense molecules have been widely identified in vertebrates and invertebrates, and extensively investigated as antimicrobial agents against pathogenic microorganisms, especially drug-resistant microorganisms [21]. Plants are constantly exposed to diverse environments harboring various pathogenic microorganisms, such as fungi, bacteria, viruses, protozoa, and nematodes. Therefore, AMPs derived from plants can be used as effective defense molecules against pathogenic microorganisms [22]. The major groups of AMPs found in plants include thionins [22,23], defensins [22,23], and lipid transfer proteins [23,24]; however, reactive oxygen species (ROS)-related proteins were recently included as a new AMP group [6,19]. Among the several subgroups of AtTrx, including Trx-m, -f, -x, -y, -z, -o, and -h, the most abundant H-type AtTrxs have been extensively studied for their disulfide reductase activity and use as a molecular chaperone [11]. Although the antimicrobial activity of AtTrx-H5 was reported [6], in this study, we sought to compare the antifungal activities and action modes of the recombinant AtTrx-H2 and -H3 proteins, as well as their hybrid proteins 2N3C and 3N2C, obtained by swapping the N- and C-terminal sequences of H2 and H3, respectively (Figure 1A). The H2, H3, 2N3C, and 3N2C proteins were expressed in E. coli and purified using affinity and size-exclusion chromatography (data not shown).
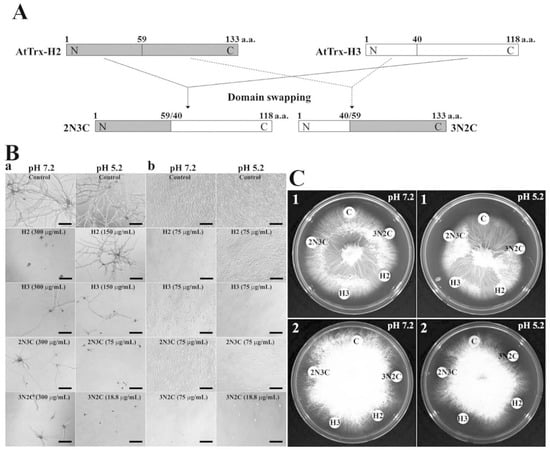
Figure 1.
Growth inhibition of Trx-H2, -H3, -2N3C, and -3N2C proteins in liquid (B) and solid (C) cultivation. (A) Schematic representation of domain swapping between AtTrx-H2 and -H3. a: F. solani, b: C. albicans, 1: F. moniliform, 2: T. ruburum.
3.2. Antifungal Activities of AtTrx-H2, -H3, -2N3C and -3N2C Proteins
To investigate the antifungal activities of AtTrx-H2, -H3, -2N3C, and 3N2C proteins, microtiter and agar germination assay were used for 11 mold and 5 yeast strains. Table 1 and Figure 1 show the antifungal activities of the four proteins. Several previous studies have shown that pH plays an important role in regulating the antimicrobial activity of AMPs. In particular, low pH increases the net charge, number of bound metal ions, and protonation of histidine, aspartic acid, and glutamic acid residues in many eukaryotic AMPs [25]. Therefore, we evaluated the antifungal activities of all four proteins at pH 5.2 and 7.2. As shown in Table 1, MICs of AtTrx-H2 and -H3, the parent proteins, were ranged from 37.5 to 300 μg/mL or over 300 μg/mL. In particular, their antifungal activities were better in Candida species than others. Swapped 2N3C protein with MICs ranging from 37.5 to 300 μg/mL exerted more good antifungal activity in pH 5.2 than pH 7.2. 3N2C protein inhibited mold and yeast growth at MICs ranging from 9.4 to 37.5 μg/mL (Table 1). Moreover, its antifungal activity at pH 5.2 was better than that at pH 7.2. Especially, the antifungal activities of two swapped proteins were stronger than those of melittin and OsTDX in F. oxysprum and F. solani cells [19]. We propose that the good antifungal activities of the swapped proteins compared to parent H2 and H3 proteins may be due to the alternation of their hydrophobicity and antifungal mechanisms. Furthermore, we suggest that the differences in the MIC values at different pH values are caused by changes of protein stability and protonation of amino acids. Figure 1 shows the antifungal activities of these proteins against F. solani (Figure 1B(a)) and C. albicans (Figure 1B(b)) in liquid culture, and F. moniliform (Figure 1C(1)) and T. ruburum (Figure 1C(2)) in solid culture, visualized by the inhibition of the hyphal growth of mold strains.

Table 1.
Antifungal activity of Trx H2/H3/2N3C/3N2C proteins against mold and yeast fungal cells.
3.3. Intracellular Localization of FAM-Labeled AtTrx Proteins in Fungal Cells
To investigate the cellular distribution of H2, H3, 2N3C and 3N2C proteins in C. gloeosporioides cells, the localization of FAM-labeled proteins was visualized under CLSM. The results showed a different aspect depending on proteins. Although all proteins accumulated in the cytosol, FAM-labeled 2N3C and 3N2C showed fluorescence on the cell surface and in the cytosolic space, caused by intracellular damage (Figure 2). These results suggest that swapping of the functional domains of the proteins can induce a change in the antifungal mechanism.
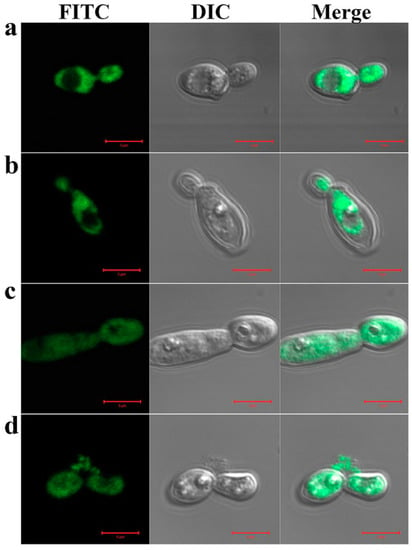
Figure 2.
Cellular distribution of Trx-H2/H3/2N3C/3N2C proteins in C. gloeosporioides cells. FAM-labeled Trx-H2 (a), -H3 (b), -2N3C (c), or -3N2C (d) proteins were incubated with spores of C. gloeosporioides at corresponding minimum inhibitory concentrations (MICs) in pH 5.2 for 2 h at 28 °C, following which, cellular populations of FAM-labeled proteins were observed under a confocal laser scanning microscope.
3.4. Intracellular ROS Generation in Fungal Cells
In previous study [6], AtTrx-H5, another Trx h-type protein, exhibits antifungal activity via cellular ROS generation. Therefore, we investigated intracellular ROS generation of Trx-H2, H3, 2N3C, and 3N2C proteins in C. albicans cells. As shown in Figure 3, H2 and H3 proteins produced the cellular ROS, cells treated with H2 and H3 proteins significantly produced ROS (Figure 3a,b, respectively), whereas 2N3C and 3N2C did not generate ROS (Figure 3c,d, respectively). We suggest that H2 and H3 proteins exhibit the fungal growth via intracellular ROS generation, but swapped proteins show a potent antifungal activity via another mode of action.
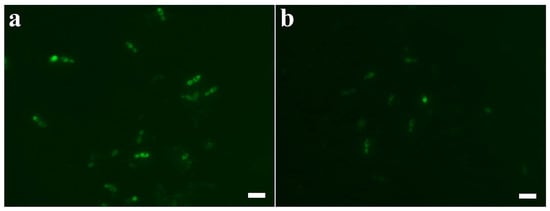
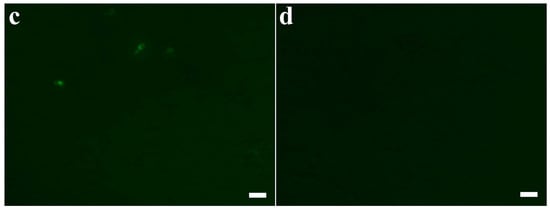
Figure 3.
Reactive oxygen species (ROS) generation of Trx-H proteins and their swapped proteins in C. albicans. Cells were treated with the proteins at their MIC concentrations in pH 5.2 for 8 h, after which they were stained with 100 μM DCF-DA for 1 h. Cells with ROS generation were visualized by fluorescence microscope. (a): Trx-H2, (b): Trx-H3, (c): Trx-2N3C, (d): Trx-3N2C. Scale bar: 10 μm.
3.5. Membrane-Permeable Effects of AtTrx Proteins in Fungal Cells
Next, we tested the membrane-permeable activities of H2, H3, 2N3C, and 3N2C proteins using the SYTOX Green uptake assay. SYTOX Green, a membrane-impermeant dye, emits green fluorescence upon binding to nucleic acids. Moreover, this dye can penetrate the cell membrane upon damage by antifungal agents. We used florescence microscopy and flow cytometry analysis to measure membrane permeability in the presence of H2, H3, 2N3C, and 3N2C proteins. As shown in Figure 4A, SYTOX Green uptake in a large population of C. albicans and C. gloeosporioides cells treated with 3N2C, similarly to melittin, a membranolytic peptide, shifted to the right in flow cytometry. The percentage of 3N2C-positive cells was higher than that of 2N3C-positive cells, suggesting that the degrees of SYTOX green uptake correspond to their antifungal activities. In contrast, no cellular green fluorescence was detected in the presence of H2 and H3 because these proteins did not induce membrane damage. These results correspond to those observed under the fluorescence microscope (Figure 4B). Green fluorescence in fungal cells treated with H2 and H3 was not detected, whereas 3N2C-treated cells facilitated SYTOX Green uptake into the cytosol, indicating that this protein destabilizes the fungal cell membrane before internalization into the cytosol.
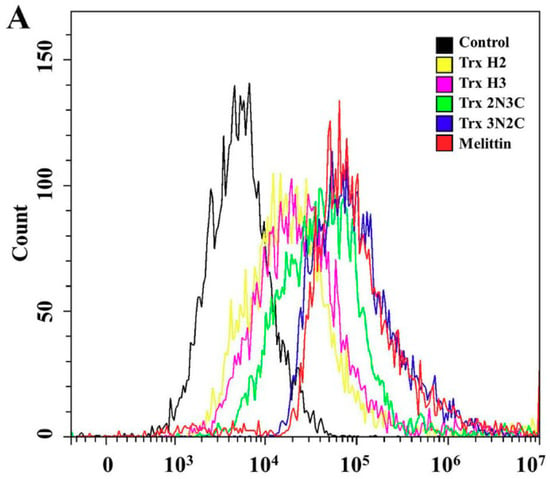
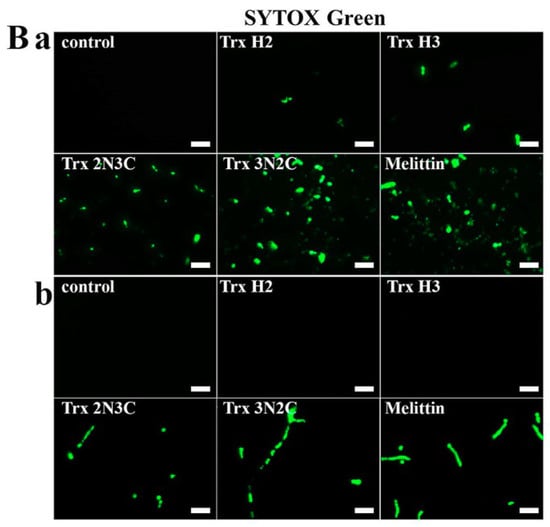
Figure 4.
Effects of Trx-H2/H3/2N3C/3N2C on fungal membrane permeability. (A) After 1 h incubation of each protein with SYTOX Green-pretreated C. albicans cells in pH 5.2, cells were analyzed using flow cytometry. (B) Each protein was added to C. albicans (a) and C. gloeosporioides (b) cells pretreated with SYTOX Green, after which the cells were visualized under a fluorescence microscope. Scale bar: 10 μm.
3.6. Morphological Changes of C. albicans Cells Caused by AtTrx Proteins
The morphological changes of C. albicans cells upon treatment with AtTrx-H2, H3, 2N3C, and 3N2C proteins were observed using SEM. Cells treated with each protein at their MICs exhibited morphological changes in comparison to untreated cells (Figure 5). The treatment with H2, H3, and 2N3C proteins caused cell surface wrinkling along with accumulation of cell debris; however, treatment with 3N2C formed irregular-sized holes in the cell surface similar to those formed by treatment with mellitin. These results indicate that 3N2C facilitates the formation of toroidal pores and increases membranolytic activity. In conclusion, treatment with 3N2C protein enhanced antifungal activity against mold and yeast cells. We demonstrated that domain-swapping between the N- and C-terminal regions of Trx-H2 and -H3 affect their mechanism of action.
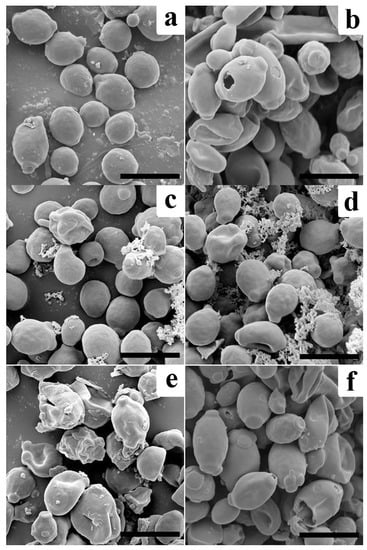
Figure 5.
Morphological changes of C. albicans cells with proteins in pH 5.2. (a): control, (b): melittin (37.5 μg/mL), (c): Trx-H2 (300 μg/mL), (d): H3 (37.5 μg/mL), (e): 2N3C (75 μg/mL), and (f): 3N2C (18.8 μg/mL). Scale bar: 5 μm.
4. Conclusions
In summary, we demonstrated the pH-dependent antifungal activities of two H-type Trxs of A. thaliana and investigated their mechanism of action that enhances their antifungal activities via N- and C-terminal swapping. Two hybrid proteins, 2N3C and 3N2C, interacted with the fungal cell wall and disrupted the cell membrane, compared to H2 and H3 proteins. This study demonstrates that the swapping technique can effectively increase antimicrobial activity.
Author Contributions
Conceptualization, J.-Y.K., Y.H.C., S.-C.P., S.Y.L., and J.R.L.; methodology, J.-Y.K., S.-C.P., M.-K.J., and J.R.L.; validation, J.-Y.K., and S.-C.P.; formal analysis, Y.H.C., S.-C.P., and J.R.L.; investigation, J.-Y.K., Y.H.C., S.-C.P., I.R.K., H.K., and J.H.J.; resources, Y.H.C., S.-C.P. and J.R.L.; data curation, J.-Y.K., S.-C.P., and J.R.L.; writing—original draft preparation, J.-Y.K., S.C.P. and J.R.L.; writing—review and editing, M.-K.J., S.Y.L., and J.R.L.; visualization, J.-Y.K., and S.-C.P.; supervision, S.Y.L., and J.R.L.; funding acquisition, J.-Y.K., and J.R.L.
Funding
This research was supported by a grant from the National Institute of Ecology, funded by the Ministry of Environment (MOE) of the Republic of Korea (NIE-A-2019-07); the Center for Women In Science, Engineering and Technology (WISET), Ministry of Science, ICT & Future Planning of Korea (MSIP) under the Program for Returners into R and D; and by a grant from the NG-BioGreen 21 Program (SSAC, PJ01317301), RDA, Korea, and by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2018R1D1A1B07047990).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Dos Santos, C.V.; Rey, P. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci. 2006, 11, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Arsova, B.; Hoja, U.; Wimmelbacher, M.; Greiner, E.; Üstün, Ş.; Melzer, M.; Petersen, K.; Lein, W.; Börnke, F. Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: Evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana. Plant Cell 2010, 22, 1498–1515. [Google Scholar] [CrossRef] [PubMed]
- Ueoka-Nakanishi, H.; Sazuka, T.; Nakanishi, Y.; Maeshima, M.; Mori, H.; Hisabori, T. Thioredoxin h regulates calcium dependent protein kinases in plasma membranes. FEBS J. 2013, 280, 3220–3231. [Google Scholar] [CrossRef] [PubMed]
- Sweat, T.A.; Wolpert, T.J. Thioredoxin h5 is required for victorin sensitivity mediated by a CC-NBS-LRR gene in Arabidopsis. Plant Cell 2007, 19, 673–687. [Google Scholar] [CrossRef]
- Chi, Y.H.; Paeng, S.K.; Kim, M.J.; Hwang, G.Y.; Melencion, S.M.B.; Oh, H.T.; Lee, S.Y. Redox-dependent functional switching of plant proteins accompanying with their structural changes. Front Plant Sci. 2013, 4, 277. [Google Scholar] [CrossRef]
- Park, S.C.; Jung, Y.J.; Kim, I.R.; Lee, Y.; Kim, Y.M.; Jang, M.K.; Lee, J.R. Functional characterization of thioredoxin h type 5 with antimicrobial activity from Arabidopsis thaliana. Biotechnol. Bioprocess Eng. 2017, 22, 129–135. [Google Scholar] [CrossRef]
- Park, S.K.; Jung, Y.J.; Lee, J.R.; Lee, Y.M.; Jang, H.H.; Lee, S.S.; Park, J.H.; Kim, S.Y.; Moon, J.C.; Lee, S.Y. Heat-shock and redox-dependent functional switching of an h-type Arabidopsis thioredoxin from a disulfide reductase to a molecular chaperone. Plant Physiol. 2009, 150, 552–561. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Reichheld, J.P.; Mestres-Ortega, D.; Laloi, C.; Meyer, Y. The multigenic family of thioredoxin h in Arabidopsis thaliana: Specific expression and stress response. Plant Physiol. Biochem. 2002, 40, 685–690. [Google Scholar] [CrossRef]
- Wong, J.H.; Cai, N.; Balmer, Y.; Tanaka, C.K.; Vensel, W.H.; Hurkman, W.J.; Buchanan, B.B. Thioredoxin targets of developing wheat seeds identified by complementary proteomic approaches. Phytochemistry 2004, 65, 1629–1640. [Google Scholar] [CrossRef]
- Jung, Y.J.; Chi, Y.H.; Chae, H.B.; Shin, M.R.; Lee, E.S.; Cha, J.Y.; Paeng, S.K.; Lee, Y.; Park, J.H.; Kim, W.Y. Analysis of Arabidopsis thioredoxin-h isotypes identifies discrete domains that confer specific structural and functional properties. Biochem. J. 2013, 456, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Liu, C.; Kim, B.G.; Lee, D.Y. Synthetic fusion protein design and applications. Biotechnol. Adv. 2015, 33, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.; Wade, D.; Boman, I.; Wåhlin, B.; Merrifield, R. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989, 259, 103–106. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, N.H.; Lee, J.W.; Jang, J.S.; Park, Y.H.; Park, S.C.; Jang, M.K. Novel chimeric peptide with enhanced cell specificity and anti-inflammatory activity. Biochem. Biophys. Res. Commun. 2015, 463, 322–328. [Google Scholar] [CrossRef]
- Che, Y.Z.; Li, Y.R.; Zou, H.S.; Zou, L.F.; Zhang, B.; Chen, G.Y. A novel antimicrobial protein for plant protection consisting of a Xanthomonas oryzae harpin and active domains of cecropin A and melittin. Microb. Biotechnol. 2011, 4, 777–793. [Google Scholar] [CrossRef]
- Erbse, A.; Yifrach, O.; Jones, S.; Lund, P.A. Chaperone activity of a chimeric GroEL protein that can exist in a single or double ring form. J. Biol. Chem. 1999, 274, 20351–20357. [Google Scholar] [CrossRef]
- Manoharadas, S.; Witte, A.; Bläsi, U. Antimicrobial activity of a chimeric enzybiotic towards Staphylococcus aureus. J. Biotechnol. 2009, 139, 118–123. [Google Scholar] [CrossRef]
- Wheeldon, I.R.; Campbell, E.; Banta, S. A chimeric fusion protein engineered with disparate functionalities—Enzymatic activity and self–assembly. J. Mol. Biol. 2009, 392, 129–142. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, I.R.; Hwang, J.E.; Kim, J.Y.; Jung, Y.J.; Choi, W.; Lee, Y.; Jang, M.K.; Lee, J.R. Functional mechanisms underlying the antimicrobial activity of the Oryza sativa Trx-like protein. Int. J. Mol. Sci. 2019, 20, 1413. [Google Scholar] [CrossRef]
- Semighini, C.P.; Harris, S.D. Methods to detect apoptotic-like cell death in filamentous fungi. Methods Mol. Biol. 2010, 638, 269–279. [Google Scholar]
- Zasloff, M. Antimicrobial peptides of multicellular organisms: My perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [PubMed]
- Tang, S.S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals (Basel) 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Marion, D.; Bakan, B.; Elmorjani, K. Plant lipid binding proteins: Properties and applications. Biotechnol. Adv. 2007, 25, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. PH dependent antimicrobial peptides and proteins, their mechanisms of action and potential as therapeutic agents. Pharmaceuticals (Basel) 2016, 9, 67. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).