Mapping Thyroarytenoid and Cricothyroid Activations to Postural and Acoustic Features in a Fiber-Gel Model of the Vocal Folds
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
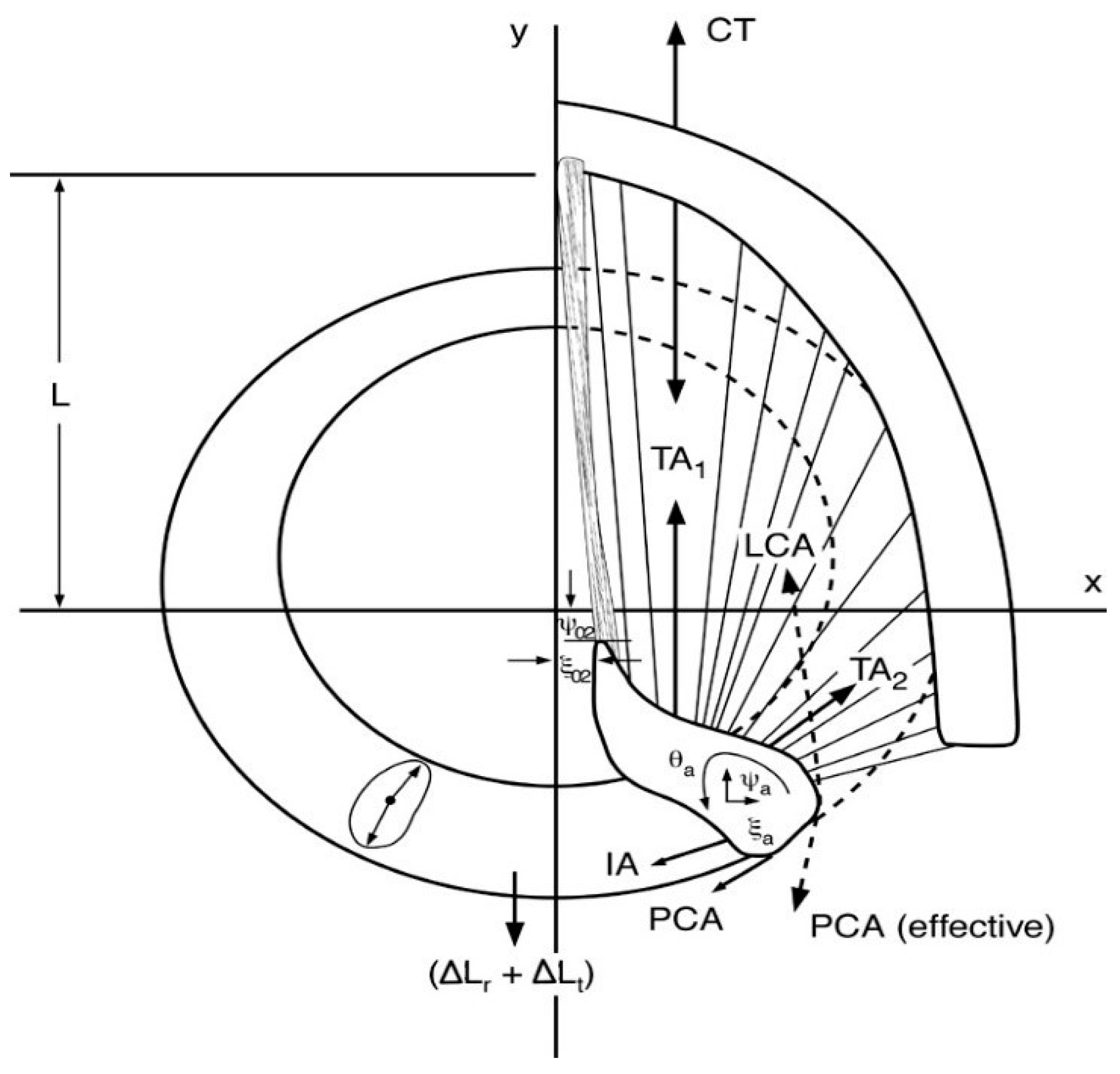
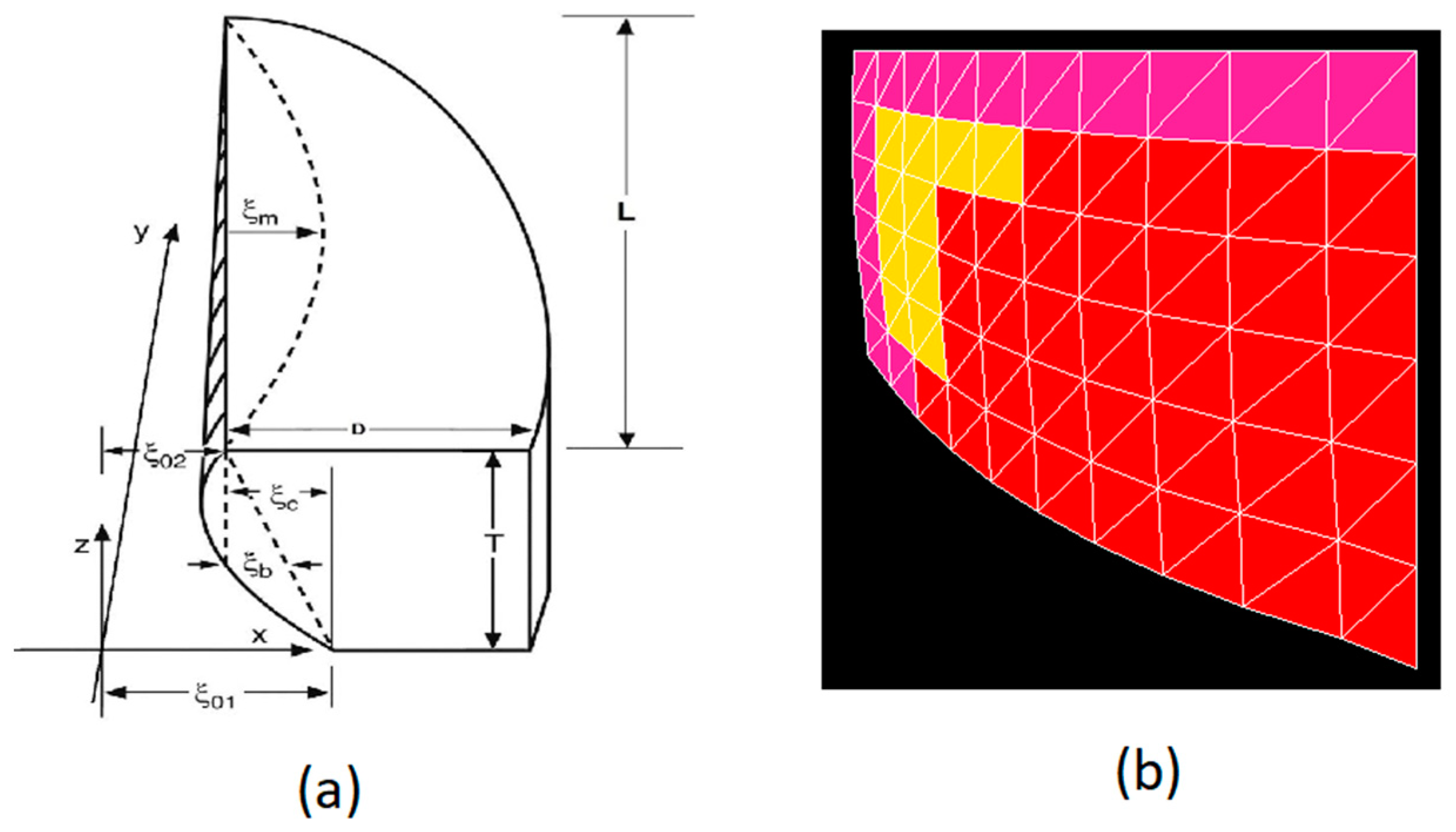
2.1. Fiber-Gel Finite Element Model
2.2. Data Collection
3. Results
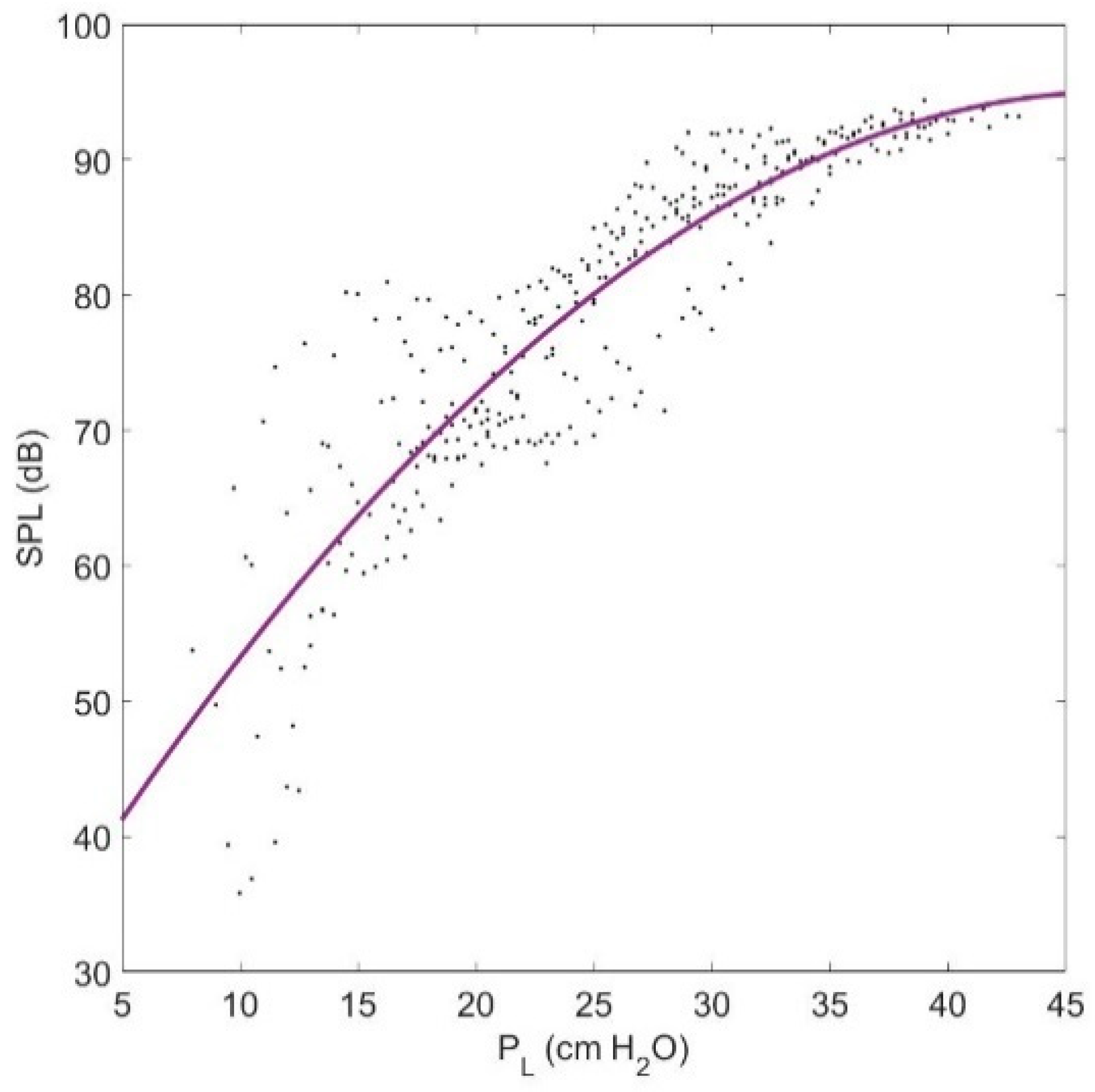
3.1. Lung Pressure
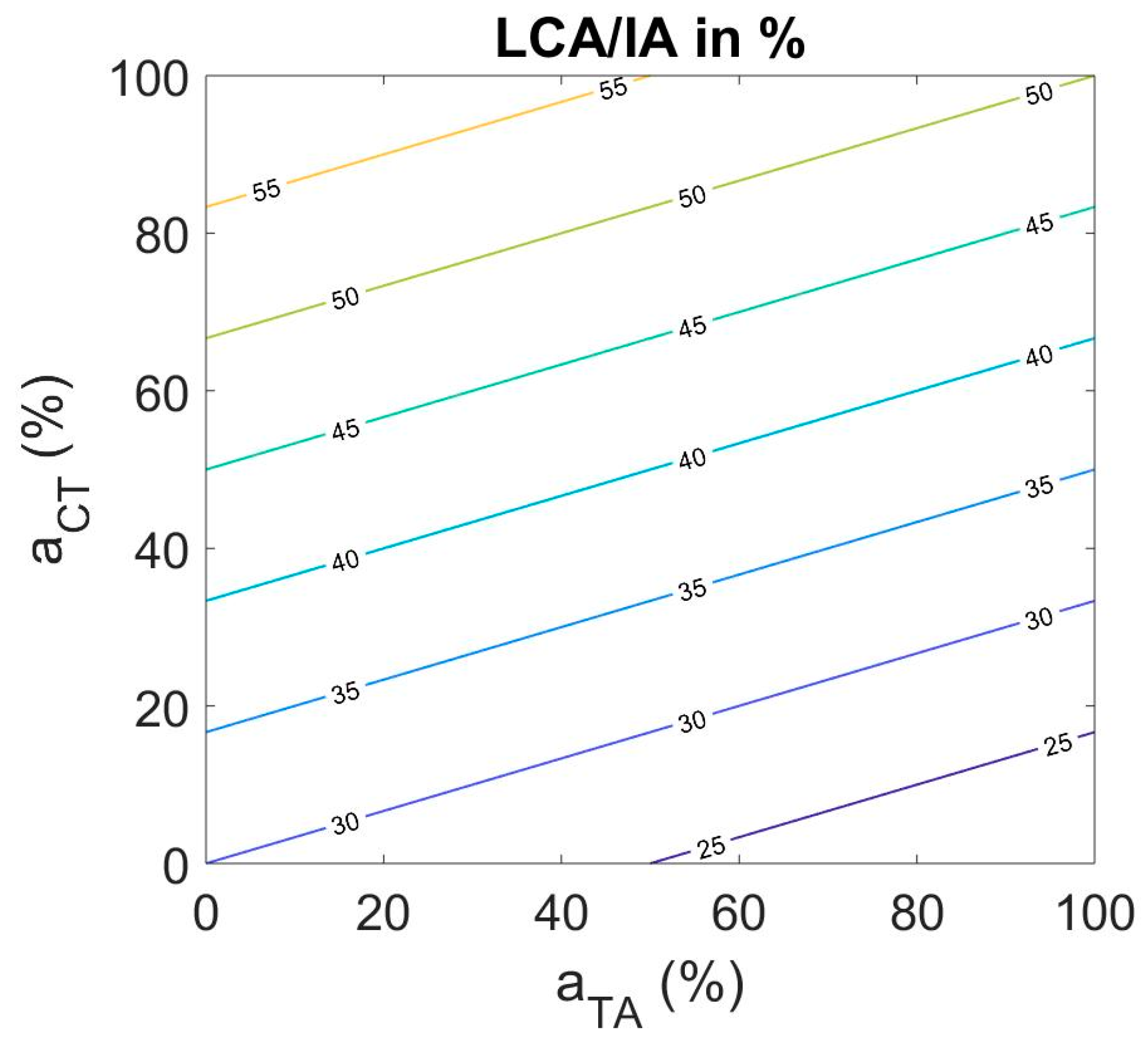
3.2. Muscle Activation for Adduction
3.3. Acoustic Features
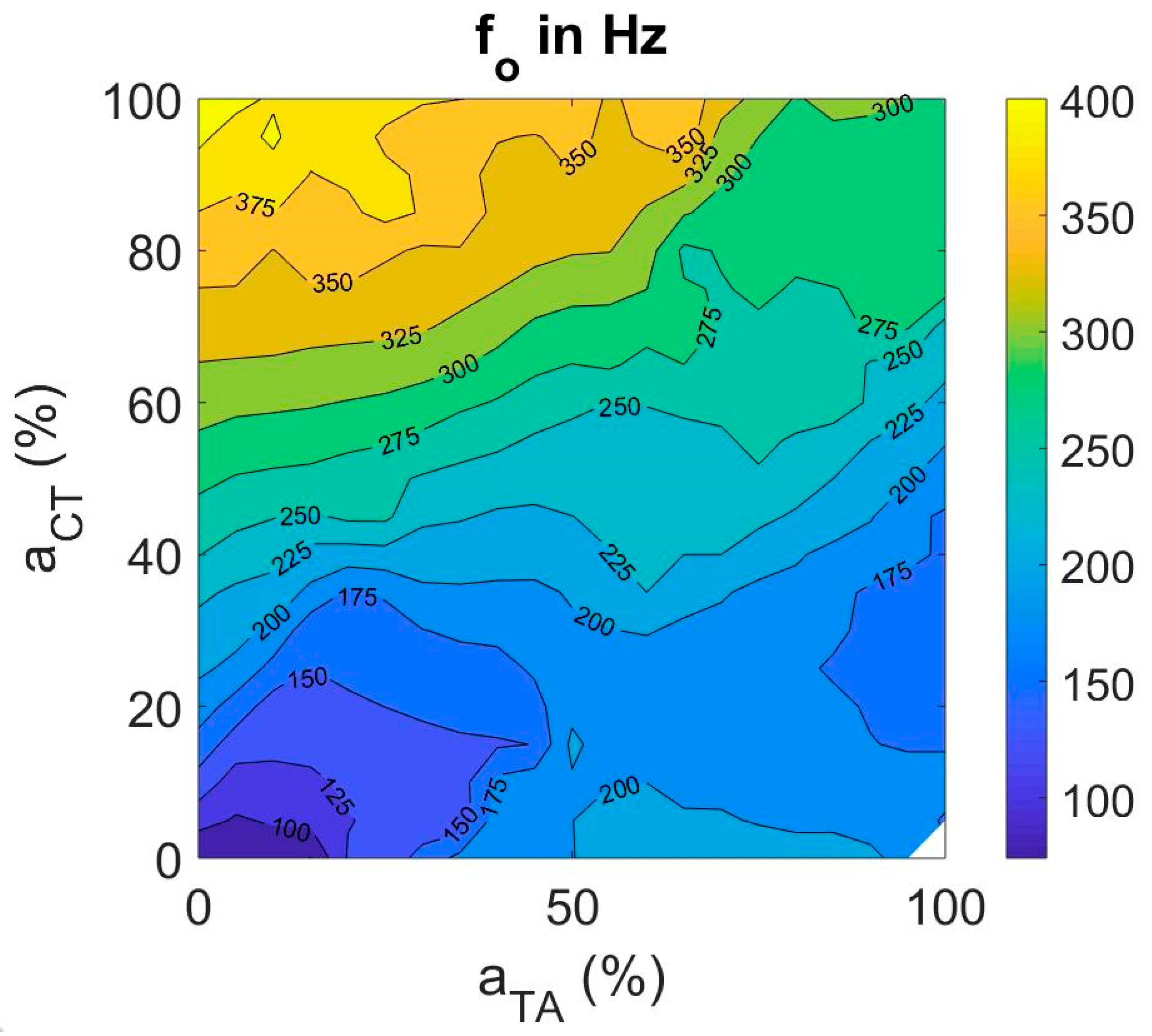
3.3.1. Fundamental Frequency
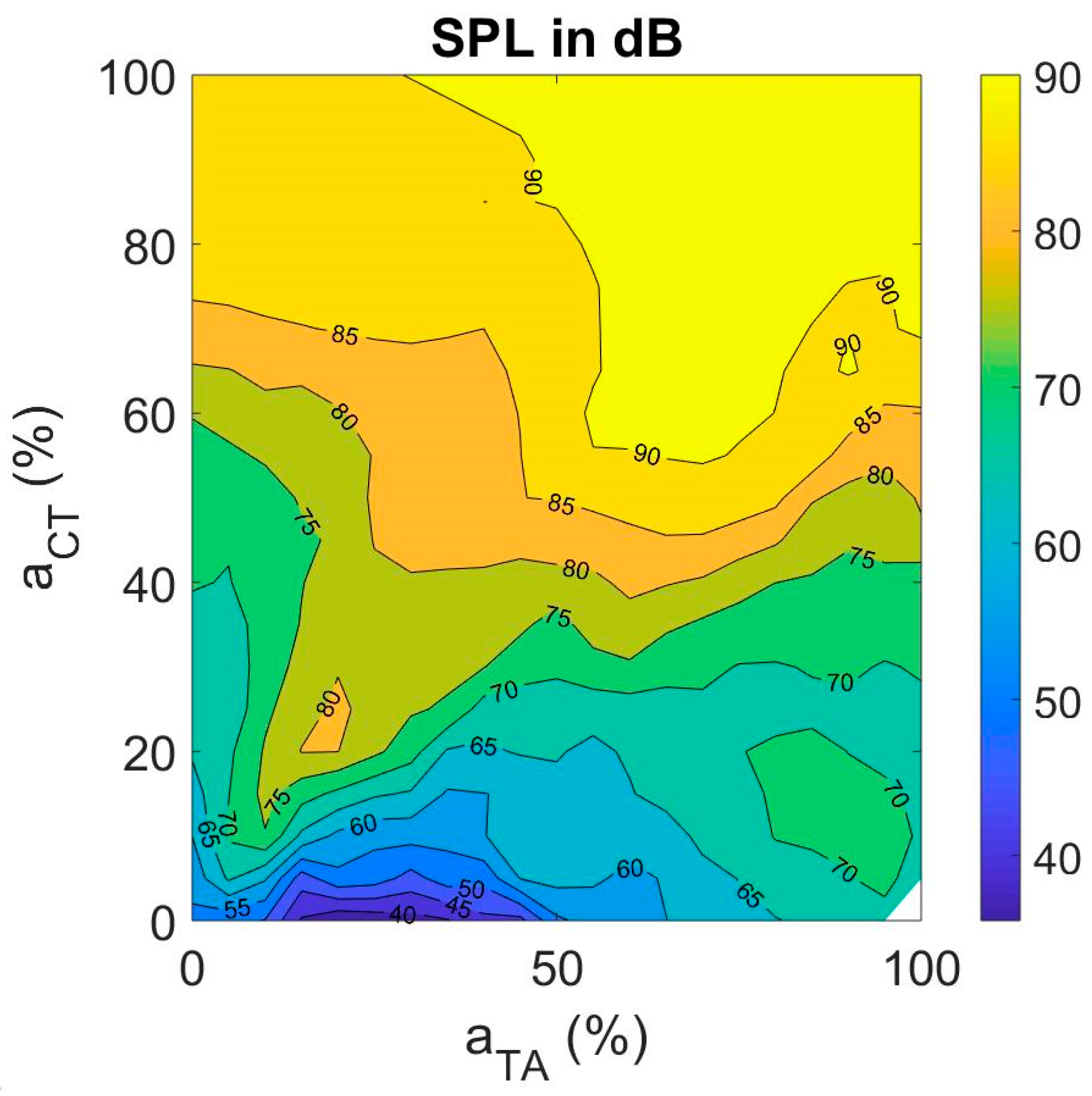
3.3.2. Sound Pressure Level (SPL)
3.3.3. Brightness of a Sound
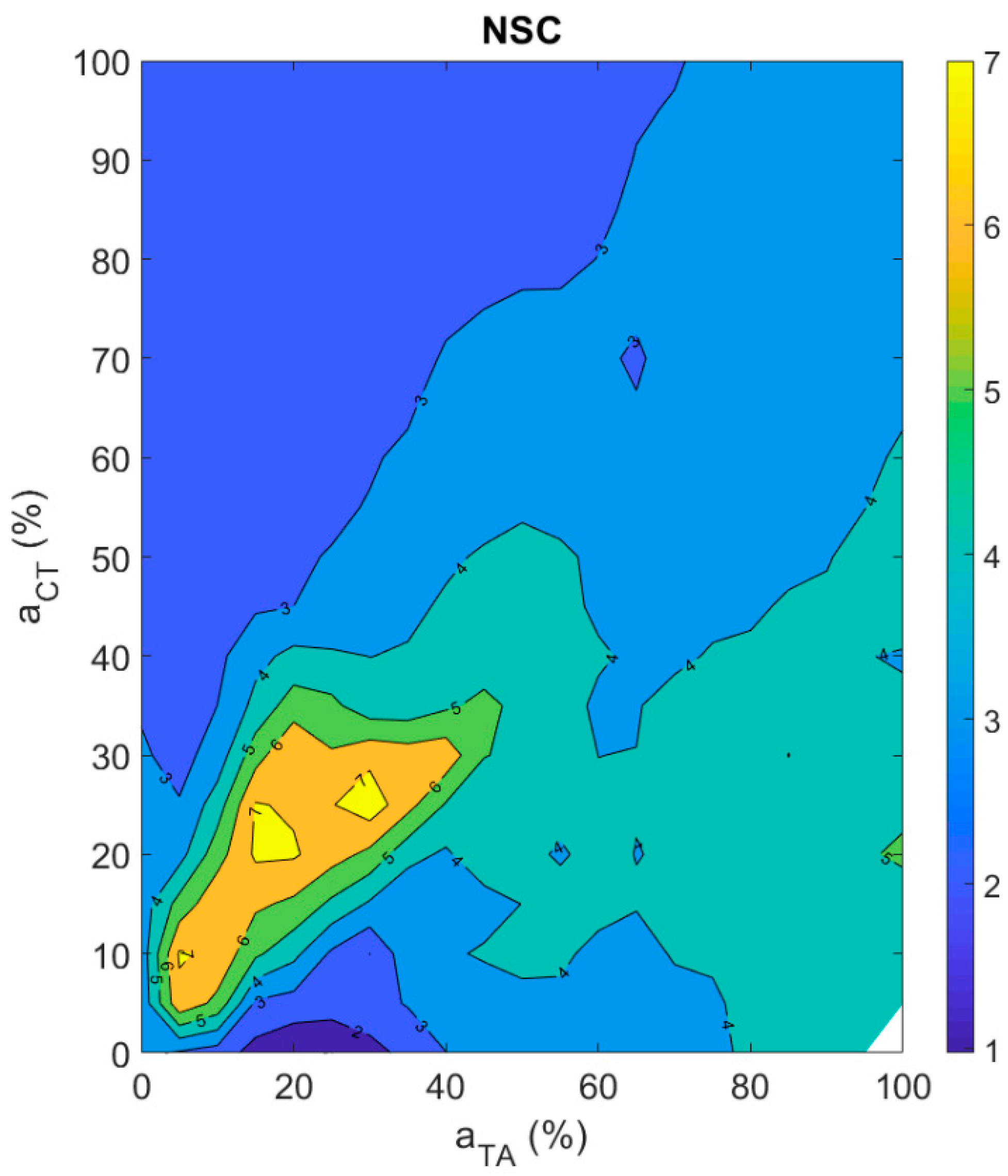
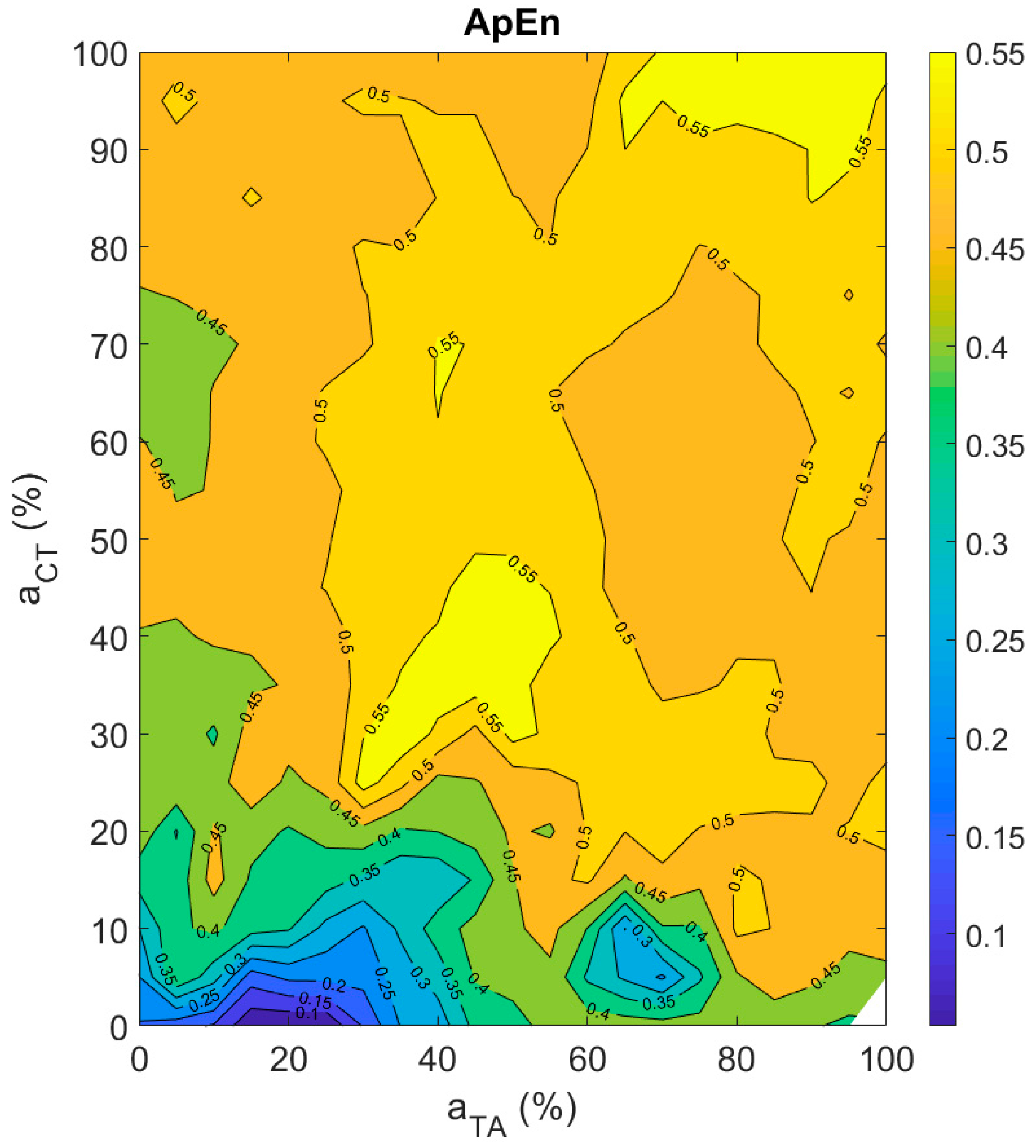
3.3.4. Aperiodicity Roughness in the Vocal Sound
3.4. Posturing Features
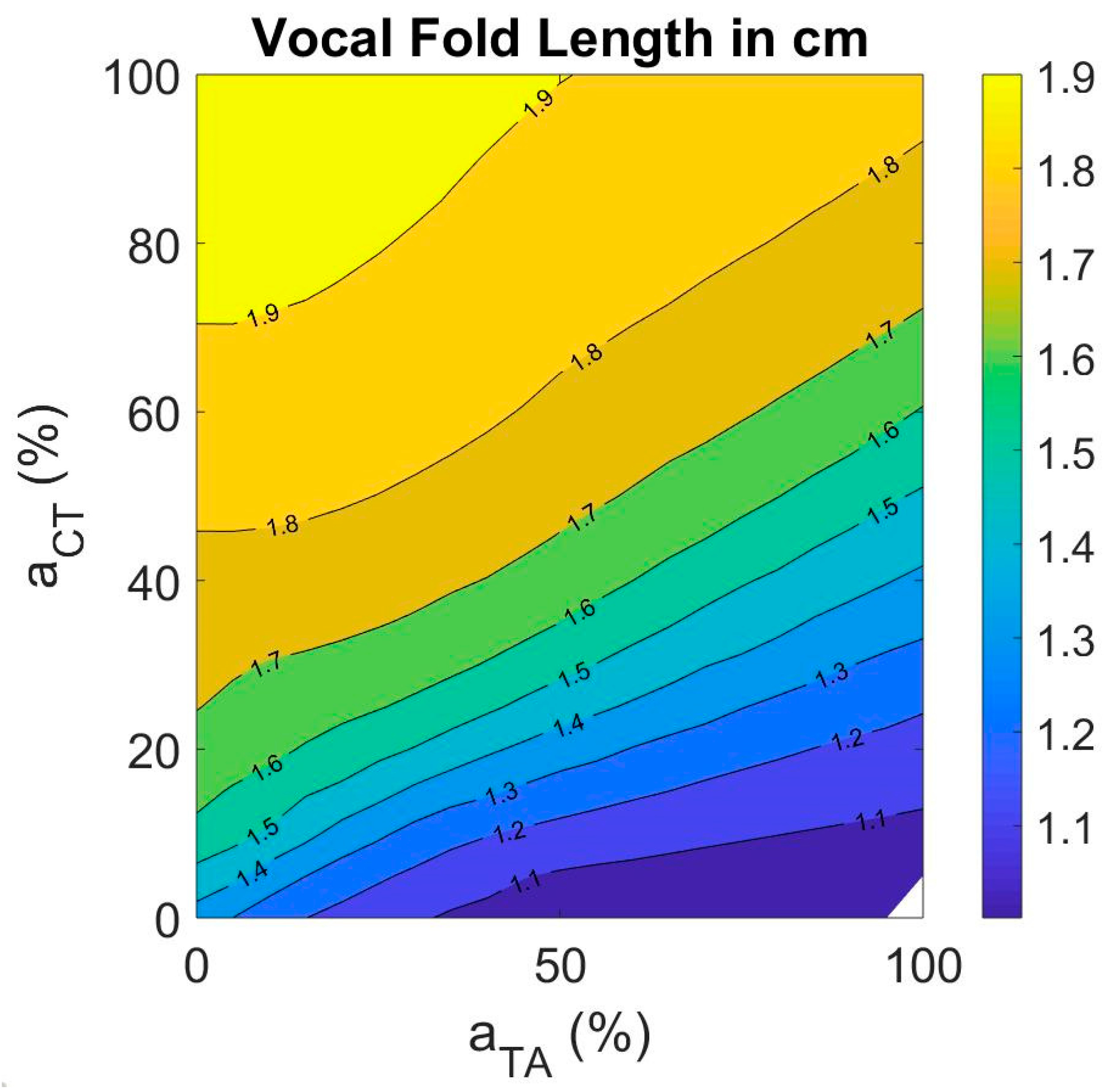
3.4.1. Vocal Fold Length
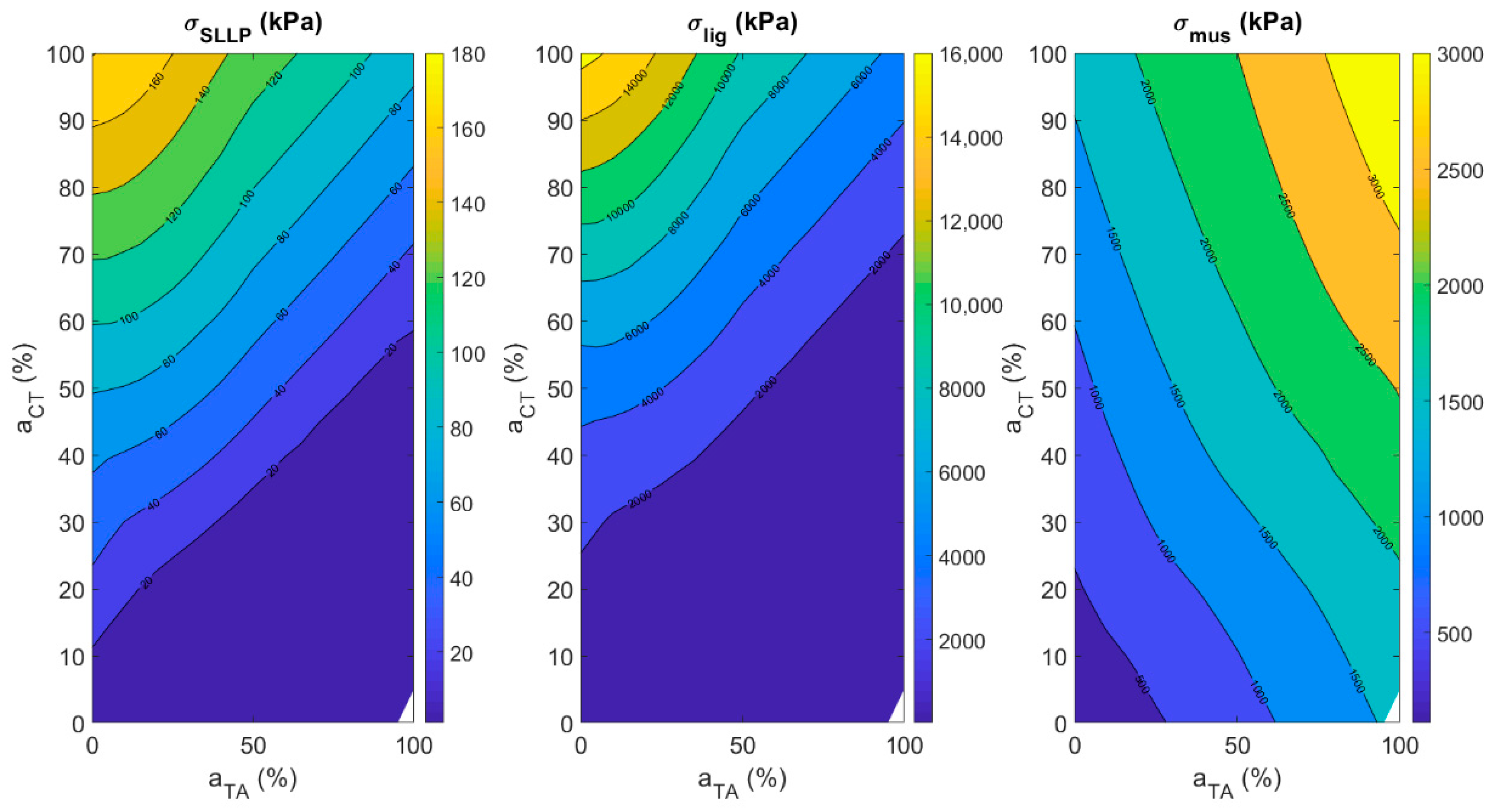
3.4.2. Fiber Stress in Vocal Fold Layers
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Callaghan, C. Auditory Perception, Winter 2016th ed.; Zalta, E.N., Ed.; Metaphysics Research Lab, Stanford University: Stanford, CA, USA, 2016. [Google Scholar]
- Schubert, E.; Wolfe, J. Does Timbral Brightness Scale with Frequency and Spectral Centroid? Acta Acust. United Acust. 2006, 92, 820–825. [Google Scholar]
- Daniel, P.; Weber, R. Psychoacoustical roughness: Implementation of an optimized model. Acta Acust. United Acust. 1997, 83, 113–123. [Google Scholar]
- Bodden, M. Instrumentation for sound quality evaluation. Acta Acust. United Acust. 1997, 83, 775–783. [Google Scholar]
- De Cheveigne, A.; Kawahara, H. YIN, a fundamental frequency estimator for speech and music. J. Acoust. Soc. Am. 2002, 111, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Harris, J.G. A sawtooth waveform inspired pitch estimator for speech and music. J. Acoust. Soc. Am. 2008, 124, 1638–1652. [Google Scholar] [CrossRef]
- Titze, I.R. Principles of Voice Production; Prentice-Hall, Engle-Wood Cliffs: Bergen, NJ, USA, 1994; pp. 220–222. [Google Scholar]
- Shmilovitz, D. On the definition of total harmonic distortion and its effect on measurement interpretation. IEEE Trans. Power Deliv. 2005, 20, 526–528. [Google Scholar]
- Carral, S.; Vergez, C.; Nederveen, C.J. Toward a single reed mouthpiece for the oboe. Arch. Acoust. 2011, 36, 267–282. [Google Scholar] [CrossRef]
- Zwicker, E.; Fastl, H. Psychoacoustics: Facts and Models; Springer: Berlin, Germany, 1990. [Google Scholar]
- Eddinsa, D.A.; Kopf, L.M.; Shrivastav, R. The psychophysics of roughness applied to dysphonic voice. J. Acoust. Soc. Am. 2015, 138, 3820–3825. [Google Scholar] [CrossRef]
- Bergan, C.C.; Titze, I.R. Perception of pitch and roughness in vocal signals with subharmonics. J. Voice 2001, 15, 165–175. [Google Scholar] [CrossRef]
- Horii, Y. Jitter and Shimmer differences among sustained vowel phonations. J. Speech Lang. Hear. Res. 1982, 25, 12–14. [Google Scholar] [CrossRef]
- Fraile, R.; Godino-Llorente, J.I. Cepstral peak prominence: A comprehensive analysis. Biomed. Signal Process. Control 2014, 14, 42–54. [Google Scholar] [CrossRef]
- Heman-Ackah, Y.D.; Heuer, R.J.; Michael, D.D.; Ostrowski, R.; Horman, M.; Baroody, M.M.; Hillenbrand, J.; Sataloff, R.T. Cepstral peak prominence: A more reliable measure of dysphonia. Ann. Otol. Rhinol. Laryngol. 2003, 112, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Latoszek, B.B.; Maryn, Y.; Gerrits, E.; De Bodt, M. A meta-analysis: Acoustic measurement of roughness and breathiness. J. Speech Lang. Hear. Res. 2018, 61, 298–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Polce, E.; Sprott, J.C.; Jiang, J.J. Applied chaos level test for validation of signal conditions underlying optimal performance of voice classification methods. J. Speech Lang. Hear. Res. 2018, 61, 1130–1139. [Google Scholar] [CrossRef]
- MacCallum, J.K.; Cai, L.; Zhang, Y.; Jiang, J.J. Acoustic analysis of aperiodic voice: Perturbation and nonlinear dynamic properties in esophageal phonation. J. Voice 2009, 23, 283–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fabris, C.; De Colle, W.; Sparacino, G. Voice disorders assessed by (cross-) sample entropy of electroglottogram and microphone signals. Biomed. Signal Process. Control 2013, 8, 920–926. [Google Scholar] [CrossRef]
- Titze, I.R.; Palaparthi, A. Sensitivity of Source-Filter Interaction to specific vocal tract shapes. IEEE/ACM Trans. Audio Speech Lang. Process. 2016, 24, 2507–2515. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Neubauer, J.; Berry, D.A. Graded activation of the intrinsic laryngeal muscles for vocal fold posturing. J. Acoust. Soc. Am. 2010, 127, EL127–EL133. [Google Scholar] [CrossRef]
- Faaborg-Andersen, K. Electromyographic investigation of intrinsic laryngeal muscles in humans. Acta Physiol. Scand. 1957, 41, 1–149. [Google Scholar]
- Faaborg-Andersen, K. Electromyography of laryngeal muscles in humans. Technics and results. Aktuel Probl. Phoniatr. Logop. 1965, 12, 1–72. [Google Scholar]
- Hirano, M.; Ohala, J.; Vennard, W. The function of laryngeal muscles in regulating fundamental frequency and intensity of phonation. J. Speech Hear. Res. 1969, 12, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Gay, T.; Hirose, H.; Strome, M.; Sawashima, M. Electromyography of the intrinsic laryngeal muscles during phonation. Ann. Otol. Rhinol. Laryngol. 1972, 81, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kochis-Jennings, K.A.; Finnegan, E.M.; Hoffman, H.T.; Jaiswal, S. Laryngeal muscle activity and vocal fold adduction during chest, chestmix, headmix, and head registers in females. J. Voice 2012, 26, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Nasri, S.; Beizai, P.; Sercarz, J.A.; Kreiman, J.; Graves, M.C.; Berke, G.S. Function of the Interarytenoid muscle in a canine laryngeal model. Ann. Otol. Rhinol. Laryngol. 1994, 103, 975–982. [Google Scholar] [CrossRef]
- Choi, H.S.; Ye, M.; Berke, G.S. Function of the interarytenoid (IA) muscle in phonation: In vivo laryngeal model. Yonsei Med. J. 1995, 36, 58–67. [Google Scholar] [CrossRef]
- Choi, H.S.; Berke, G.S.; Ye, M.; Kreiman, J. Function of the posterior cricoarytenoid muscle in phonation: In vivo laryngeal model. Otolaryngol. Head Neck Surg. 1993, 109, 1043–1051. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Neubauer, J.; Berry, D.A. Neuromuscular control of fundamental frequency and glottal posture at phonation onset. J. Acoust. Soc. Am. 2012, 131, 1401–1412. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Park, S.J. Interactions of subglottal pressure and neuromuscular activation on fundamental frequency and intensity. Laryngoscope 2016, 126, 1123–1130. [Google Scholar] [CrossRef]
- Titze, I.R.; Luschei, E.S.; Hirano, M. Role of the thyroarytenoid muscle in regulation of fundamental frequency. J. Voice 1989, 3, 213–224. [Google Scholar] [CrossRef]
- Farley, G.R. A biomechanical laryngeal model of voice F0 and glottal width control. J. Acoust. Soc. Am. 1996, 100, 3794–3812. [Google Scholar] [CrossRef]
- Titze, I.R.; Story, B.H. Rules for controlling low-dimensional vocal fold models with muscle activation. J. Acoust. Soc. Am. 2002, 112, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.M.; Luschei, E.S.; Hoffman, H.T. Modulations in respiratory and laryngeal activity associated with changes in vocal intensity during speech. J. Speech Lang. Hear. Res. 2000, 43, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.K.; Ramig, L.O.; Sapir, S.; Luschei, E.S.; Smith, M.E. Control of vocal loudness in young and old adults. J. Speech Lang. Hear. Res. 2001, 44, 297–305. [Google Scholar] [CrossRef]
- Perlman, A.L. Electromyography and the study of Oropharyngeal Swallowing. Dysphagia 1993, 8, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Hillel, A.D. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope 2001, 111, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Poletto, C.J.; Verdun, L.P.; Strominger, R.; Ludlow, C.L. Correspondence between laryngeal vocal fold movement and muscle activity during speech and nonspeech gestures. J. Appl. Physiol. 2004, 97, 858–866. [Google Scholar] [CrossRef]
- Lowell, S.Y.; Story, B.H. Simulated effects of cricothyroid and thyroarytenoid muscle activation on adult-male vocal fold vibration. J. Acoust. Soc. Am. 2006, 120, 386–397. [Google Scholar] [CrossRef]
- Vahabzadeh-Hagh, A.M.; Zhang, Z.; Chhetri, D.K. Quantitative evaluation of the in vivo vocal fold medial surface shape. J. Voice 2017, 31, 513.e15–513.e23. [Google Scholar] [CrossRef]
- Titze, I.R.; Alipour, F.; Blake, D.; Palaparthi, A. Comparison of a fiber-gel finite element model of vocal fold vibration to a transversely isotropic stiffness model. J. Acoust. Soc. Am. 2017, 142, 1376–1383. [Google Scholar] [CrossRef]
- Titze, I.R. The Myoelastic Aerodynamic Theory of Phonation; National Center for Voice and Speech: Denver, CO, USA, 2006; pp. 1–337. [Google Scholar]
- Story, B.H.; Titze, I.R.; Hoffman, E.A. Vocal tract area functions from magnetic resonance imaging. J. Acoust. Soc. Am. 1996, 100, 537–554. [Google Scholar] [CrossRef]
- Hirano, M. Phonosurgery: Basic and clinical investigations. Otol. Fukuoka 1975, 21, 239–440. [Google Scholar]
- Sato, K.; Hirano, M. Histological investigation of the macula flava of the human vocal fold. Ann. Otol. Rhinol. Laryngol. 1995, 104, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.D.; Alipour, F.; Titze, I.R. Biomechanical and histological observations of vocal fold fibrous proteins. Ann. Otol. Rhinol. Laryngol. 2000, 109, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Berry, D.A.; Titze, I.R. A finite element model of vocal fold vibration. J. Acoust. Soc. Am. 2000, 108, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Liljencrants, J. Speech Synthesis with a Reflection-Type Line Analog. Ph.D. Thesis, Department of Speech Communication and Music Acoustics, Royal Institute of Technology, Stockholm, Sweden, 1985. [Google Scholar]
- Story, B.H. Physiologically Based Speech Simulation Using an Enhanced Wave Reflection Model of the Vocal Tract. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 1995. [Google Scholar]
- Titze, I.R.; Palaparthi, A.; Smith, S. Benchmarks for time-domain simulation of sound propagation in soft-walled airways: Steady configurations. J. Acoust. Soc. Am. 2014, 136, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R.; Palaparthi, A. Radiation efficiency for long-range vocal communication in mammals and birds. J. Acoust. Soc. Am. 2018, 143, 2813–2824. [Google Scholar] [CrossRef]
- Palaparthi, A.; Riede, T.; Titze, I.R. Combining multiobjective optimization and cluster analysis to study vocal fold functional morphology. IEEE Trans. Biomed. Eng. 2014, 61, 2199–2208. [Google Scholar] [CrossRef]
- Palaparthi, A.; Smith, S.; Mau, T.; Titze, I.R. A computational study of depth of vibration into vocal fold tissues. J. Acoust. Soc. Am. 2019, 145, 881–891. [Google Scholar] [CrossRef]
- Flanagan, J.L. Speech Analysis, Synthesis, and Perception; Springer: New York, NY, USA, 1972. [Google Scholar]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef]
- Titze, I.R. The physics of small-amplitude oscillation of the vocal folds. J. Acoust. Soc. Am. 1988, 83, 1536–1552. [Google Scholar] [CrossRef]
- Titze, I.R.; Sundberg, J. Vocal intensity in speakers and singers. J. Acoust. Soc. Am. 1992, 91, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, S.; Sundberg, J. Relationship between subglottal pressure and sound pressure level in untrained singers. J. Voice 2016, 30, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R.; Riede, T.; Mau, T. Predicting fundamental frequency ranges in vocalization across species. PLoS Comput. Biol. 2016, 12, e1004907. [Google Scholar] [CrossRef] [PubMed]

| CT | LCA | TA | IA | PCA | LIG | SLLP | |
|---|---|---|---|---|---|---|---|
| 5 | 59 | 1.5 | 30 | 55 | 1 | ||
| B | 7 | 4 | 6.5 | 3.5 | 5.3 | 12.8 | 6.5 |
| −0.9 | −0.9 | −0.9 | −0.9 | −0.9 | −0.9 | −0.9 | |
| −0.06 | 0.05 | −0.5 | 0 | 0.1 | −0.5 | −0.2 | |
| 400 | 140 | 180 | 140 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaparthi, A.; Smith, S.; Titze, I.R. Mapping Thyroarytenoid and Cricothyroid Activations to Postural and Acoustic Features in a Fiber-Gel Model of the Vocal Folds. Appl. Sci. 2019, 9, 4671. https://doi.org/10.3390/app9214671
Palaparthi A, Smith S, Titze IR. Mapping Thyroarytenoid and Cricothyroid Activations to Postural and Acoustic Features in a Fiber-Gel Model of the Vocal Folds. Applied Sciences. 2019; 9(21):4671. https://doi.org/10.3390/app9214671
Chicago/Turabian StylePalaparthi, Anil, Simeon Smith, and Ingo R. Titze. 2019. "Mapping Thyroarytenoid and Cricothyroid Activations to Postural and Acoustic Features in a Fiber-Gel Model of the Vocal Folds" Applied Sciences 9, no. 21: 4671. https://doi.org/10.3390/app9214671
APA StylePalaparthi, A., Smith, S., & Titze, I. R. (2019). Mapping Thyroarytenoid and Cricothyroid Activations to Postural and Acoustic Features in a Fiber-Gel Model of the Vocal Folds. Applied Sciences, 9(21), 4671. https://doi.org/10.3390/app9214671





