Optical Properties of PbS Quantum Dots deposited on Glass Employing a Supercritical CO2 Fluid Process
Abstract
1. Introduction
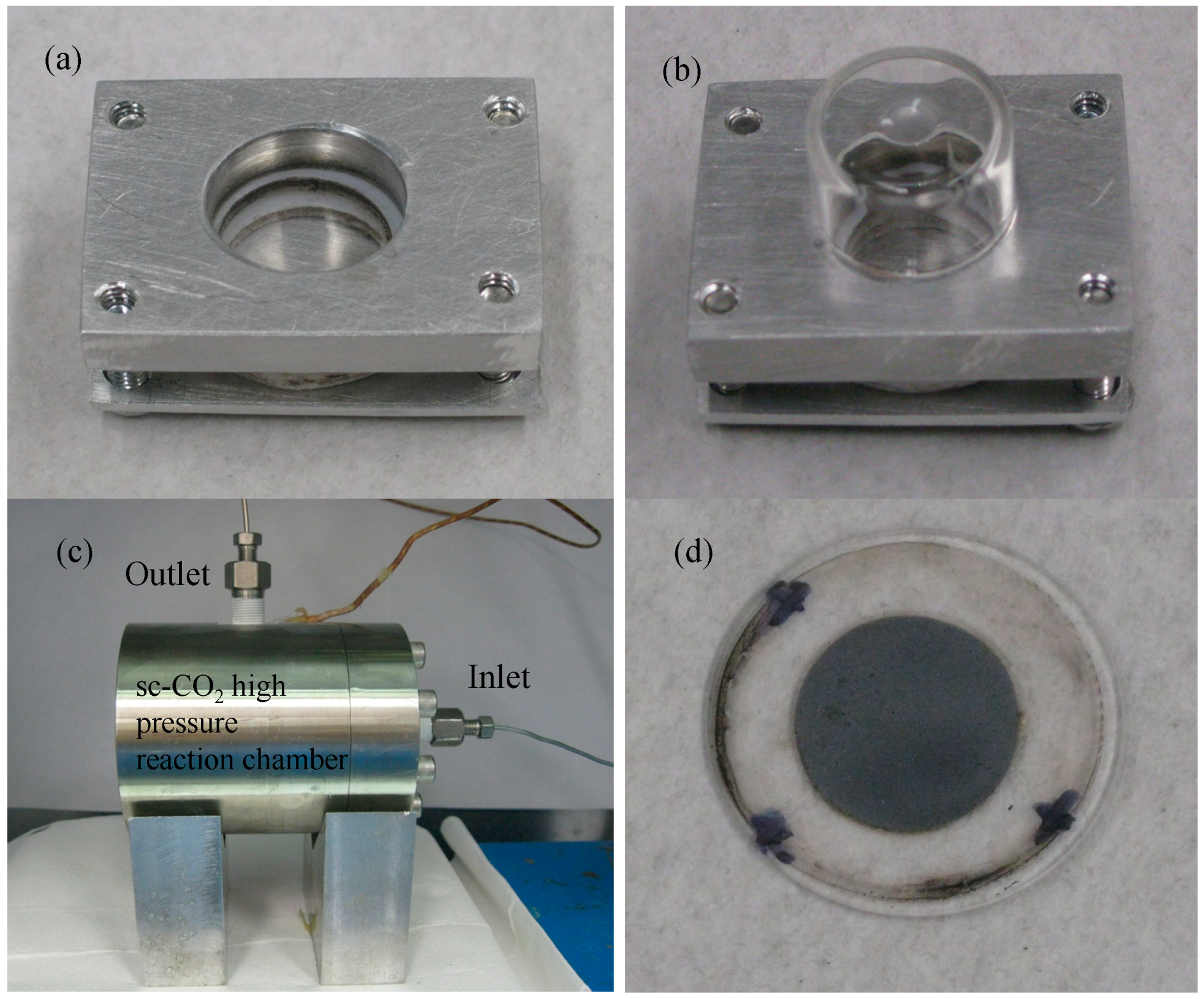
2. Experimental Section
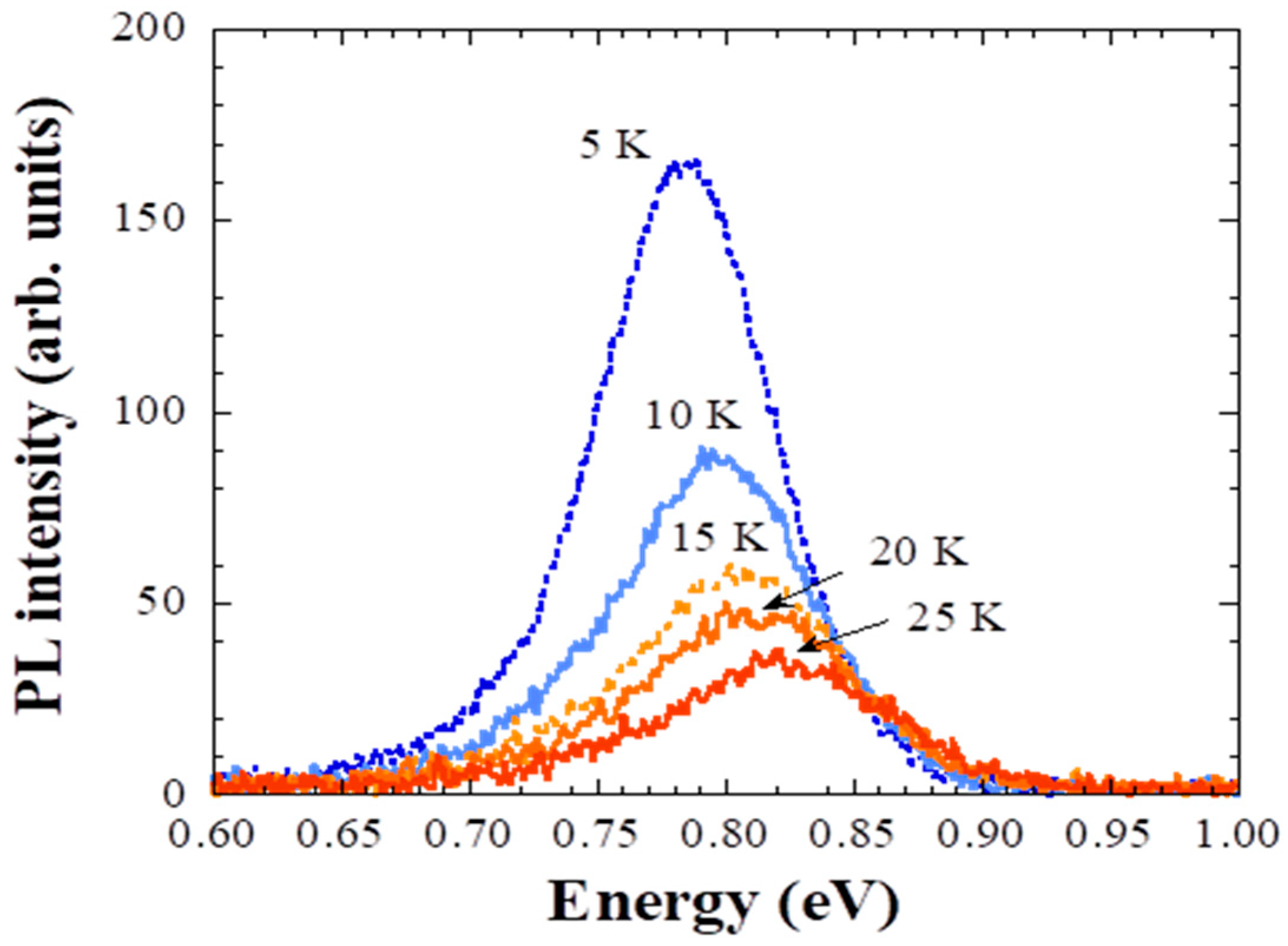
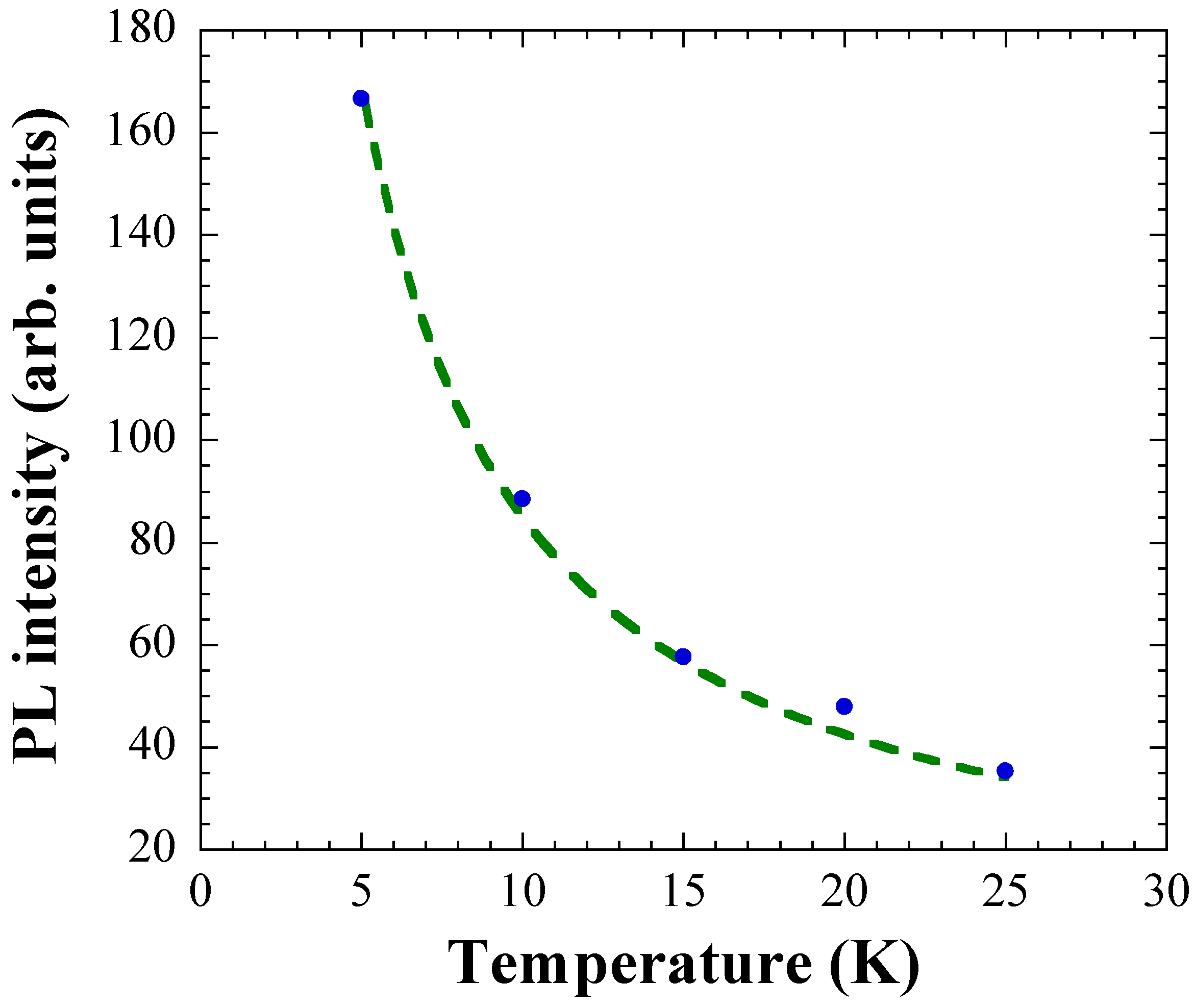
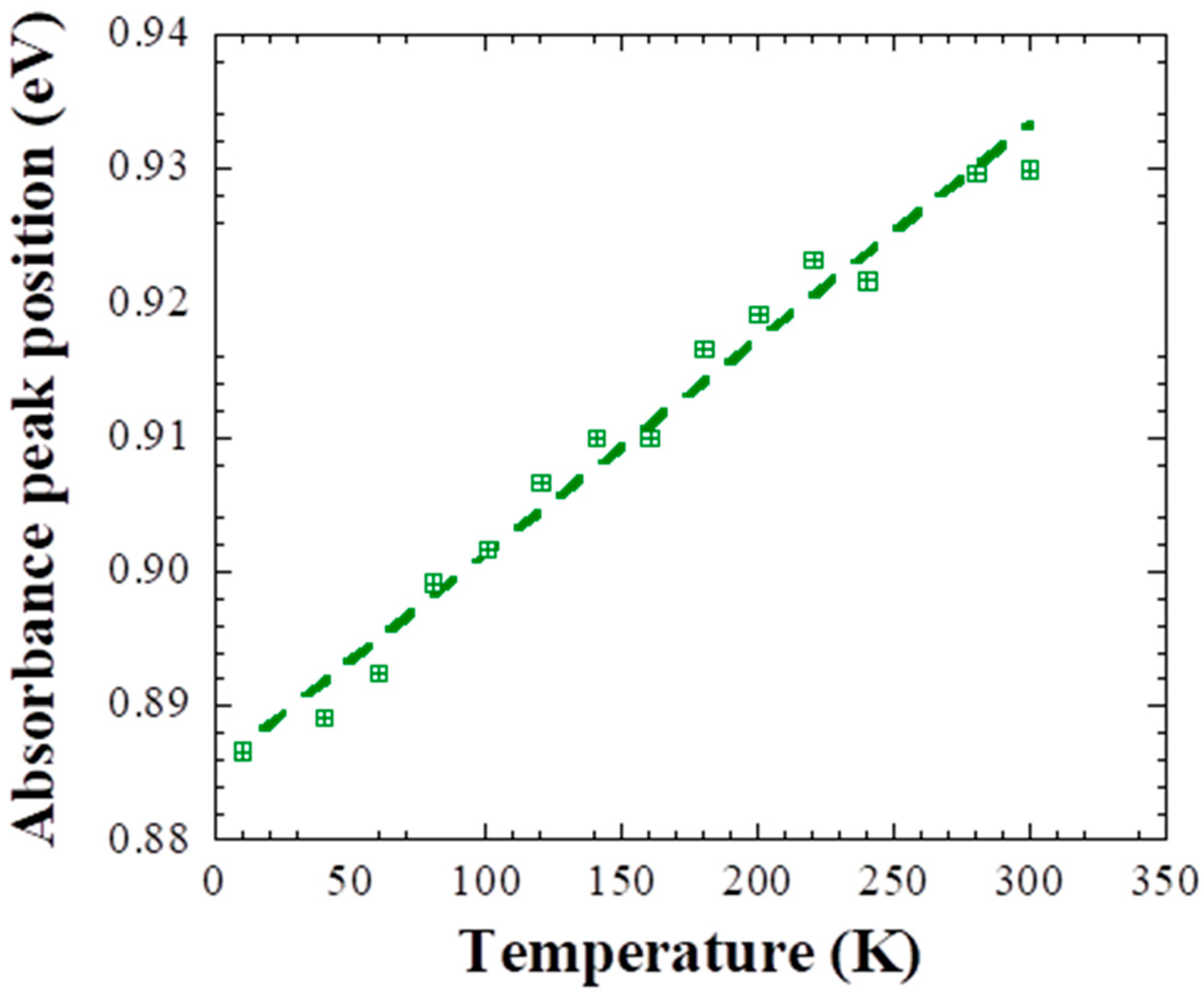
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rakher, M.T.; Bose, R.; Wong, C.W.; Srinivasan, K. Spectroscopy of 1.55 μm PbS quantum dots on Si photonic crystal cavities with a fiber taper waveguide. Appl. Phys. Lett. 2010, 96, 161108. [Google Scholar] [CrossRef]
- Gaponenko, M.S.; Lutich, A.A.; Tolstik, N.A.; Onushchenko, A.A.; Malyarevich, A.M.; Petrov, E.P.; Yumashev, K.V. Temperature-dependent photoluminescence of PbS quantum dots in glass: Evidence of exciton state splitting and carrier trapping. Phys. Rev. B 2010, 82, 125320. [Google Scholar] [CrossRef]
- Singh, A.K.; Barik, P.; Ullrich, B. Magneto-optical controlled transmittance alteration of PbS quantum dots by moderately applied magnetic fields at room temperature. Appl. Phys. Lett. 2014, 105, 242410. [Google Scholar] [CrossRef]
- Wise, F.W. Lead salt quantum dots: The limit of strong quantum confinement. Acc. Chem. Res. 2000, 33, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Lü, W.; Kamiya, I.; Ichida, M.; Ando, H. Temperature dependence of electronic energy transfer in PbS quantum dot films. Appl. Phys. Lett. 2009, 95, 83102. [Google Scholar] [CrossRef]
- Lü, W.; Yamada, F.; Kamiya, I. Self-assembled colloidal PbS quantum dots on GaAs substrates. J. Phys. Conf. Ser. 2009, 245, 012069. [Google Scholar] [CrossRef]
- Peterson, J.J.; Krauss, T.D. Fluorescence spectroscopy of single lead sulfide quantum dots. Nano Lett. 2006, 6, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Turyanska, L.; Patanè, A.; Henini, M.; Hennequin, B.; Thomas, N.R. Temperature dependence of the photoluminescence emission from thiol-capped PbS quantum dots. Appl. Phys. Lett. 2007, 90, 101913. [Google Scholar] [CrossRef]
- Lu, W.; Yamada, F.; Kamiya, I. Fabrication and coupling investigation of films of PbS quantum dots. J. Vac. Sci. Technol. B 2010, 28, C5E8–C5E12. [Google Scholar] [CrossRef]
- Ullrich, B.; Xiao, X.Y.; Brown, G.J. Photoluminescence of PbS quantum dots on semi-insulating GaAs. J. Appl. Phys. 2010, 108, 13525. [Google Scholar] [CrossRef]
- Brown, G.J.; Ullrich, B.; Wang, J.S. Analysis of thermal band gap variations of PbS quantum dots by Fourier transform transmission and emission spectroscopy. Appl. Phys. Lett. 2011, 99, 81901. [Google Scholar]
- Fan, H.Y. Temperature dependence of the energy gap in semiconductors. Phys. Rev. 1951, 82, 900. [Google Scholar] [CrossRef]
- Bhowmick, M.; Xi, H.; Androulidaki, M.; Ullrich, B. Mathematical assessment of the thermal band gap variation of semiconductors. Phys. Scr. 2019, 94, 085701. [Google Scholar] [CrossRef]
- Smetana, A.B.; Wang, J.S.; Boeckl, J.J.; Brown, G.J.; Wai, C.M. Deposition of ordered arrays of gold and platinum nanoparticles with an adjustable particle size and interparticle spacing using supercritical CO2. J. Phys. Chem. C 2008, 112, 2294–2297. [Google Scholar] [CrossRef]
- Liu, J.; Anand, M.; Roberts, C.B. Synthesis and extraction of β-d-glucose-stabilized Au nanoparticles processed into low-defect, wide-area thin films and ordered arrays using CO2-expanded liquids. Langmuir 2006, 22, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Suna, A.; Mahler, W.; Kasowski, R. PbS in polymers. From molecules to bulk solids. J. Chem. Phys. 1987, 87, 7315–7322. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Li, B.; Chen, X.; Dong, Y.; Zhang, M. Size-dependent radiative emission of PbS quantum dots embedded in Nafion membrane. Appl. Phys. A 2009, 95, 173–177. [Google Scholar] [CrossRef]
- Zemel, J.N.; Jensen, J.D.; Schoolar, R.B. Electrical and optical properties of epitaxial films of PbS, PbSe, PbTe, and SnTe. Phys. Rev. 1965, 140, 330–342. [Google Scholar] [CrossRef]
- Van Roosbroeck, W.; Shockley, W. Photon-radiative recombination of electrons and holes in germanium. Phys. Rev. 1954, 94, 1558–1560. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullrich, B.; Wang, J. Optical Properties of PbS Quantum Dots deposited on Glass Employing a Supercritical CO2 Fluid Process. Appl. Sci. 2019, 9, 4567. https://doi.org/10.3390/app9214567
Ullrich B, Wang J. Optical Properties of PbS Quantum Dots deposited on Glass Employing a Supercritical CO2 Fluid Process. Applied Sciences. 2019; 9(21):4567. https://doi.org/10.3390/app9214567
Chicago/Turabian StyleUllrich, Bruno, and Joanna Wang. 2019. "Optical Properties of PbS Quantum Dots deposited on Glass Employing a Supercritical CO2 Fluid Process" Applied Sciences 9, no. 21: 4567. https://doi.org/10.3390/app9214567
APA StyleUllrich, B., & Wang, J. (2019). Optical Properties of PbS Quantum Dots deposited on Glass Employing a Supercritical CO2 Fluid Process. Applied Sciences, 9(21), 4567. https://doi.org/10.3390/app9214567




