Facile Preparation of Multilayered Graphene with CO2 as a Carbon Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials
2.1.2. Experiment Equipment
2.2. Pretreatment of Materials
2.3. Synthesis of Multilayered Graphene
2.3.1. Synthesis Process
2.3.2. Yield Rate and Yield of Multilayered Graphene
2.3.3. Conversion between the Amount of Substance and Velocity of CO2
3. Results
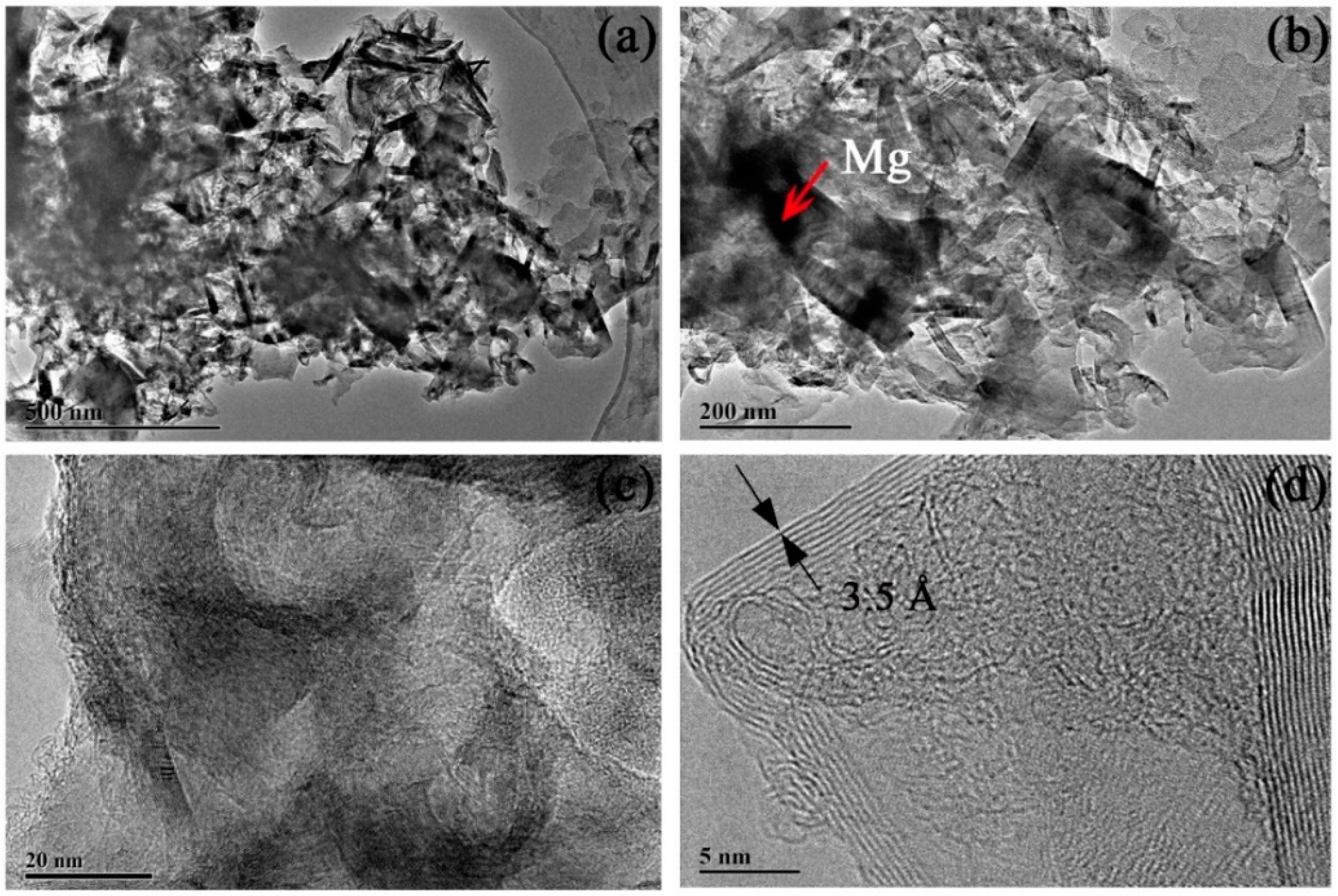
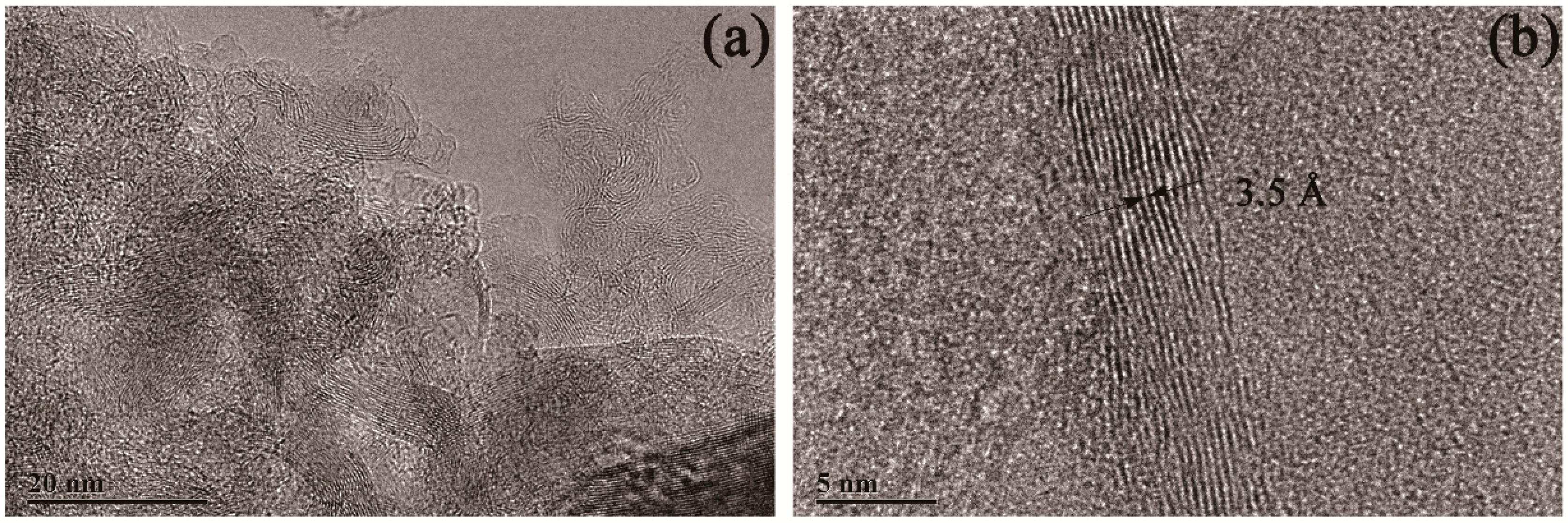
3.1. Mg Ribbon and Mg Powder
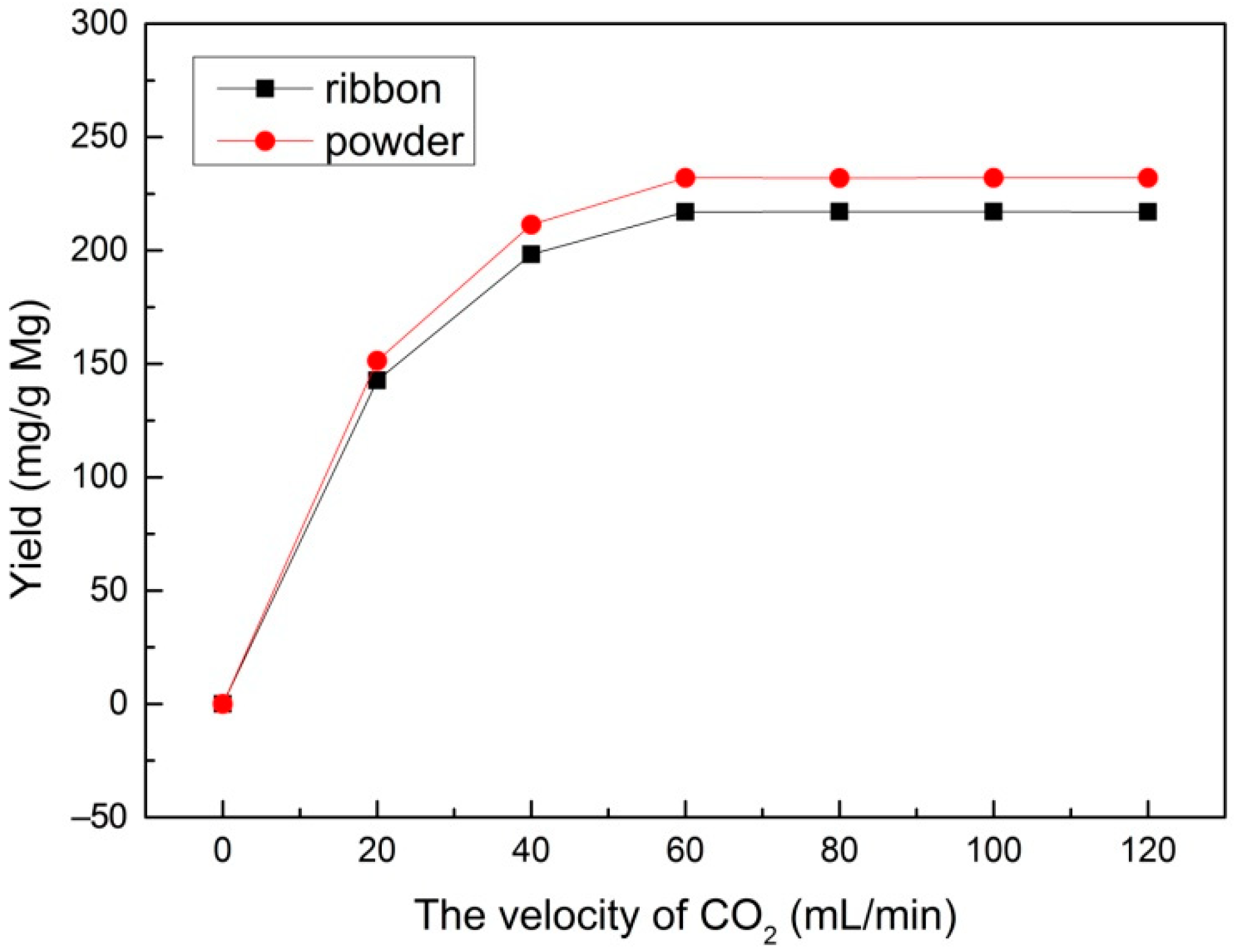
3.2. Multilayered Graphene under Different Velocities of CO2 Flow
3.3. Multilayered Graphene under Different Reaction Temperatures
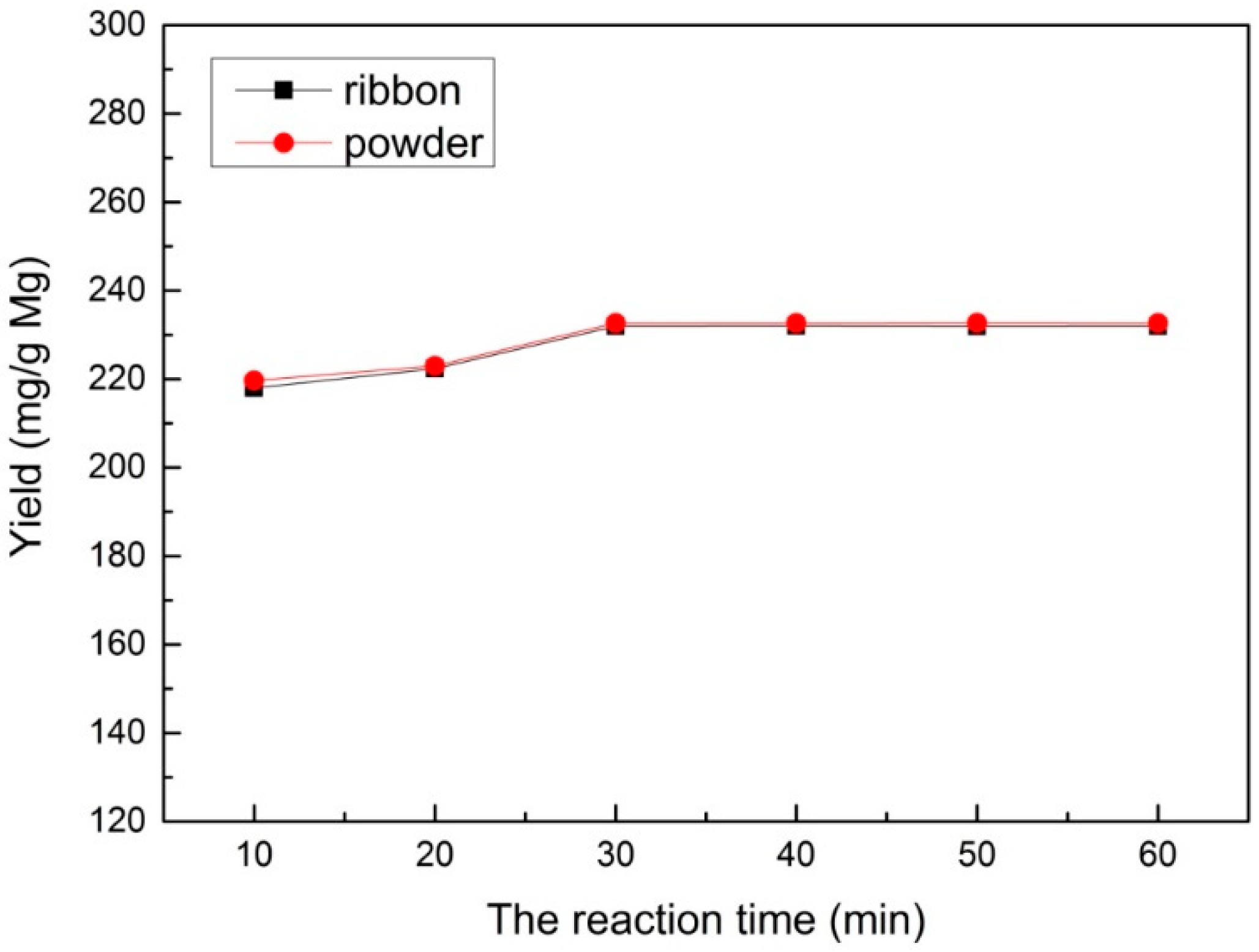
3.4. Multilayered Graphene under Different Reaction Times
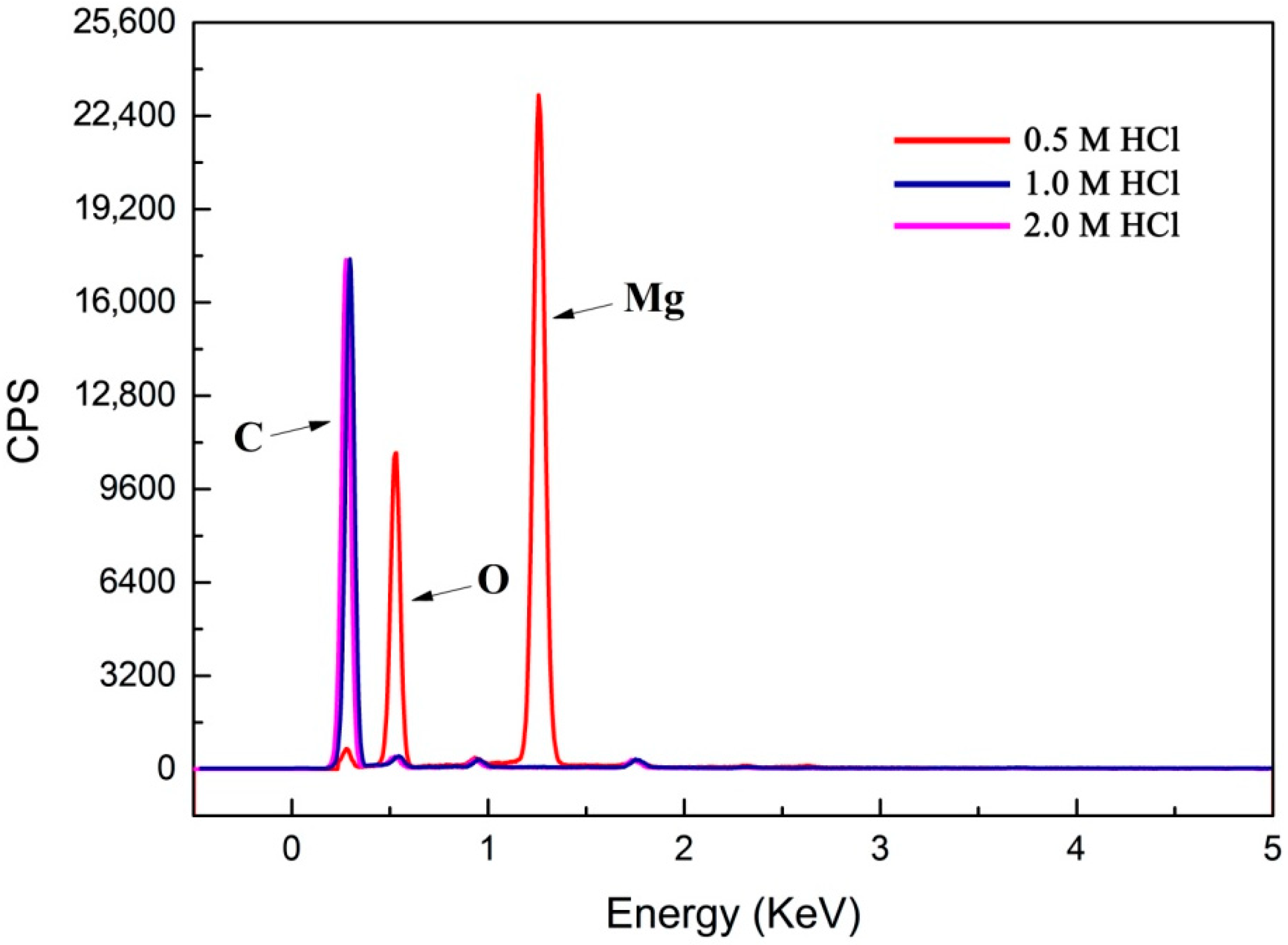
3.5. Multilayered Graphene under Different Concentrations of HCl Solution for Sample Post-Processing
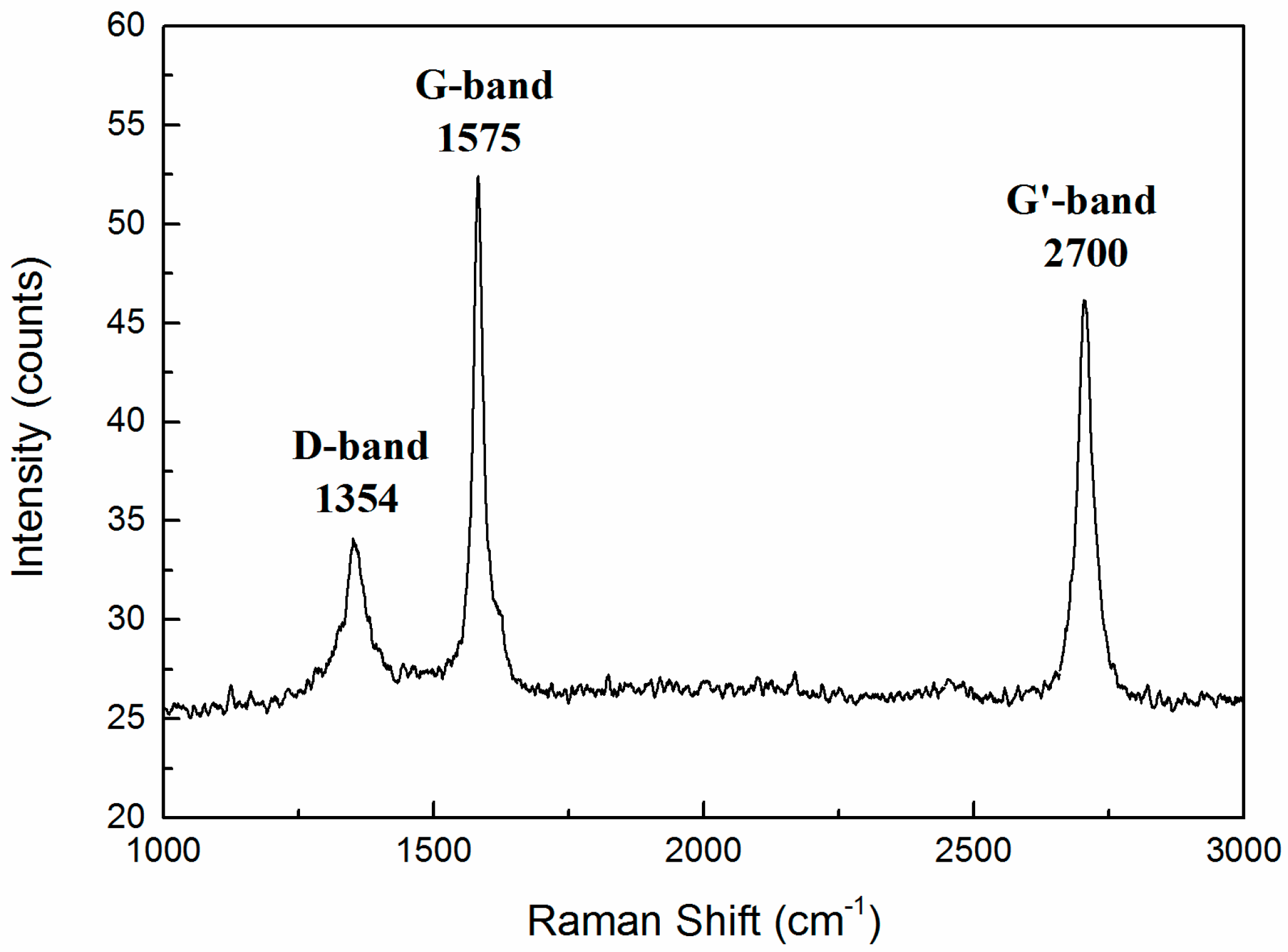
3.6. Raman Spectroscopy Characterization
3.7. XRD Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhong, X.; Jiang, S.; Huang, Y.; Duan, X. Graphene nanomesh. Nat. Nanotechnol. 2010, 5, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, P.; Athanassiou, A.; Bayer, I.S. Graphene nanoplatelets-based advanced materials and recent progress in sustainable applications. Appl. Sci. 2018, 8, 1438. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Kashif, M.F.; Bianco, G.V.; Stomeo, T.; Vincenti, M.A.; de Ceglia, D.; de Vittorio, M.; Scalora, M.; Bruno, G.; D’Orazio, A.; Grande, M. Graphene-based cylindrical pillar gratings for polarization-insensitive optical absorbers. Appl. Sci. 2019, 9, 2528. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.V.; Tene, T.; Guevara, M.; Usca, G.T.; Colcha, D.; Brito, H.; Molina, R.; Bellucci, S.; Tavolaro, A. Preparation of few-layer graphene dispersions from hydrothermally expanded graphite. Appl. Sci. 2019, 9, 2539. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Direct synthesis of graphene quantum dots with different fluorescence properties by oxidation of graphene oxide using nitric acid. Appl. Sci. 2018, 8, 1303. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Hu, T.; Liu, X.; Ma, D.; Wei, R.; Xu, K.; Ma, F. Formation of micro-and nano-trenches on epitaxial graphene. Appl. Sci. 2018, 8, 2518. [Google Scholar] [CrossRef]
- Kun, P.; Weber, F.; Balazsi, C. Preparation and examination of multilayer graphene nanosheets by exfoliation of graphite in high efficient attritor mill. Cent. Eur. J. Chem. 2011, 9, 47–51. [Google Scholar] [CrossRef]
- Sierra, U.; Alvarez, P.; Blanco, C.; Granda, M.; Santamaria, R.; Menendez, R. New alternatives to graphite for producing graphene materials. Carbon 2015, 93, 812–818. [Google Scholar] [CrossRef]
- Lian, P.; Liu, H.; Li, H.; Zhang, Y.; Mei, Y. A facile and mild route to synthesize ultralight and flexible 3D functionalized graphene. J. Porous Mater. 2018, 25, 905–911. [Google Scholar] [CrossRef]
- Ryu, S.K.; Jin, H.; Gondy, D.; Pusset, N.; Ehrburger, P. Activation of carbon-fibers by steam and carbon-dioxide. Carbon 1993, 31, 841–842. [Google Scholar] [CrossRef]
- Yang, S.; Qiao, Y.; He, P.; Liu, Y.; Cheng, Z.; Zhu, J.-J.; Zhou, H. A reversible lithium-CO2 battery with Ru nanoparticles as a cathode catalyst. Energy Environ. Sci. 2017, 10, 972–978. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Zhao, C.; Li, S.; Song, Y.; Ge, R.; Wei, W.; Sun, Y. Exclusive formation of formic acid from CO2 electroreduction by a tunable Pd-Sn alloy. Angew. Chem. Int. Ed. 2017, 56, 12219–12223. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Lu, J.; Skrabutenas, J.C.; Xu, T.; Xiao, Z.; Maguire, J.A.; Hosmane, N.S. Conversion of carbon dioxide to few-layer graphene. J. Mater. Chem. 2011, 21, 9491–9493. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Large scale synthesis of N-doped multi-layered graphene sheets by simple arc-discharge method. Carbon 2010, 48, 255–259. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Price, R.J.; Ladislaus, P.I.; Smith, G.C.; Davies, T.J. A novel ‘bottom-up’ synthesis of few-and multi-layer graphene platelets with partial oxidation via cavitation. Ultrason. Sonochem. 2019, 56, 466–473. [Google Scholar] [CrossRef]
- Kim, H.Y.; Dawood, O.M.; Monteverde, U.; Sexton, J.; Li, Z.L.; Britnell, L.; Migliorato, M.A.; Young, R.J.; Missous, M. Multilayer stacking and metal deposition effects on large area graphene on GaAs. Carbon 2016, 96, 83–90. [Google Scholar] [CrossRef]
- Johnson, J.L.; Behnam, A.; Pearton, S.J.; Ural, A. Hydrogen sensing using Pd-functionalized multi-layer graphene nanoribbon networks. Adv. Mater. 2010, 22, 4877–4880. [Google Scholar] [CrossRef]
- Biswas, S.; Drzal, L.T. Multi layered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Mohanty, A.; Janowska, I. Tuning the structure of in-situ synthesized few layer graphene/carbon composites into nanoporous vertically aligned graphene electrodes with high volumetric capacitance. Electrochim. Acta 2019, 308, 206–216. [Google Scholar] [CrossRef]
- Maffucci, A.; Miano, G. Electrical properties of graphene for interconnect applications. Appl. Sci. 2014, 4, 305–317. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Y.; Tian, H.; Li, M.; Jian, J.; Wei, Y.; Tian, Y.; Wang, D.-Y.; Pang, Y.; Geng, X.; et al. Multilayer graphene epidermal electronic skin. ACS Nano 2018, 12, 8839–8846. [Google Scholar] [CrossRef] [PubMed]
- Khurram, A.; He, M.F.; Gallant, B.M. Tailoring the discharge reaction in Li-CO2 batteries through incorporation of CO2 capture chemistry. Joule 2018, 2, 2649–2666. [Google Scholar] [CrossRef]
- Ramachandran, P.A.; Doraiswamy, L.K. Modeling of non-catalytic gas-solid reactions. AlChE J. 1982, 28, 881–900. [Google Scholar] [CrossRef]
- Gadsby, J.; Long, F.J.; Sleightholm, P.; Sykes, K.W. The mechanism of the carbon dioxide-carbon reaction. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1948, 193, 356–376. [Google Scholar]
- Reif, A.E. The mechanism of the carbon dioxide carbon reaction. J. Phys. Chem. 1952, 56, 785–788. [Google Scholar] [CrossRef]
- Cancado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Jibrael, R.I.; Mohammed, M.K.A. Production of graphene powder by electrochemical exfoliation of graphite electrodes immersed in aqueous solution. Optik 2016, 127, 6384–6389. [Google Scholar] [CrossRef]
- Banerjee, I.; Faris, T.; Stoeva, Z.; Harris, P.G.; Chen, J.; Sharma, A.K.; Ray, A.K. Graphene films printable on flexible substrates for sensor applications. 2D Mater. 2017, 4, 015036. [Google Scholar] [CrossRef]
- Reina, A.; Son, H.; Jiao, L.; Fan, B.; Dresselhaus, M.S.; Liu, Z.; Kong, J. Transferring and identification of single-and few-layer graphene on arbitrary substrates. J. Phys. Chem. C 2008, 112, 17741–17744. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ni, Z.H.; Yu, T.; Shen, Z.X.; Wang, H.M.; Wu, Y.H.; Chen, W.; Wee, A.T.S. Raman studies of monolayer graphene: The substrate effect. J. Phys. Chem. C 2008, 112, 10637–10640. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ni, Z.H.; Shen, Z.X.; Wang, H.M.; Wu, Y.H. Interference enhancement of Raman signal of graphene. Appl. Phys. Lett. 2008, 92, 043121. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Zhang, J.; Jin, X.; Liu, J.-Y.; Li, Q.; Li, M.-H.; Cai, W.; Wu, D.-Y.; Zhan, D.; Ren, B. Quantitative correlation between defect density and heterogeneous electron transfer rate of single layer graphene. J. Am. Chem. Soc. 2014, 136, 16609–16617. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Seehra, M.S.; Geddam, U.K.; Schwegler-Berry, D.; Stefaniak, A.B. Detection and quantification of 2H and 3R phases in commercial graphene-based materials. Carbon 2015, 95, 818–823. [Google Scholar] [CrossRef]
- Seehra, M.S.; Narang, V.; Geddam, U.K.; Stefaniak, A.B. Correlation between X-ray diffraction and Raman spectra of 16 commercial graphene-based materials and their resulting classification. Carbon 2017, 111, 380–385. [Google Scholar] [CrossRef]
- Andonovic, B.; Grozdanov, A.; Paunovic, P.; Dimitrov, A.T. X-ray diffraction analysis on layers in graphene samples obtained by electrolysis in molten salts: A new perspective. Micro Nano Lett. 2015, 10, 683–685. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Guo, S.; Zhao, L. Facile Preparation of Multilayered Graphene with CO2 as a Carbon Source. Appl. Sci. 2019, 9, 4482. https://doi.org/10.3390/app9214482
Dong H, Guo S, Zhao L. Facile Preparation of Multilayered Graphene with CO2 as a Carbon Source. Applied Sciences. 2019; 9(21):4482. https://doi.org/10.3390/app9214482
Chicago/Turabian StyleDong, Hongyu, Shaoqing Guo, and Liangfu Zhao. 2019. "Facile Preparation of Multilayered Graphene with CO2 as a Carbon Source" Applied Sciences 9, no. 21: 4482. https://doi.org/10.3390/app9214482
APA StyleDong, H., Guo, S., & Zhao, L. (2019). Facile Preparation of Multilayered Graphene with CO2 as a Carbon Source. Applied Sciences, 9(21), 4482. https://doi.org/10.3390/app9214482




