1. Introduction
The electrodeposition of copper has been investigated for a variety of applications, and the anodic dissolution of metallic copper has been thoroughly studied in ionic liquid (IL) and deep eutectic solvent (DES) media [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. Previously, the anodic dissolution of metallic copper has been thoroughly examined in aqueous media [
11,
12,
13]. This invaluable metal has wide applications, including electronics, decorating, pre-coating, and electro-catalysis [
14,
15,
16,
17,
18,
19].
Gu et al. studied electrodeposition and the corrosion behaviour of copper in a choline chloride-ethylene glycol DES. In the electrodeposition process, a rough surface was obtained with the addition of ethylene diamine; the corrosion decreased and a relatively uniform surface was gained [
5]. Many research groups have tried to deal with the physical properties of ethaline (choline chloride–ethylene glycol). it was found that the mass transport could be increased with an increasing temperature; when the temperature was increased to 50 °C, the viscosity decreased by 65% [
7]. Concerning the physical properties of the electrolyte, such as conductivity and viscosity, it was found that it is insensitive to metal-salt addition but strongly temperature dependent [
9]. The speciation of copper is well-defined in choline-based DESs. It was determined to be [CuCl
3]
2− and [CuCl
4]
2− for Cu(I) and Cu(II), respectively [
20]. The dissolution of bulk copper was studied previously in these media, in the present work attempt was directed to deal with copper in more detail [
21]. Deep eutectic solvents (DESs) are attracting significant research interest as electrochemical media, especially for electrodeposition, as they share many of the useful properties of ILs, but are typically greener and cheaper [
22]. The influence of water and the changing potential on the interfacial region between a platinum electrode and a DES were studied. It was found that the nanostructural feature of interface decreased in the presence of small amount of water and was also strongly potential dependent [
23].
The aim of this work is to investigate the dissolution mechanism of copper in a DES and its comparable IL and compare it to the aqueous system. This is important for counter-electrode processes during electrodeposition, for electropolishing, and also to understand whether metallic copper corrodes in the DESs.
2. Experimental
The DES was prepared by mixing choline chloride (ChCl) (Aldrich, 99%) and EG (Aldrich, >99%) in a stoichiometric molar ratio of 1:2 (ChCl:EG). Then, it was heated to 60°C with continuous stirring until a clear liquid was produced. The IL one was purchased from (Aldrich, 99%). The 1-Butyl-3-methylimidazolium chloride, (C4mim)(Cl), was dried under vacuum before use, but had a water content of ca. 0.1 wt.% (thermogravimetric analysis, Mettler Toledo TGA/DSC1 STARe system) which enabled it to be liquid at 70°C.The copper wire was purchased from Alfa Aesar (99.9% purity).
Regarding electrochemical measurements, both cyclic voltammetry and linear sweep voltammetry were conducted by means of both stationary and rotating disk electrodes. The galvanostatic and the AC impedance were performed using an Autolab PGSTAT 12: controlling by GPES software and then fitting with an FRA impedance module. The impedance spectra acquisition was in the frequency range of 1–65,000 Hz with small amplitude of 10 mV of the AC signal. All electrochemical measurements were carried out in a three electrode cell system involving a 1 mm diameter metal disc Cu working electrode, sealed in glass; a platinum flag (1 cm2 area) as a counter electrode; and Ag/AgCl (0.1 M in 1:2ChCl:EG) as the reference electrode. All measurements are performed at 20°C and 70°C at a 5 mV·s−1 scan rate except for the determination of time considered necessary for copper electrode to reach passivation. The sweep rate was changed from 5 up to 50.5 mV·s−1.
The UV spectra were recorded by means of a Shimadzu model Uv-1601 spectrophotometer with a cell path length of 10 mm.
The morphological examinations were conducted using atomic force microscopy (AFM). The acquisition of images was by means of a Digital Instruments Nanoscope IV Dimension 300 (Veeco) atomic force microscope with a 100 mm scanning head contact mode. The controlling software was Nanoscope version 6.13 during image acquisition in air.
3. Results and Discussion
3.1. Anodic Dissolution Mechanism
Cyclic and Linear Sweep Voltammetry
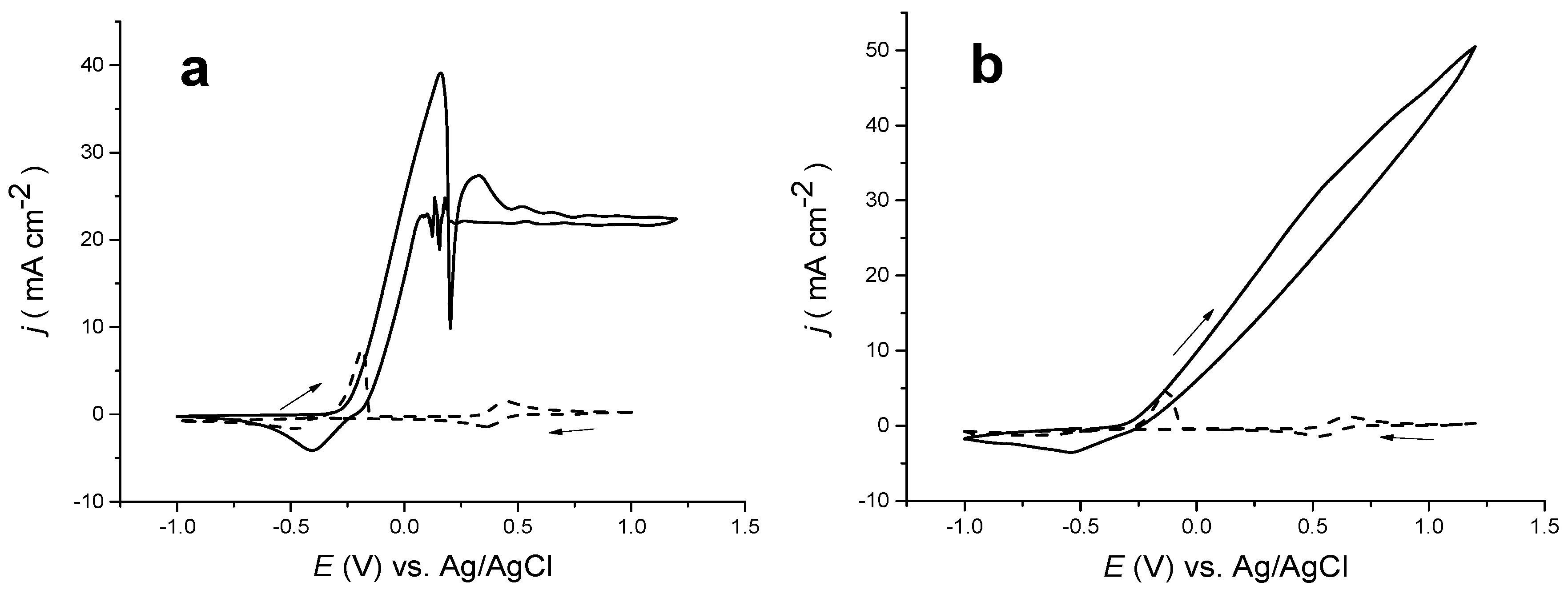
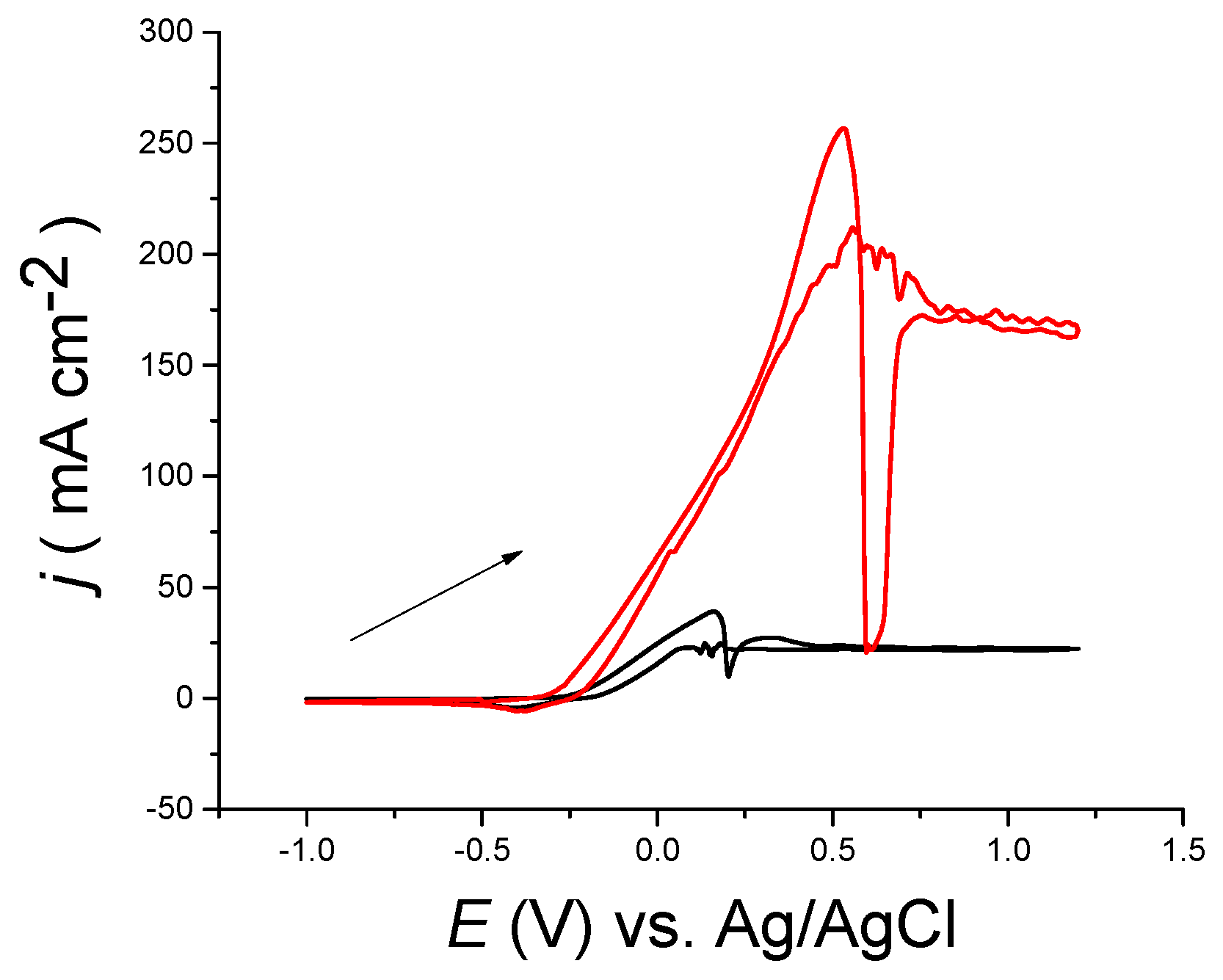
Figure 1a exhibits the cyclic voltammetric response a of metallic, copper disc electrode in choline chloride-based IL at 20 °C. Within the anodic potential range, two oxidation processes can be clearly seen. The anodic current begins to increase at −0.3 V, peaking at ca. 0 V. The current sharply drops in a manner which is a characteristic of a quasi-passivation process. This might be due primarily to presence of EG, and partly, the chloride ion. The second anodic current rises to a peak at +0.3 V and falls down to a steady state current of approximately 23 mA·cm
−2. The second anodic peak beyond +0.25 V could be linked to extra oxidation of copper from Cu(I) to Cu(II). The other ting peaks are artefacts that might be linked to complexity of dissolution process of bulk copper metal within such a viscous liquid.
On the cathodic scan, the current was approximately constant until ca. 0.2 V, when a noisy phenomenon occurred at the same potential that passivation film formation occurred: on the reversed sweep. Indeed, the process is not an artefact but is very reproducible. The main cathodic process begins at ca. 0.0 V, peaking at −0.4 V, with a shoulder at −0.2 V. The noise is likely owed to redissolution of the Cu(II) species formed on the electrode’s surface.
In the interpretation of the whole processes that occurs, it is helpful to compare the cyclic voltammograms of the metallic copper electrode in choline chloride-based DES with that for at Pt electrode in a solution of 0.1 M CuCl
2·2H
2O in the electrolyte. Abbott et al. documented the electrodeposition of copper using CuCl
2·2H
2O in the electrolyte, observing that two distinct processes take place, with Cu(II) undergoing a one-electron reduction to Cu(I)at +0.43 V, followed by a one-electron reduction to metallic copper at −0.45 V [
24]. The significance of this finding is that both processes are reversible. It is worth-mentioning, however, that direct comparison between the redox potentials in
Figure 1 and the previously reported study are not helpful due to the use of considerably different reference electrodes (a silver wire quasi-reference electrode in the latter case).
Figure 1a reveals an over-laid cyclic voltammetry CV (dashed line) of 0.1 M CuCl
2·2H
2O in choline chloride-based DES versus Ag/Ag
+ at 20 °C. It can obviously be seen that the onset potential on the anodic scan are similar to large extent, indicating that the metallic copper first dissolves as Cu(I) in the complex form. The quasi-passivation which is observed for the bulk copper electrode dissolution occurs at the same potential as the Cu(I)/(II) oxidation in solution. One can conclude that the second process in metallic copper dissolution occurs owing the change in oxidation state of the metal. When Cu(I) salts solubilise in choline chloride-based DES, the speciation was found to be in the [CuCl
2]
− form while the Cu(II) salts tend to produce [CuCl
4]
2− [
20,
24]. Both of these complexes are known to be soluble largely and so the cause of the quasi-passivation is not immediately clear.
From
Figure 1a,b, one can see the differences which are caused due to a specific cation effect or because of the lower chloride concentration in choline chloride-based DES compared to imidazolium-based IL. To confirm this further, 1-butyl-3-methylimidazolium chloride (C
4mimCl) was diluted by adding EG. The addition of EG into imidazolium-based IL one results in a shift in the oxidation onset potential of metallic copper by approximately 300 mV, as presented in
Figure 2. Similarly, a quasi-passivation profile is also observed showing that passivation is probably associated to the chloride concentration.
As the concentration of chloride declines due to addition of EG, shifting in the onset potential occurs. It is noteworthy that the chloride ion caused a higher current response.
The effect of anion concentration on copper dissolution in choline chloride-based IL was investigated as shown in
Figure 3. In the study the concentration of chloride in electrolyte (ChCl:EG 1:2) was manipulated by diluting the electrolyte with EG. As the molar ratio altered from 1:2 to 1:3 and 1:4, the passivation potential was changed to less positive potential values. This was predictable, as in such cases, there is less chloride available to the electrode’s surface, so it is more difficult to produce CuCl
42− and more likely forms CuCl
2.
3.2. UV-Visible Spectroscopy
It is of utmost significance to know about the speciation of dissolved copper in choline chloride/EG system. After metallic bulk copper’s dissolution, electrochemically, into Ethaline, a coloured solution of dissolved copper was generated. Of great importance to the dissolved bulk metal is the determination of the identity of the species that arose from copper dissolution into a chloride rich electrolyte, as that can be regarded as a key factor in dealing with the kinetics of the electrochemical processes. Herein, a facile, non-destructive technique was used to conduct this task: the UV-Vis spectroscopic technique. In the visible wavelength range, to gain information about any species, the solution must be coloured. To fulfil this condition, the chromophores/electron transition within the d-d orbitals have to exist [
22].
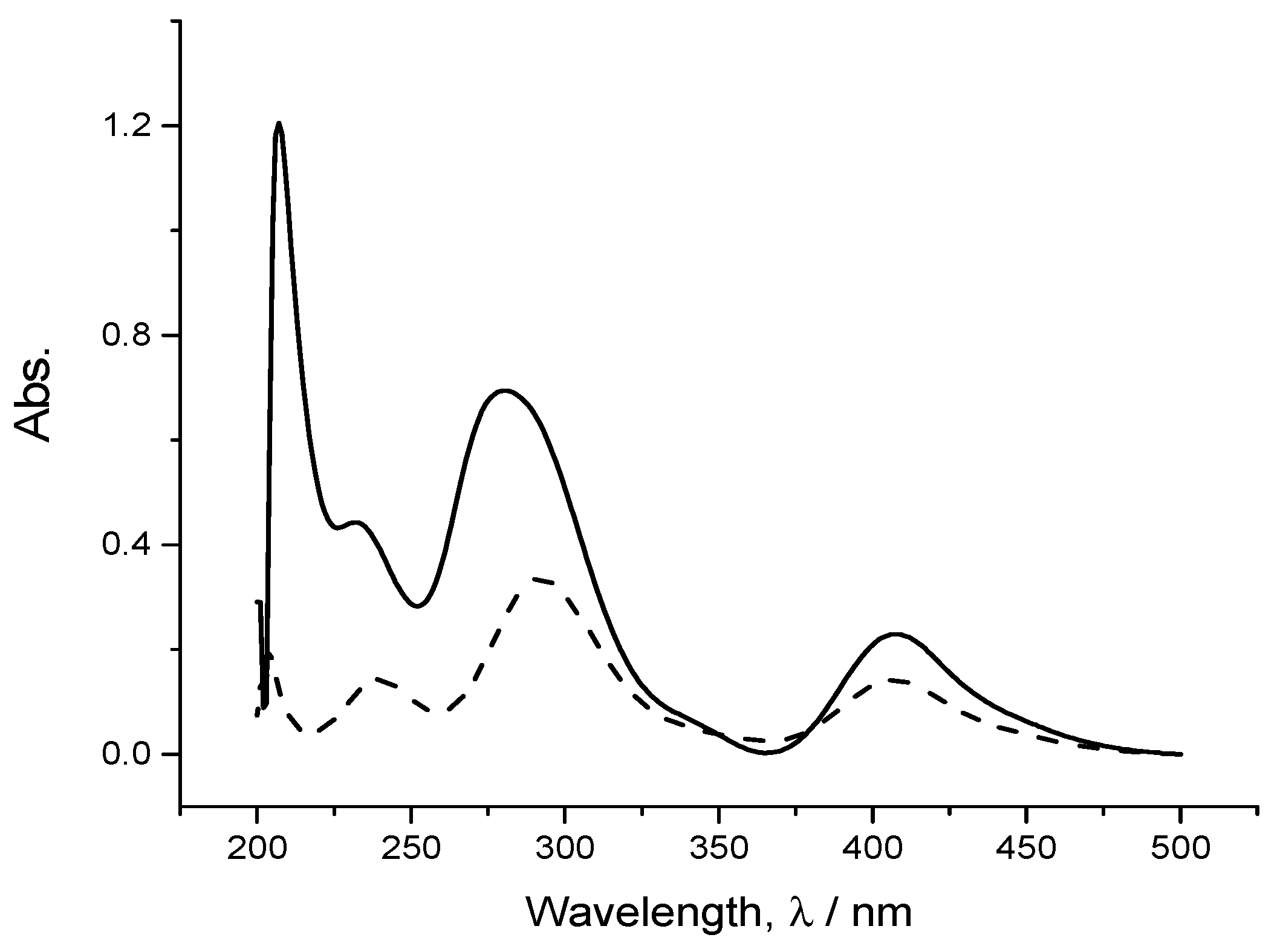
From
Figure 4, one can see similar UV-Vis spectra of 0.1 mM CuCl
2·2H
2O in ethaline and the solution of dissolved copper obtained electrochemically by holding a copper disc electrode in ethaline at a potential of 1.2 V for 1 h, from which dissolved copper ion in the electrolyte, i.e.,ethaline, was obtained at 20 °C. However, there are two observations to make: first, there is a large peak at around 220 nm present in the copper dissolved electrochemically, which is entirely missing in the control solution; next, the peak at 281 nm in the control solution underwent blue-shifting in the solution of the copper product dissolved electrochemically. These can be associated to the impurities which might be present in the used copper wire (99.9%).
The spectra obtained in both cases were identical, indicating that the speciation of stripped copper from the bulk copper metal into ethaline is exactly the same as that for the solution species obtained by dissolving CuCl
2.2H
2O in solution. The formation of [CuCl
4]
2− was emphasised from the spectra where three distinct peaks were seen at 233, 281, and 407 nm and this was confirmed using EXAFS [
20,
22].
3.3. The Effect of Temperature
Figure 1a exhibits the corresponding experiments in the IL, C
4mimCl; in other words, Cu electrochemical dissolution and the voltammetry of 0.1 M CuCl
2·2H
2O on a Pt electrode. This experiment had to be performed at 70 °C owing to the high melting point of C
4mimCl. Clearly, it is seen that a comparable current density was observed for copper dissolution to that of choline chloride-based IL. Moreover, the same onset potential for electrochemical copper dissolution was also obtained. However, the most noticeable difference between the electrochemical dissolution responses in choline chloride-based IL and imidazolium-based IL is that there is no quasi-passivation response in the latter and there is an inflection point occurring the identical potential as the Cu(I)/(II) process. It is also worth noting that the voltammogram for CuCl
2·2H
2O in C
4mimCl is quite comparable to that in ethaline, showing two one-electron reductions and oxidation processes.
Basically, the ligands for copper’s dissolution electrochemically, should be the same in both liquids (Cl−). However, there is a discrepancy in the electrochemical behaviour in both electrolytes, which can, therefore, only be due to kinetic factors (diffusion of copper from the electrode and Cl− to the electrode) or thermodynamic factors owing to the solubility of the chlorometallate complexes in the both electrolytes.
From the literature survey, one can see a huge number of studies on copper dissolution and deposition in a various electrolyte solutions. The kinetics of the electrodeposition of a copper salt in choline chloride-based IL was studied, and it was determined that the Cu(I)/II process is quasi-reversible with a rate constant of 9.5 ± 10
−4 cm·s
−1 [
25]. For copper to be deposited from its salt in the electrolyte, the amount of copper deposited on the electrode’s surface must be quite small so it can all be dissolved in the anodic sweep (returning the current to approximately zero), and a diffusion-limited current is seen for the oxidation of Cu(I) to Cu(II). When copper dissolution is conducted electrochemically at a bulk copper electrode, the copper is effectively at infinite concentration. The solution close to the electrode’s surface, i.e., at the interface region, could become saturated. It is proposed that that is what occurred in
Figure 1a at the potential about +0.2 V. Saturating the solution at the interfacial region with the copper complex will result in a decrease in the oxidative current as the electrode becomes blocked with dissolution product, and as a consequence, an asymmetric peak is gained.
The number of moles involved in the phase transitions at first oxidation peaks have been computed and are presented in
Table 1. One can conclude that copper’s conversion from metallic copper to Cu(I) in Ethaline is about 90 times higher than stripping of electrodeposited copper one in choline chloride-based and imidazolium based electrolytes, using platinum as a substrate at 20 °C and 70 °C, respectively. The number of moles copper stripped electrochemically in the choline chloride-based IL at 20 °C is slightly lower than the moles calculated in the imidazolium based IL at 70 °C. This might be owing to higher viscosity of imidazolium-based IL even at 70 °C (142 cP) to choline chloride-based IL (36 cP) at 20 °C [
26].
The quasi-passivation process in the electrochemical dissolution of bulk copper in choline based IL at 20 °C could be as a result of the low solubility of CuCl
2. So as to verify this, the experiment was repeated in the choline-based IL at 70 °C to create a comparison with the imidazolium-based IL experiment and the result are shown in
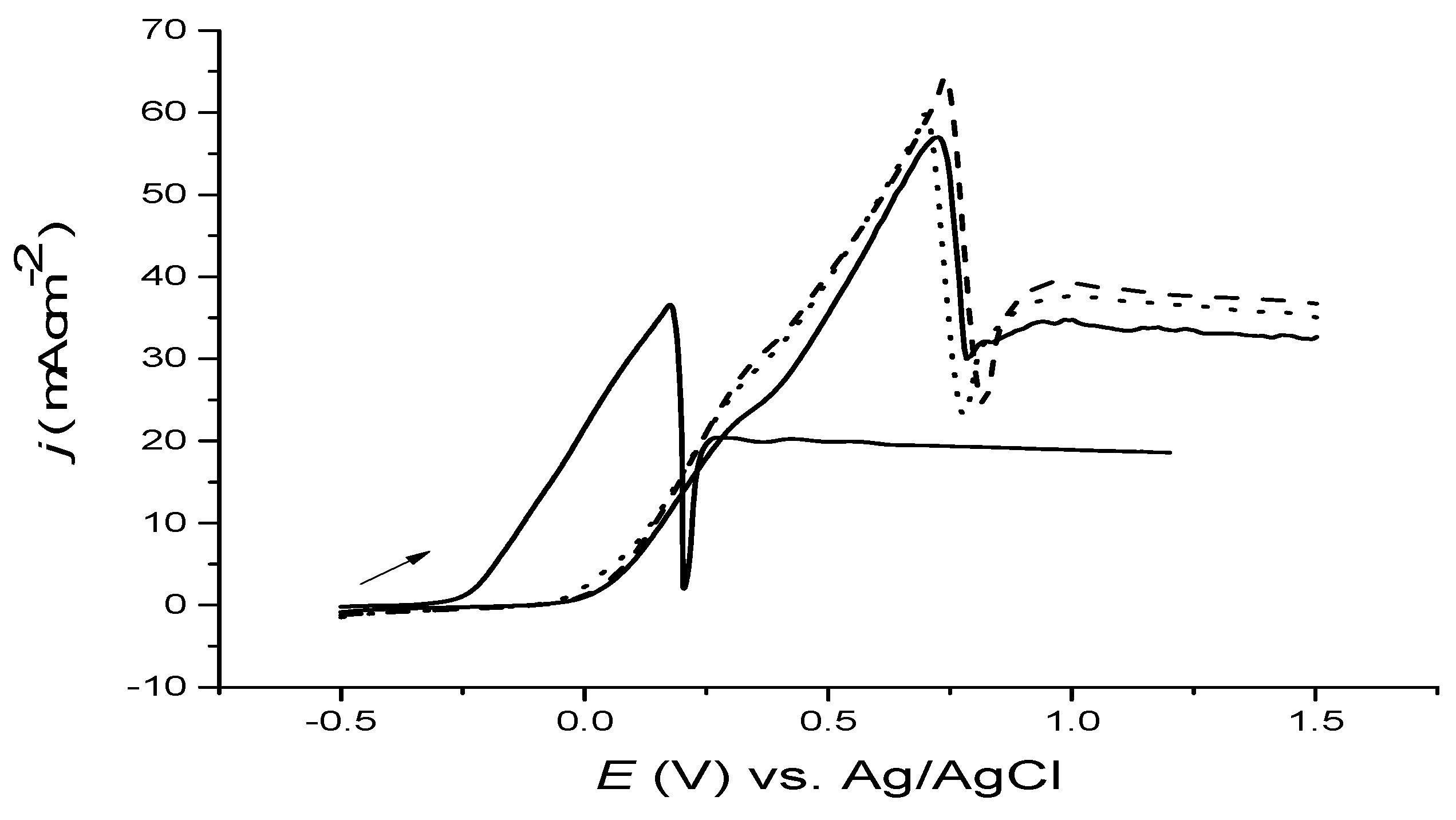
Figure 5. It was anticipated that as the temperature raised, so would the anodic current (approximately 10 fold) because of the lowering of the solution viscosity. It ought to, however, be noticed that the sharp decline in current still occurred at 0.6 V instead of 0.2 V. This is presumably as a result of the super-saturation at elevated temperature; i.e., higher concentration relative to saturated one, and the diffusion of chloride was also higher.
One thing that has to be well-known is the oxidation onset potential that shifted to be more negative; i.e., there was less anodic onset potential as the temperature is raised, demonstrating the kinetic accelerating oxidation reaction of the bulk copper. Ultimately, the overall electrochemical behaviour did not alter at that elevated temperature; in the other words, the passivation was still effective.
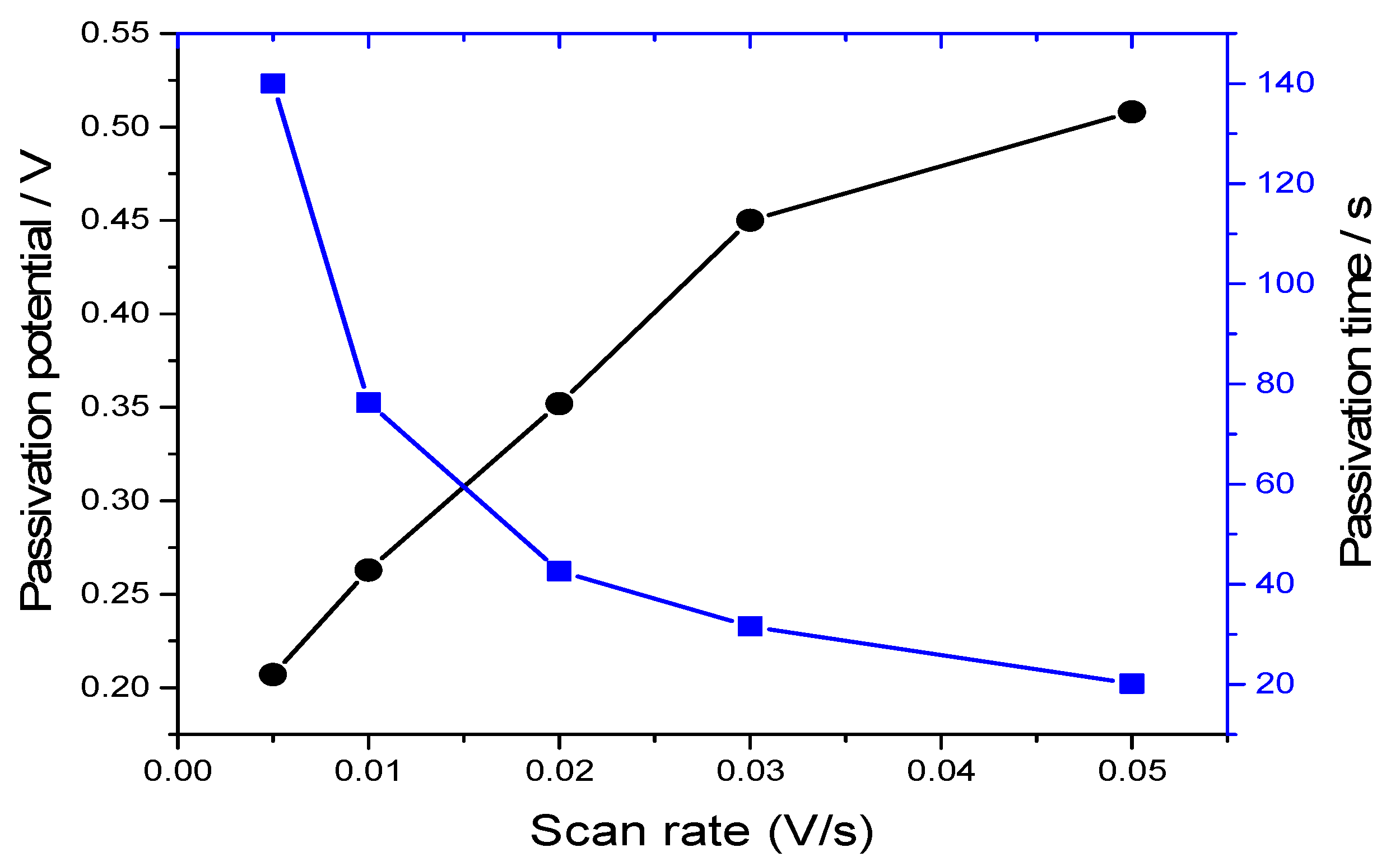
The time for the bulk copper electrode to passivate in the choline chloride-based IL as a function of scan rate is presented in
Figure 6. At faster sweep rates, a higher concentration of electrochemically dissolved copper is put more rapidly into the solution and the interfacial region between the electrode’s surface and the electrolyte saturates more quickly. It appears as if the potential of passivation increases, but this is just an artefact of the system not being at equilibrium state.
3.4. Influence of Mass Transport
When the potential of bulk copper electrode was held at +0.18 V versus Ag/Ag
+ for 10 min in choline chloride-based IL, the surface initially darkened and a green film slowly formed on the electrode’s surface, as presented in the previous work [
21]. At this potential, the most likely salt is CuCl, which is only sparingly soluble in the electrolyte. The light green colour indicates that the further oxidation of Cu(II) occurs; however, given the applied electrode potential, the oxidation would also be caused by the existence of dissolved oxygen.
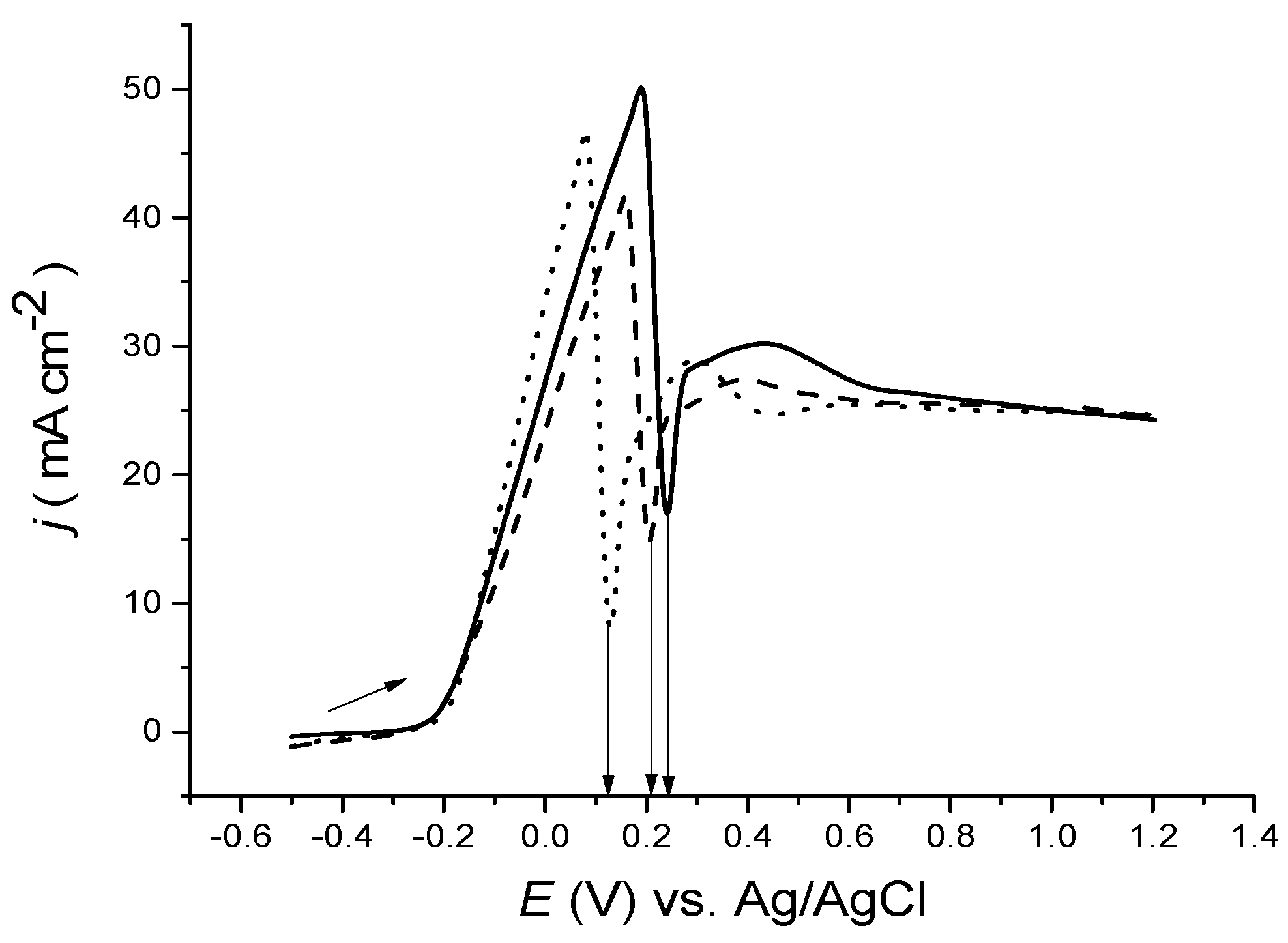
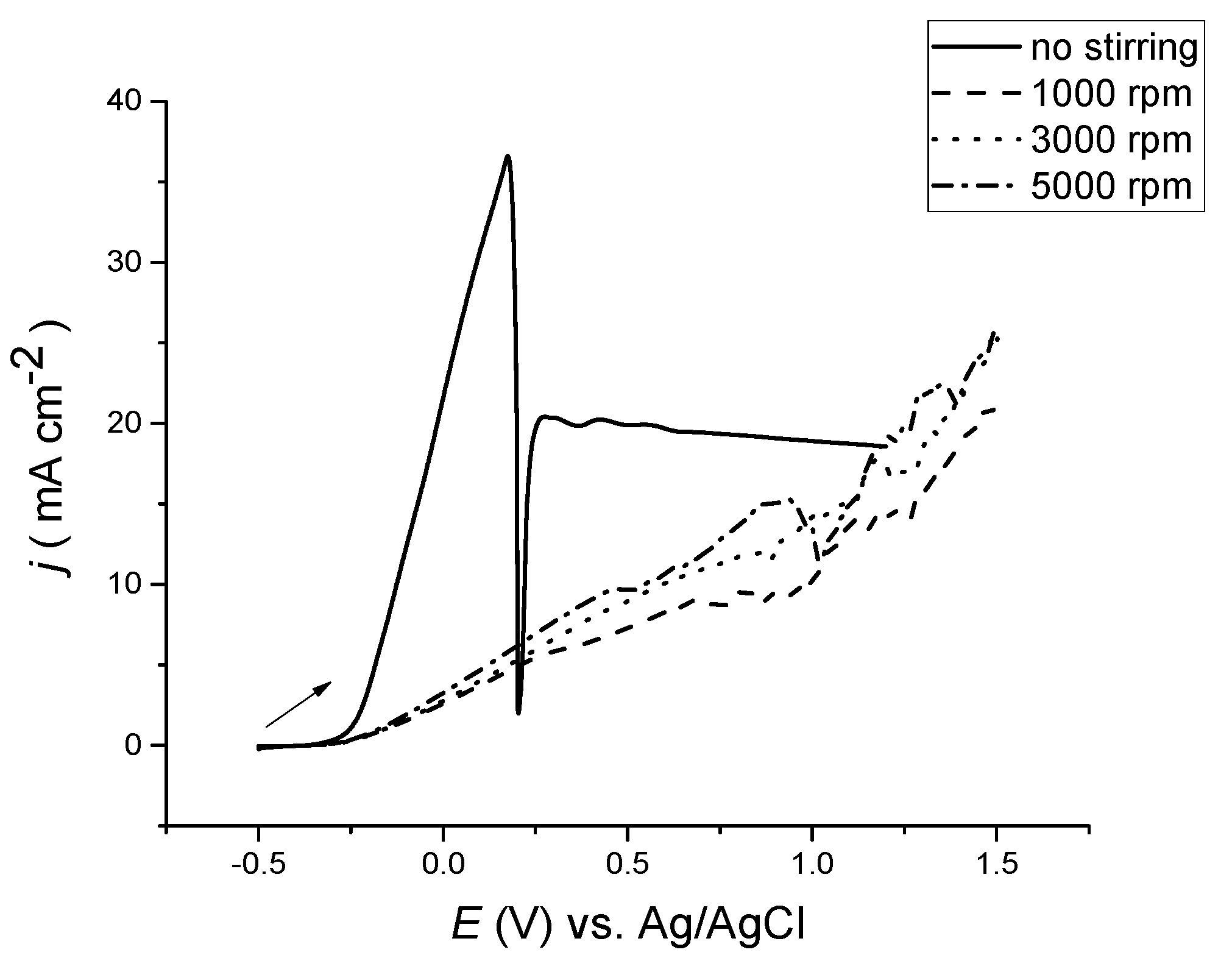
After the green film was washed off, the electrode metal was darkened quite considerably owing to surface roughening. To study the morphology of the surface, it is important to determine the role of mass transport; the experiment was repeated using a rotating disc electrode, as presented in
Figure 7. The morphology of the surface before and after anodic polarisation can be seen in later section for a bulk copper electrode with and without stirring.
The manipulation of mass transportation was tested using a rotating disc electrode, as presented in
Figure 7. It is important that in the absence of stirring, passivation occurs, even at stirring rates of up to 500 rpm. Only at rotation speeds above 1000 rpm, the passivation response was lost. As the rotation speed increased, so did the current, due to the anions provided to the surface by which more reactions occurred. The current did not, however, reach a steady state value as would be predicted for a solution based species, so it must be limited by the diffusion of oxidised copper from the electrode’s surface rather than a diffusion of chloride from the bulk electrolyte region to the electrode’s surface.
The formation of films on the electrode’s surface is known to influence the surface morphology of the dissolved copper at the surface. The process of electropolishing to small extent, i.e., surface levelling, is thought to occur because of film formation which restricts metal ions diffusing away from the electrode’s surface as a consequence of compact film formation, i.e., resistive film.
3.5. Electrochemical Impedance Spectroscopy (EIS)
EIS technique is a novel approach to examine the electrical properties of bulk and interfacial regions of various materials [
27,
28,
29].
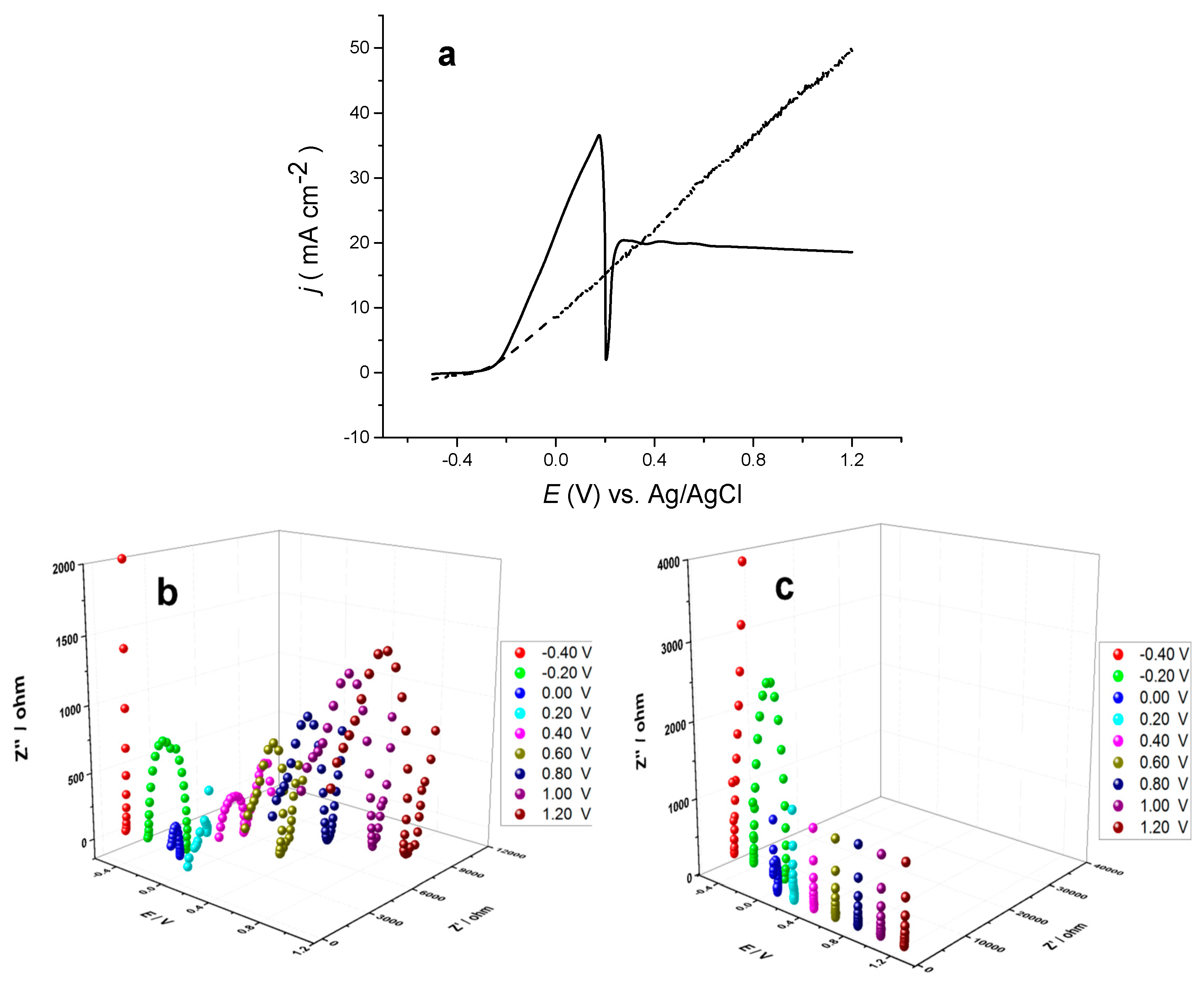
Figure 8 shows the EIS spectra of metallic copper in choline chloride-based IL at various direct current (DC) potentials. At −0.2 V, there was a single semi-circle for an electron transfer process which got smaller at 0 V indicating an increase in electron transfer rate constant. At +0.2 V, a second semi-circle was enlarged at +0.4 V, and above that voltage, dominated, suggesting that an insulating layer formed on the electrode’s surface. At +0.2 V, there were two semi-circle responses which could represent the first and second oxidations of copper.
The data acquired for impedance at different polarisation potentials in
Figure 8b were fitted to an electrical equivalent circuit (EEC) involving a two Randle’s circuits in series with Warburg impedance. EEC is a straight method to represent the behaviour of the medium with circuit elements, thus understanding the electrical properties of the system under study [
30,
31]. From the data analysis, when applying a potential of +0.8 V, the film capacitance was found to be 4.9 × 10
−6 F·cm
−2, assuming a dielectric constant of 8.0; the thickness of the film was estimated to be 1.4 µm [
32]. This is about an order of magnitude more than expected from the films formed in aqueous media. The film thickness was, however, remarkably thicker than that found for stainless steel electropolishing in the same liquid under the comparable conditions, and less thick compared to cobalt in the same electrolyte. As a consequence, the cobalt surface underwent a well-mirror-like electropolishing. The thickness was estimated to be only 16 nm for that layer [
32]. It should, however, be noted that speciation is different where for stainless steel, the iron complex formed is glycolate.
The experiment was repeated in choline chloride-based IL at 70 °C (not shown) and the same mechanism of dissolution was gained. A comparison with copper dissolution at 20 °C showed a capacitance of 2.17 ×10
−5 F·cm
−2 at 70 °C and + 0.8 V which corresponds to a layer of about 0.3 µm thick [
32]. It would seem logical to say that the diffusion layer in the duplex salt film model should be thinner at a higher temperature as the salt would be more soluble.
The structure of the double layer at the electrode’s surface in molecular solvent systems is completely different from that in ILs, in such a way that in the former, the electrode charge is compensated by both adsorbed counter ions and the diffuse layer, while in the latter, the structure may involve a monolayer of counter ions as compensation, followed by a multilayer involving cations and anions adjacent to each other [
33,
34].
In
Figure 8c the impedance of a copper electrode in imidazolium-based IL was measured at 70 °C as a function of DC potential. A single semicircle was observed at −0.2 V corresponding to the process of electron transfer that was likely the oxidation of copper. In this experiment, the polarisation potential was altered over the potential window from negative to positive. As the DC potential was shifted from negative to 0.0 V, the semicircle lessened its width as a result of a faster electron transfer process that was predicted given the increase in overall potential. From +0.4 to +1.2 V, a vertical straight line was observed, signifying the characteristic of a series RC circuit which was caused by an insulating layer on the electrode’s surface. The response of the series RC circuit did not alter with potential, indicating that once the film forms, it is not permeable and insulates the electrode’s surface. It would therefore, be expected that this would make the electrode unelectropolished. This reveals how the speciation of dissolved copper can affect the behaviour of the electrode. From examining the linear sweep voltammetry for the imidazolium-based IL system, it can be seen that a diagonal line is gained, indicating a resistor. This can be attributed to a layer of copper chloride formation on the copper metal.
3.6. Electropolishing
The electropolishing can basically be described as a controlled electrochemical dissolution process of a surface in an attempt to construct it less roughly at the macroscale (levelling) >1 µm and microscale (brightening) <1 µm [
35]. The basic principles of this process are film formation and a mass transport limited current plateau from the polarisation response. So as to achieve the conditions for macrosmoothing, the ohmic control or the mass transport control has to be conducted, while for microsmoothing, the mass transport mechanism is sufficient [
35].
In the present work, metallic copper was electropolished in choline chloride-based IL at 20 °C at a potential of +1.2 V, as exhibited in
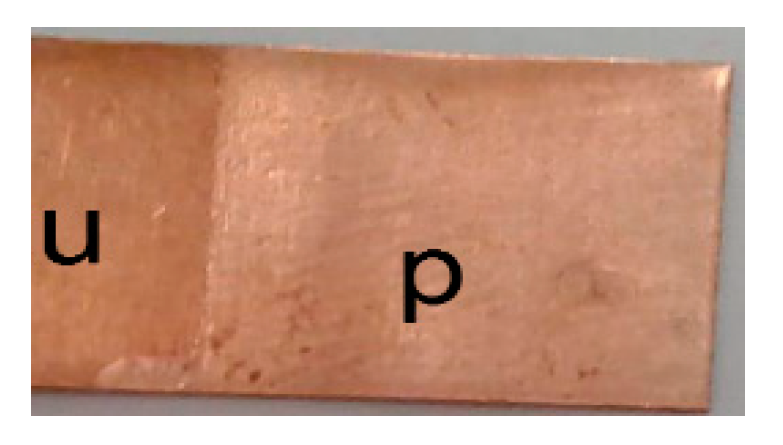
Figure 9. This is the first time that a DES has been shown to be useful electropolishing electrolyte for a single metal.
Figure 9 exhibits a metallic copper surface which is brighter but there are obvious signs of pitting on the surface. It could actually be questioned whether this is truly electropolished or just brightened alone.
3.7. Atomic Force Microscopy (AFM)
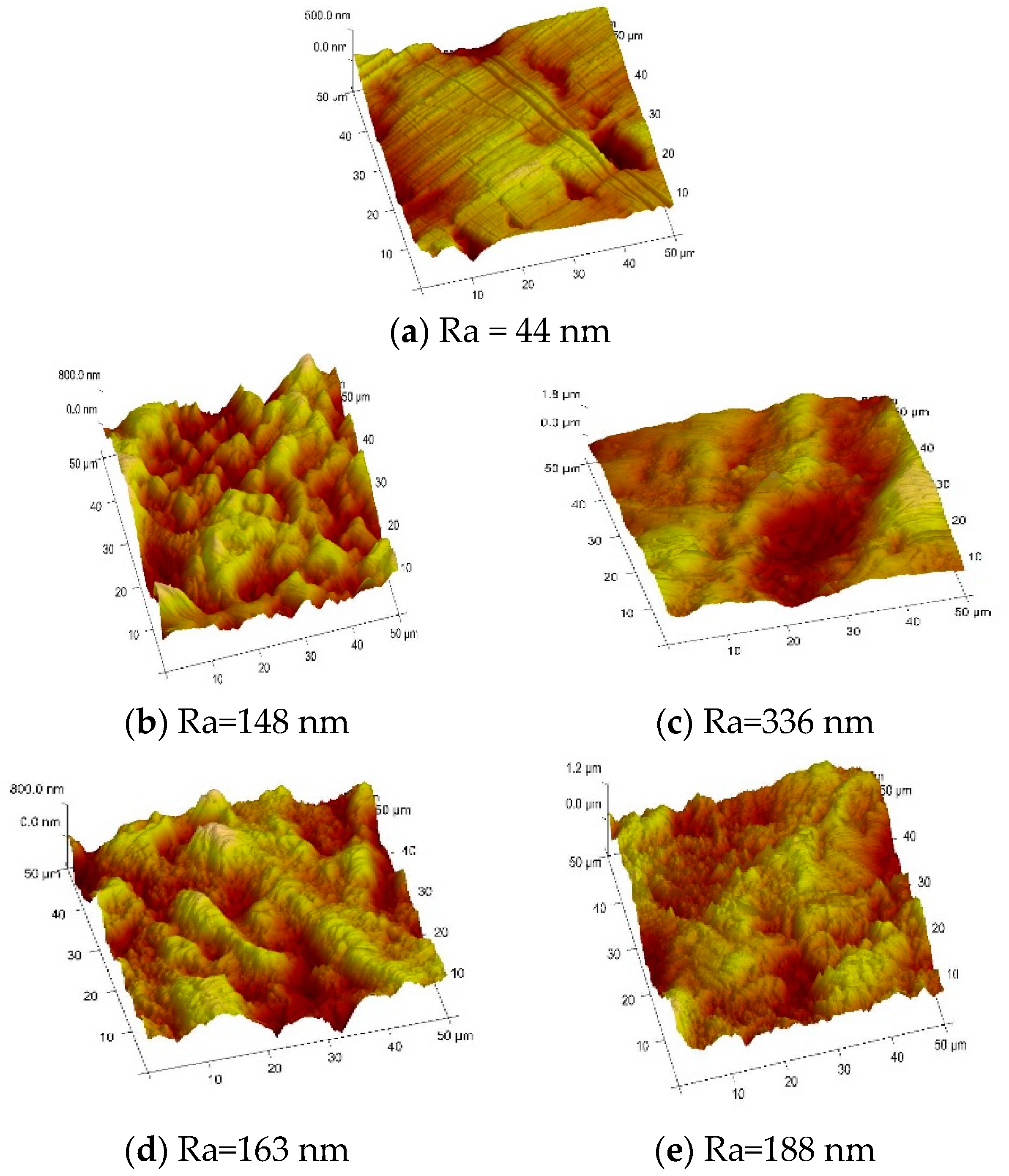
The AFM images for a bulk copper sheet before and after electrochemical dissolution in choline chloride-based IL are presented in
Figure 10. It can be seen that metallic copper undergoes pitting under several conditions and electropolishing under the others, noticing that on the native (unpolished) surface, machining marks and scratches can clearly be seen. Over the anodic sweep, it can be seen that the average surface roughness value, Ra, of the electrode prior to anodic polarisation was 0.75 µm, while the value after polarisation was 3.75 µm. When the electrode was rotated at 3000 rpm, the 0.63 µm was gained. If the liquid was stirred (by rotating the electrode at 3000 rpm) then no film was produced at the electrode’s surface and the solution became green.
Galvanostatic etching for short times resulted in pitting taking place, leading to an uneven surface, whereas increasing the etch time led to a visibly brighter surface with less microscopic roughness. The machining marks were removed by the electro polishing process. Similarly, the same pattern was observed for the electropolishing of stainless steel in choline chloride-based IL [
36]. In commercial electropolishing electrolytes, it is well-known that levelling only really takes place once the electrolyte is saturated with metal ions. Electropolishing the metallic copper samples with 0.81 M CuCl
2 added to the solution caused two different morphologies on the electrode’s surface. It is worth noting that at low etch times, pitting was more observable, whereas longer timescales gave more even surface finishes. It is worth mentioning the hydrogen evolution occurs at the cathode during the anodic dissolution of metallic copper in choline chloride-based IL at 20°C. On the one hand, a negligible gas evolution at low current density (<20 mA·cm
−2) is observed in the form of bubble. On the other hand, at higher current density the evolution is remarkable in the addition of water at any amount. This can be related to either the electrolysis of a trace of water or EG [
36]. The hydrogen gas evolution resulted in a lowering of the current efficiency of the electrochemical dissolution process, which is undesirable. DESs are generally insensitive to moisture compared to ILs, but the impact of water on the electrochemical process is still effective [
37].
On the one hand, there is an alteration in morphology of metallic copper surface from dark to surface brightness, as shown in
Figure 10. On the other side, roughness is revealed in
Figure 10; the copper sheet after dissolution made the subjects rougher, but the fewer crevices are considered the key feature of the copper sheet. So as to make a decision on whether electropolishing occurred or not, herein at least, the brightness accomplished had to be sufficient. Moreover, this is desirable from the electropolishing perspective that can be linked to the kind of electrolyte because the electropolishing of copper robustly depends on the nature of the electrolyte.
It is also impressive to notice that the nature of interfacial region governs the nature of electrochemical-polishing which is different to large extent from that of aqueous counterparts. It is well-reported that electrochemical polishing occurs at the interface region between the electrode and the electrolyte where dissolved ions diffuse from the electrode’s surface into bulk electrolyte [
38,
39].
4. Conclusions
In the present work, the study of mechanism of metallic copper dissolution in two chloride- containing electrolytes, choline chloride-based deep eutectic solvent, ethaline, and imidazolium-based IL, C4mimCl, has revealed that CuClads and CuCl2ads formed in the first oxidation region which was a compact film, and that was followed by a second oxidation resulting in the complexation of oxidised copper in the form CuCl3− and CuCl42− producing a porous film which then diffused away from the metallic copper surface. In other words, the release of dissolved copper from the bulk solid phase into both electrolytes in the form Cu(II)rather than Cu(I) occurred.
The Cu(I)/Cu(II) process can be identified by means of an asymmetric peak at the anodic regime as a result of passivation of the copper surface primarily with saturated corrosion products. This is evidenced by comparing the voltammogram of CuCl2·2H2O and metallic copper disc in both electrolytes. This can be linked with the chemistry of what actually happens at the interface, considering the composition properties (composition and structure). It was also seen that EG was responsible for saturation at the interface region, resulting in withdrawing chloride ions into the interfacial region. The quasi-passivation of the copper in this electrolyte depends upon the EG.
The postulated mechanism involves the electrochemical formation of CuClads and CuCl2−, followed by oxidation to CuCl2 leading to super-saturation of the interfacial region with [CuCl3]− and [CuCl4]2− restricted by the availability of Cl−. It is probable that the Cl− ions interact with oxidised metallic copper, creating insulating CuCl and CuCl2 which may not cover the entire surface, and some parts keep free of coverage in non-stoichiometric proportions which are vulnerable to solvation with Cl− ions, and as a result, diffusion away from the surface occurs.
Additionally, the kinetics of metallic copper dissolution in both electrolytes of interest was studied to some extent. The temperature influence and use of RDE have demonstrated that electrochemical dissolution raised as the temperature elevated and the mass transport effect changed towards faster electrochemical metallic copper dissolution.
Finally, the electropolishing of copper in deep eutectic fashion was accomplished to some extent, which cannot be acquired by means of aqueous chloride electrolyte. The electropolishing of copper in this electrolyte (ChCl:EG 1:2) involves levelling (macrosmoothing) and to some extent brightening with a diminution in the surface roughness. The use of dissolved copper as a counter electrode in a range of experiments to produce copper ions slowly on the working electrode on a large scale level is another advantage of studying the anodic behaviour of copper metal.
Author Contributions
Conceptualisation, W.O.K.; formal analysis, W.O.K.; investigation, W.O.K.; methodology, W.O.K. project administration, S.B.A.; validation, M.A.B., R.M.A., and M.F.Z.K.; writing—original draft, W.O.K.; writing—review and editing, S.B.A., M.A.B., R.M.A., and M.F.Z.K.
Funding
This research was funded by University of Leicester, Leicester, UK and Ministry of Higher Education and Scientific Research-Kurdish National Research Council (KNRC), Kurdistan Regional Government/Iraq. The financial support from the University of Sulaimani and Komar Research Center (KRC), Komar University of Science and Technology is greatly appreciated.
Acknowledgments
The authors gratefully acknowledge the financial support for this study from Ministry of Higher Education and Scientific Research, Kurdistan Regional Government/Iraq and the University of Leicester, Leicester, UK. The authors are grateful to scientist Andrew P. Abbott for fruitful discussion regarding the dissolution of bulk copper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, P.-Y.; Chang, Y.-T. Voltammetric study and electrodeposition of copper in 1-butyl-3-methylimidazolium salicylate ionic liquid. Electrochim. Acta 2012, 75, 339–346. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, S. Electrochemical copper deposition from an ethaline-CuCl2·2H2O DES. Surf. Coat. Technol. 2014, 238, 165–173. [Google Scholar] [CrossRef]
- Mandroyan, A.; Mourad-Mahmoud, M.; Doche, M.-L.; Hihn, J.-Y. Effects of ultrasound and temperature on copper electro reduction in Deep Eutectic Solvents (DES). Ultrason. SonoChem. 2014, 21, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.P.; Hihn, J.-Y.; Mason, T.J. Sono-electrodeposition (20 and 850 kHz) of copper in aqueous and deep eutectic solvents. Electrochim. Acta 2008, 53, 4248–4256. [Google Scholar] [CrossRef]
- Gu, C.D.; You, Y.H.; Wang, X.L.; Tu, J.P. Electrodeposition, structural, and corrosion properties of Cu films from a stable deep eutectics system with additive of ethylene diamine. Surf. Coat. Technol. 2012, 209, 117–123. [Google Scholar] [CrossRef]
- Popescu, A.-M.; Cojocaru, A.; Donath, C.; Constantin, V. Electrochemical study and electrodeposition of copper (I) in ionic liquid-reline. Chem. Res. China Univ. 2013, 29, 991–997. [Google Scholar] [CrossRef]
- Ghosh, S.; Ryder, K.; Roy, S. Electrochemical and transport properties of Ethaline containing copper and tin chloride. Trans. Inst. Metal Finshing 2014, 92, 41–46. [Google Scholar] [CrossRef]
- Ali, M.R.; Rahman, M.Z.; Saha, S.S. Electroless and electrolytic deposition of nickel from deep eutectic solvents based choline chloride. J. Electrochem. 2014, 23, 210–215. [Google Scholar]
- Xing, S.; Zanella, C.; Deflorian, F. Effect of pulse current on the electrodeposition of copper from choline chloride-ethylene glycol. J. Solid State Electrochem. 2014, 18, 1657–1663. [Google Scholar] [CrossRef]
- Sebastián, P.; Torralba, E.; Vallés, E.; Molina, A.; Gómez, E. Advances in Copper Electrodeposition in Chloride Excess. A Theoretical and Experimental Approach. Electrochim. Acta 2015, 164, 187–195. [Google Scholar] [CrossRef]
- Huo, J.; Solanki, R.; McAndrew, J. Study of anodic layers and their effects on electropolishing of bulk and electroplated films of copper. J. Appl. Electrochem. 2004, 34, 305–314. [Google Scholar] [CrossRef]
- Du, B.J. Water diffusion coefficients during copper electropolishing. J. Appl. Electrochem. 2004, 34, 1215–1219. [Google Scholar] [CrossRef]
- Lebedeva, O.; Dzhungurova, G.; Zakharov, A.; Kultin, D.; Kustov, L.; Krasovskii, V.; Kalmykov, K.; Dunaev, S. Surface State of Sacrificial Copper Electrode by Electropolishing in Hydrophobic Ionic Liquid 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. ACS Appl. Mater. Interfaces 2013, 5, 10551–10558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-T.; Chen, Z.; Kang, P.; Meyer, T.J. Electrocatalytic Water Oxidation with a Copper (II) Polypeptide Complex. J. Am. Chem. Soc. 2013, 135, 2048–2051. [Google Scholar] [CrossRef]
- Chen, Z.; Meyer, T. Copper (II) Catalysis of Water Oxidation. Angew. Chem. Int. Ed. 2013, 52, 700–703. [Google Scholar] [CrossRef]
- DeCiccio, D.; Ahn, S.T.; Sen, S.; Schunk, F.; Palmore, G.T.R.; Rose-Petruck, C. Electrochemical reduction of CO2 with clathrate hydrate electrolytes and copper foam electrodes. Electrochem. Commun. 2015, 52, 13–16. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Mroczka, R. Influence of chloride anions and polyethylene glycol on the morphology of electrodeposited copper layers. Electrochim. Acta 2012, 78, 316–323. [Google Scholar] [CrossRef]
- Leisner, P.; Möller, P.; Fredenberg, M.; Belov, I. Recent progress in pulse reversal plating of copper for electronics applications. Trans. Inst. Metal Finshing 2007, 85, 40–45. [Google Scholar] [CrossRef]
- Zou, L.; Shen, X.; Wang, Q.; Wang, Z.; Yang, X.; Jing, M. Preparation, magnetic and electrochemical properties of xCuFe2O4/CuO composite microfibers. J. Sol.-Gel. Sci. Technol. 2015, 75, 54–62. [Google Scholar] [CrossRef]
- Hartley, J.M.; Ip, C.-M.; Forrest, G.C.H.; Singh, K.; Gurman, S.J.; Ryder, K.S.; Abbott, A.P. EXAFS Study into the Speciation of Metal Salts Dissolved in Ionic Liquids and Deep Eutectic Solvents. Inorg. Chem. 2014, 53, 6280–6288. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Hartley, J.; Karim, W.O.; Ryder, K.S. Anodic Dissolution of Metals in Ionic Liquids. Prog. Nat. Sci. Mater. Int. 2015, 25, 595–602. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chemistry 2007, 13, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Li, H.; Westermann, C.; Al-Murshedi, A.Y.M.; Endres, F.; Abbott, A.P.; Warr, G.G.; Edler, K.J.; Atkin, R. Nanostructure of the deep eutectic solvent/platinum electrode interface as a function of potential and water content. Nanoscale Horiz. 2018, 4, 158–168. [Google Scholar] [CrossRef]
- Abbott, A.P.; el Ttaib, K.; Frisch, G.; McKenzie, K.J.; Ryder, K.S. Electrodeposition of copper composites from deep eutectic solvents based on choline chloride. Phys. Chem. Chem. Phys. 2009, 11, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Vainikka, T.; Murtomäki, L.; Kontturi, K.; Ahlberg, E. The kinetics of the Cu2+/Cu+ redox couple in deep eutectic solvents. Electrochim. Acta 2011, 56, 4942–4948. [Google Scholar] [CrossRef]
- Sastry, C.V.R.; Haranadh, C.; Murty, C.R.K. Dielectric constant of copper chloride and copper ammonium chloride. Trans. Faraday Soc. 1967, 63, 569–572. [Google Scholar] [CrossRef]
- Aziz, S.B.J. The mixed contribution of ionic and electronic carriers to conductivity in chitosan based solid electrolytes mediated by CuNt salt. J. Inorg. Organomet. Polym. 2018, 28, 1942–1952. [Google Scholar] [CrossRef]
- Aziz, S.B. Occurrence of electrical percolation threshold and observation of phase transition in chitosan(1−x): AgIx (0.05 ≤ x ≤ 0.2)-based ion-conducting solid polymer composites. Appl. Phys A 2016, 122, 706. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Rasheed, M.A.; Ahmed, H.M. Role of ion dissociation on DC conductivity and silver nanoparticle formation in PVA: AgNt based polymer electrolytes: Deep insights to ion transport mechanism. Polymers 2017, 9, 338. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x−1)(10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30–46. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Kadir, M.F.Z.; Ahmed, H.M. Non suitability of silver ion conducting polymer electrolytes based on chitosan mediated by barium titanate (BaTiO3) for electrochemical device applications. Electrochim. Acta 2019, 296, 494–507. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; McKenzie, K.J.; Glidle, A.; Ryder, K.S. Electropolishing of stainless steels in a choline chloride based ionic liquid: An electrochemical study with surface characterisation using SEM and atomic force microscopy. Phys. Chem. Chem. Phys. 2006, 8, 4214–4221. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Electroplating using Ionic Liquids. Annu. Rev. Mater. Res. 2013, 43, 200–205. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Evans, R.G.; Compton, R.G. Non-Haloaluminate Room Temperature Ionic Liquids in Electrochemistry—A Review. Chem. Phys. Chem. 2004, 5, 1106–1120. [Google Scholar] [CrossRef]
- Landolt, D. Fundamental aspects of electropolishing. Electrochim. Acta 1987, 32, 1–11. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; McKenzie, K.J.; Ryder, K.S. Voltammetric and impedance studies of the electropolishing of type 316 stainless steel in a choline chloride based ionic liquid. Electrochim. Acta 2006, 51, 4420–4425. [Google Scholar]
- Leron, R.B.; Wong, D.S.H.; Li, M.-H. Densities of a deep eutectic solvent based on choline chloride and glycerol and its aqueous mixtures at elevated pressures. Fluid Phase Equilibria 2012, 335, 32–38. [Google Scholar] [CrossRef]
- Karim, W.O.; Abbott, A.P.; Cihangir, S.; Ryder, K.S. Electropolishing of Nickel and Cobalt in deep eutectic solvents. Trans. Inst. Metal Finshing 2018, 96, 4420–4425. [Google Scholar] [CrossRef]
- Abbott, A.P.; Karim, W.O.; Ryder, K.S. ‘’Technical Aspects”. In Electrodeposition in Ionic Liquids; Endres, F., Abbott, A.P., Mac Farlance, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; ISBN 9783527336029. [Google Scholar]
Figure 1.
Cyclic voltammetric responses of a Cu disc (solid line) and 0.1 M CuCl2·2H2O (dashed line) in choline chloride-based ionic liquid (IL) on a platinum electrode at 20 °C (a), and a Cu disc (solid line) and 0.2 M CuCl2·2H2O in imidazolium-based IL (dashed line) at 70 °C (b), all at a sweep rate of 5 mV·s−1.
Figure 1.
Cyclic voltammetric responses of a Cu disc (solid line) and 0.1 M CuCl2·2H2O (dashed line) in choline chloride-based ionic liquid (IL) on a platinum electrode at 20 °C (a), and a Cu disc (solid line) and 0.2 M CuCl2·2H2O in imidazolium-based IL (dashed line) at 70 °C (b), all at a sweep rate of 5 mV·s−1.
Figure 2.
Linear sweep voltammetry (LSVs) of copper discs in choline chloride-based IL (ChCl:EG 1:2, solid line), and the other three shifted, LSV-overlapped responses of copper discs in 1-butyl-3-methylimidazolium chloride (C4mimCl):EG with ratios of 1:2, 1:3, and 1:4, represented by dashes, dots, and solid lines, respectively, at 5 mV·s−1 and 20 °C.
Figure 2.
Linear sweep voltammetry (LSVs) of copper discs in choline chloride-based IL (ChCl:EG 1:2, solid line), and the other three shifted, LSV-overlapped responses of copper discs in 1-butyl-3-methylimidazolium chloride (C4mimCl):EG with ratios of 1:2, 1:3, and 1:4, represented by dashes, dots, and solid lines, respectively, at 5 mV·s−1 and 20 °C.
Figure 3.
The influence of electrolyte composition on the equilibrium passivation potential of copper disc electrodes in ethaline ChCl:EG, 1:2 (solid line); ChCl:EG, 1:3 (dashed line); and ChCl:EG, 1:4 (dotted line).
Figure 3.
The influence of electrolyte composition on the equilibrium passivation potential of copper disc electrodes in ethaline ChCl:EG, 1:2 (solid line); ChCl:EG, 1:3 (dashed line); and ChCl:EG, 1:4 (dotted line).
Figure 4.
UV-Vis spectra of a dissolved metallic copper disc (solid line) at 50 mA·cm−2 for 1 h at ambient temperature and 0.1 mM (CuCl2)·2H2O (dash line) in 1:2EG:ChCl electrolyte (choline chloride-based IL) on a platinum electrode.
Figure 4.
UV-Vis spectra of a dissolved metallic copper disc (solid line) at 50 mA·cm−2 for 1 h at ambient temperature and 0.1 mM (CuCl2)·2H2O (dash line) in 1:2EG:ChCl electrolyte (choline chloride-based IL) on a platinum electrode.
Figure 5.
Cyclic voltammograms of bulk Cu disc in choline chloride-based IL at 20 °C (black line) and 70 °C (red line) at 5 mV·s−1.
Figure 5.
Cyclic voltammograms of bulk Cu disc in choline chloride-based IL at 20 °C (black line) and 70 °C (red line) at 5 mV·s−1.
Figure 6.
The influence of sweep rate on the equilibrium passivation potential; square-dotted and circle-dotted lines correspond to passivation time and passivation potential, respectively.
Figure 6.
The influence of sweep rate on the equilibrium passivation potential; square-dotted and circle-dotted lines correspond to passivation time and passivation potential, respectively.
Figure 7.
Anodic LSVs of Cu disc in ethaline versus Ag/AgCl, at a sweep rate of 5 mV·s−1 and at various rotation speeds.
Figure 7.
Anodic LSVs of Cu disc in ethaline versus Ag/AgCl, at a sweep rate of 5 mV·s−1 and at various rotation speeds.
Figure 8.
(a) Comparative anodic linear sweep voltammograms of a bulk copper in choline chloride-based IL at 20 °C (solid line) and imidazolium-based IL at 70 °C (dashed line) (b). Electrochemical impedance spectra of copper in choline chloride-based IL at 20 °C at various potentials with an AC amplitude of 10 mV in the frequency range of 1–65,000 Hz (c), as in (b) but using imidazolium-based IL at 70 °C.
Figure 8.
(a) Comparative anodic linear sweep voltammograms of a bulk copper in choline chloride-based IL at 20 °C (solid line) and imidazolium-based IL at 70 °C (dashed line) (b). Electrochemical impedance spectra of copper in choline chloride-based IL at 20 °C at various potentials with an AC amplitude of 10 mV in the frequency range of 1–65,000 Hz (c), as in (b) but using imidazolium-based IL at 70 °C.
Figure 9.
A photograph of a sample of metallic copper sheet after electrochemical dissolution in choline chloride-based IL after being held at +0.18 V for 10 min at 20 °C, unpolished (u) and polished (p).
Figure 9.
A photograph of a sample of metallic copper sheet after electrochemical dissolution in choline chloride-based IL after being held at +0.18 V for 10 min at 20 °C, unpolished (u) and polished (p).
Figure 10.
Resonant mode (ca. 300 kHz) AFM images (recorded in air at a scan rate of 0.5 Hz, with 256 lines) of the sample: (a) native copper, after dissolution in pure choline chloride-based IL at 50 mA·cm−2 for (b) 20 min or (c) 1 h, and under the same conditions but with the addition of 0.81 M CuCl2·2H2O to the ethaline for (d) 10 min or (e) 1 h. (Arithmetic average height, Ra: quantifies vertical deviation of a surface from normal vector.)
Figure 10.
Resonant mode (ca. 300 kHz) AFM images (recorded in air at a scan rate of 0.5 Hz, with 256 lines) of the sample: (a) native copper, after dissolution in pure choline chloride-based IL at 50 mA·cm−2 for (b) 20 min or (c) 1 h, and under the same conditions but with the addition of 0.81 M CuCl2·2H2O to the ethaline for (d) 10 min or (e) 1 h. (Arithmetic average height, Ra: quantifies vertical deviation of a surface from normal vector.)
Table 1.
The number of moles of copper stripped in the first oxidation process, calculated from the overall charge under the peak of the LSV using the GPES software.
Table 1.
The number of moles of copper stripped in the first oxidation process, calculated from the overall charge under the peak of the LSV using the GPES software.
| Electrolyte | Cu/nmole |
|---|
| Choline chloride IL | 90.6 |
| CuCl2·2H2O Choline chloride IL at 20 °C | 1.20 |
| CuCl2·2H2O C4mimCl at 70 °C | 1.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).