Genomic Study, Phytochemical Characterization, and Antiproliferative Activity of Two Different Genotypes of Jatropha curcas L. Obtained by a Breeding Program
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts
2.2. Chemicals
2.3. Genomic and Expression Analyses
2.3.1. Nucleic Acid Extraction and Complementary DNA (cDNA) Synthesis
2.3.2. Expression Analysis Was Carried out to Isolate Differentially Expressed Genomic Fractions
2.3.3. Polymerase Chain Reaction (PCR)
2.4. Phytochemical Analysis
2.5. Cell Cultures
2.6. Antiproliferative Activity—Biochemical Assays
2.6.1. MTT Assay
2.6.2. The Trypan Blue Exclusion Test
2.6.3. The Neutral Red (NR) Assay
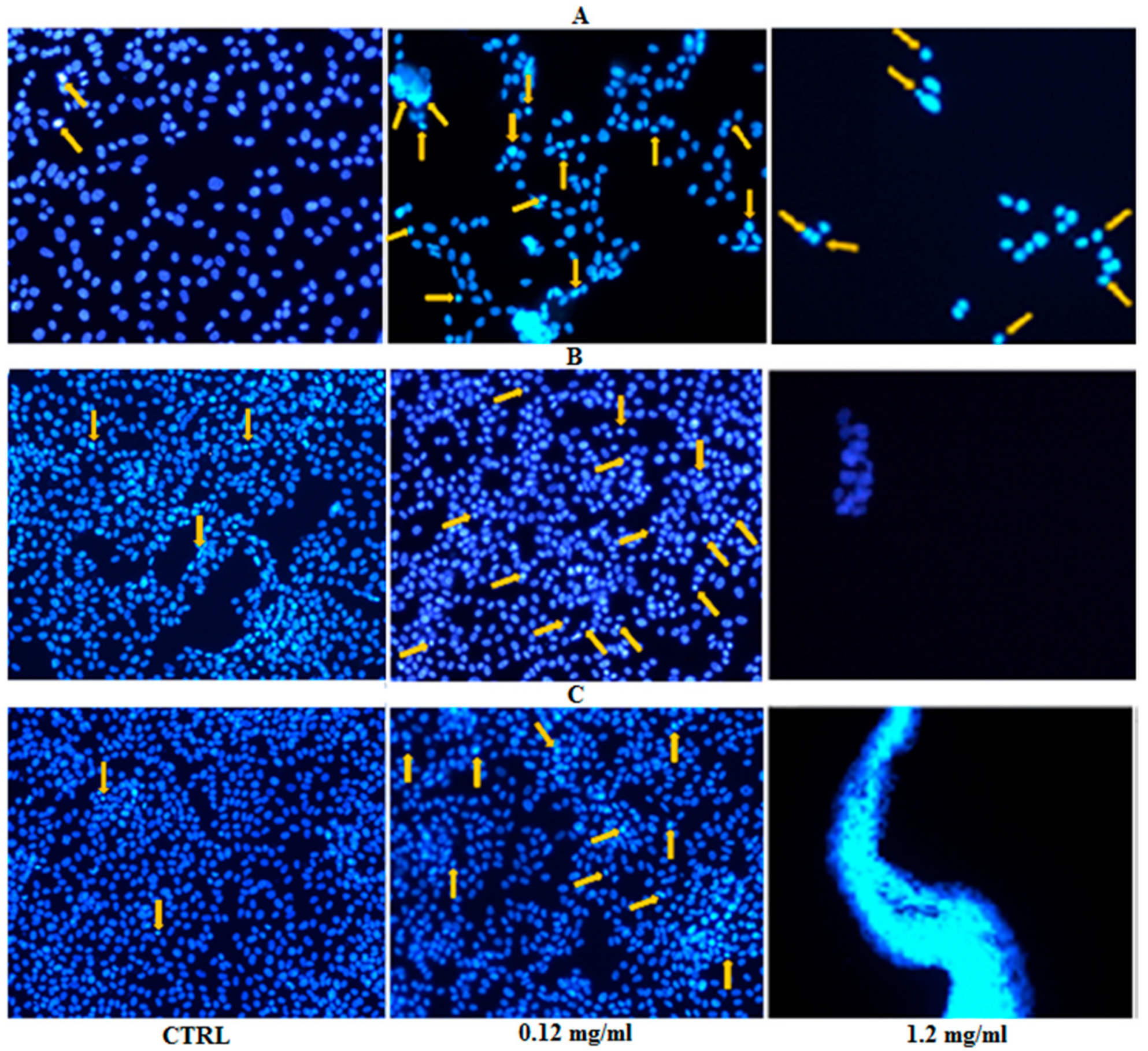
2.6.4. Chromatin Condensation Test
2.7. Statistical Analysis
3. Results
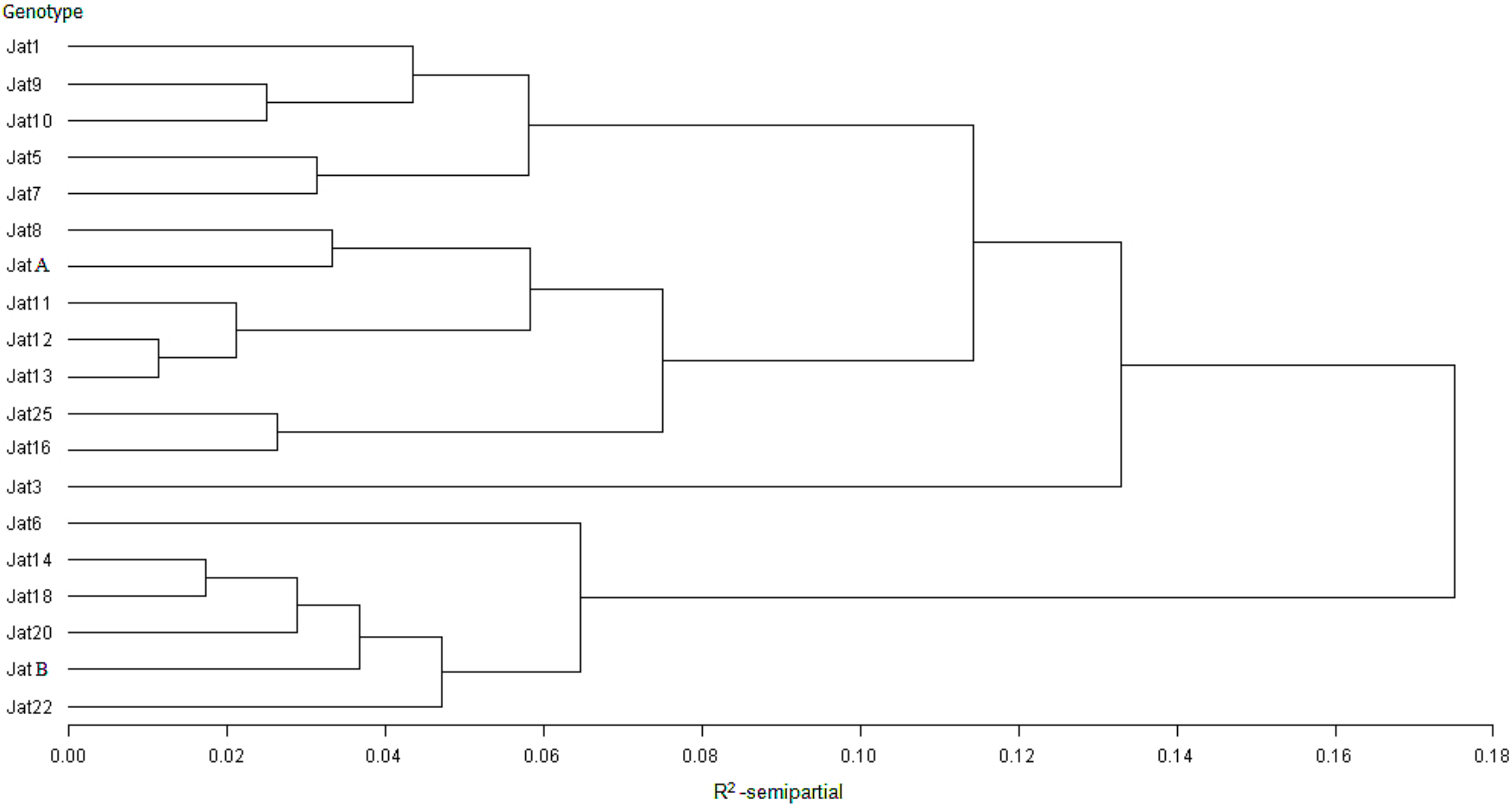
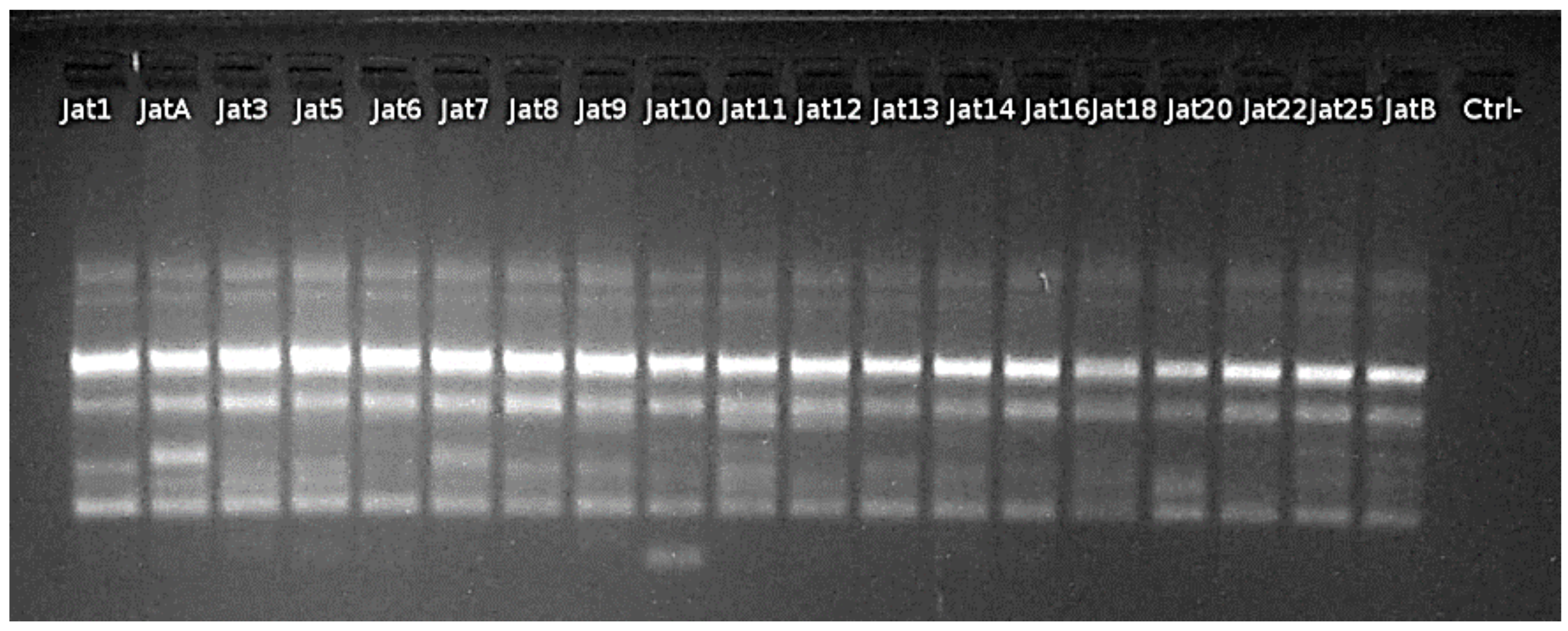
3.1. Genomic and Expression Analyses
3.1.1. Nucleic Acid Extraction and cDNA Synthesis
3.1.2. Expression Analysis
3.1.3. Polymerase Chain Reaction (PCR)
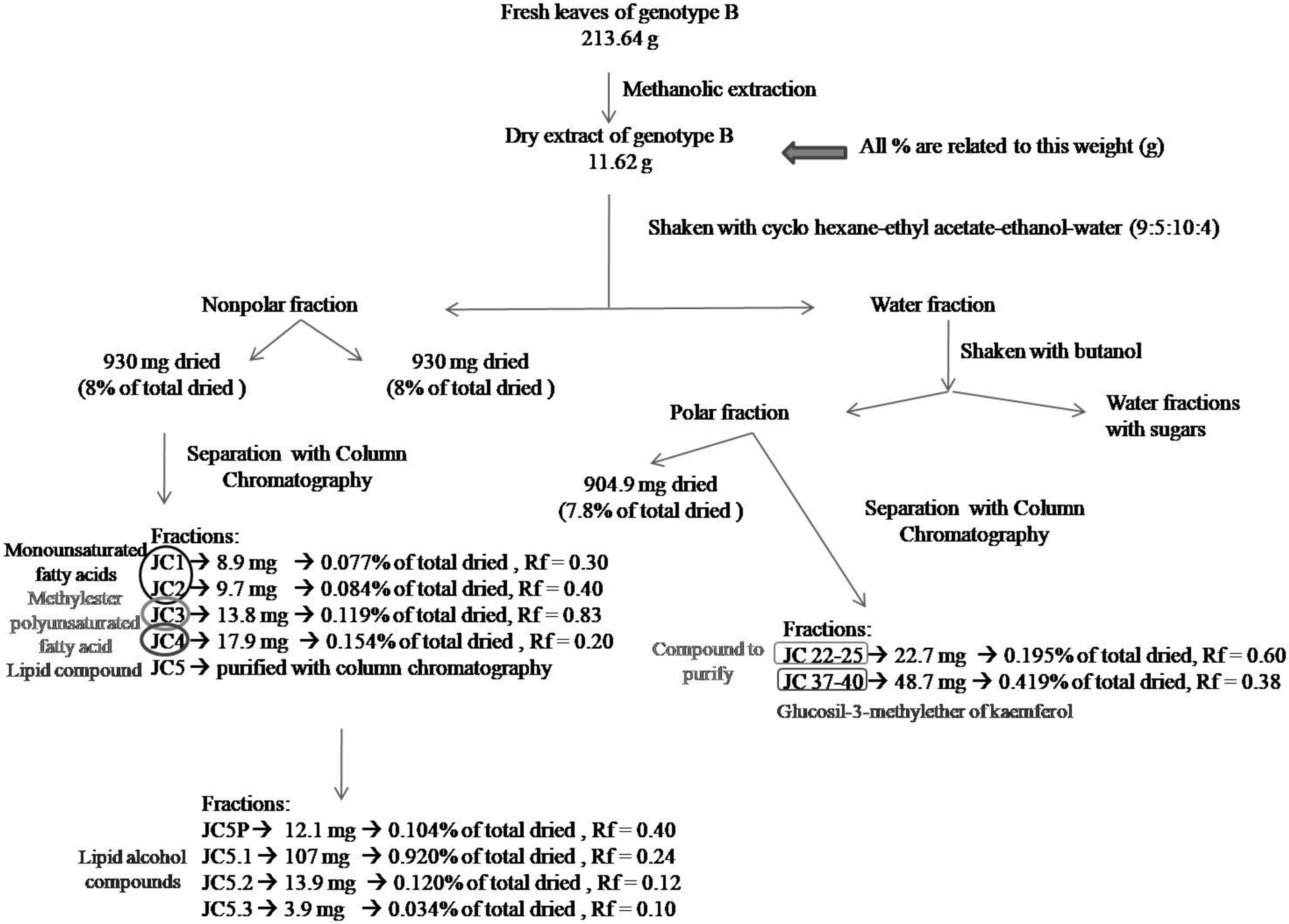
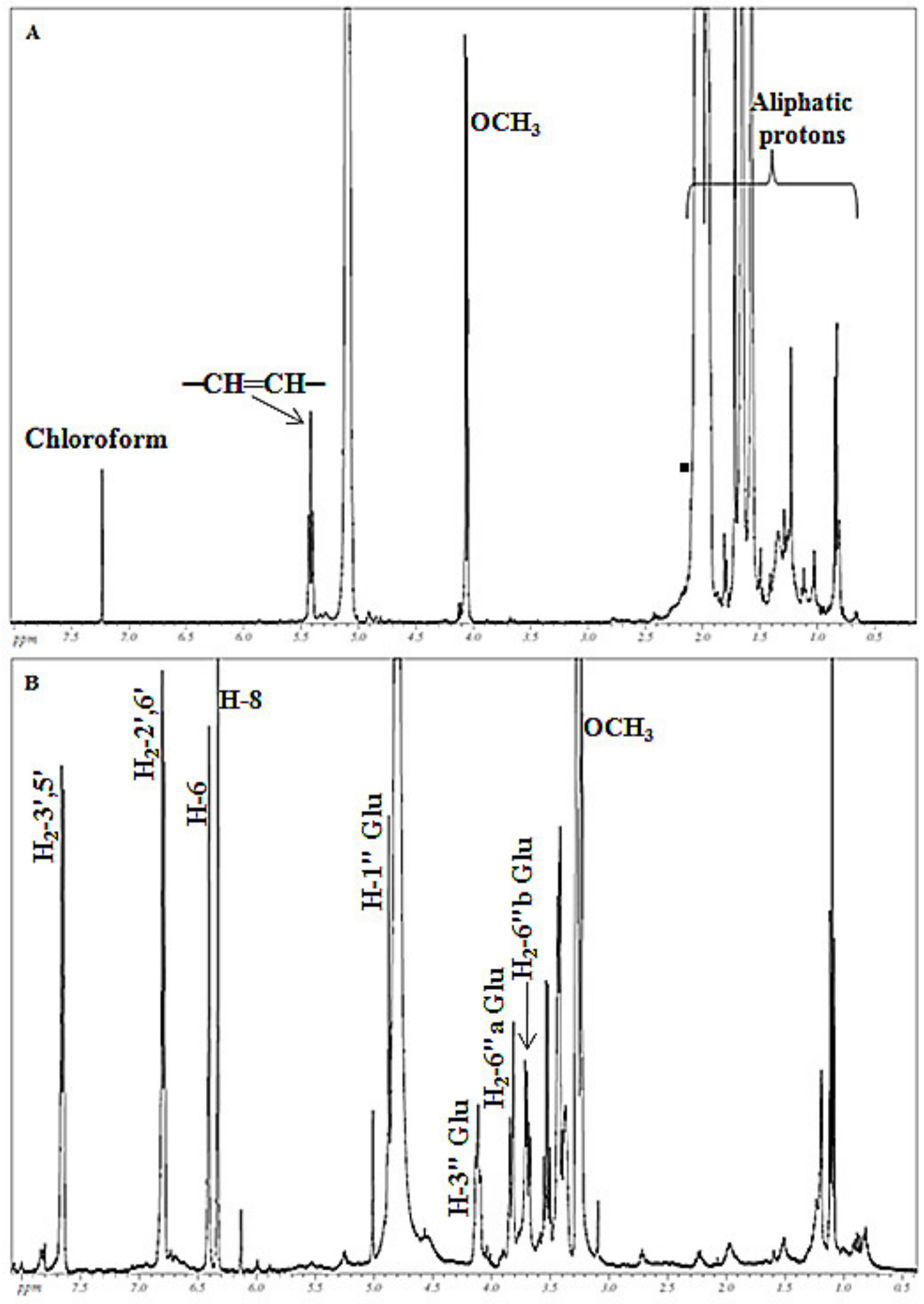
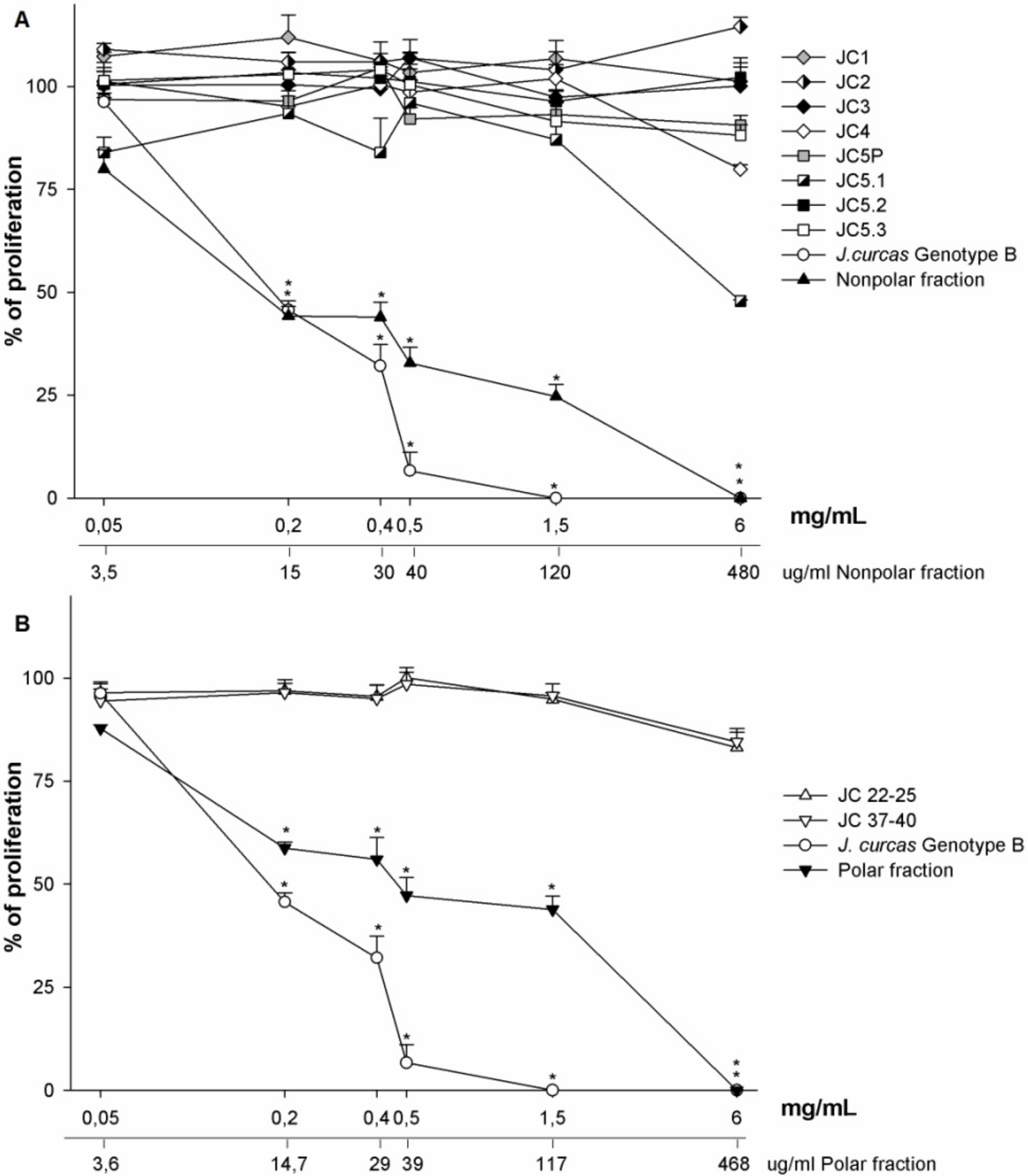
3.2. Phytochemical Analysis
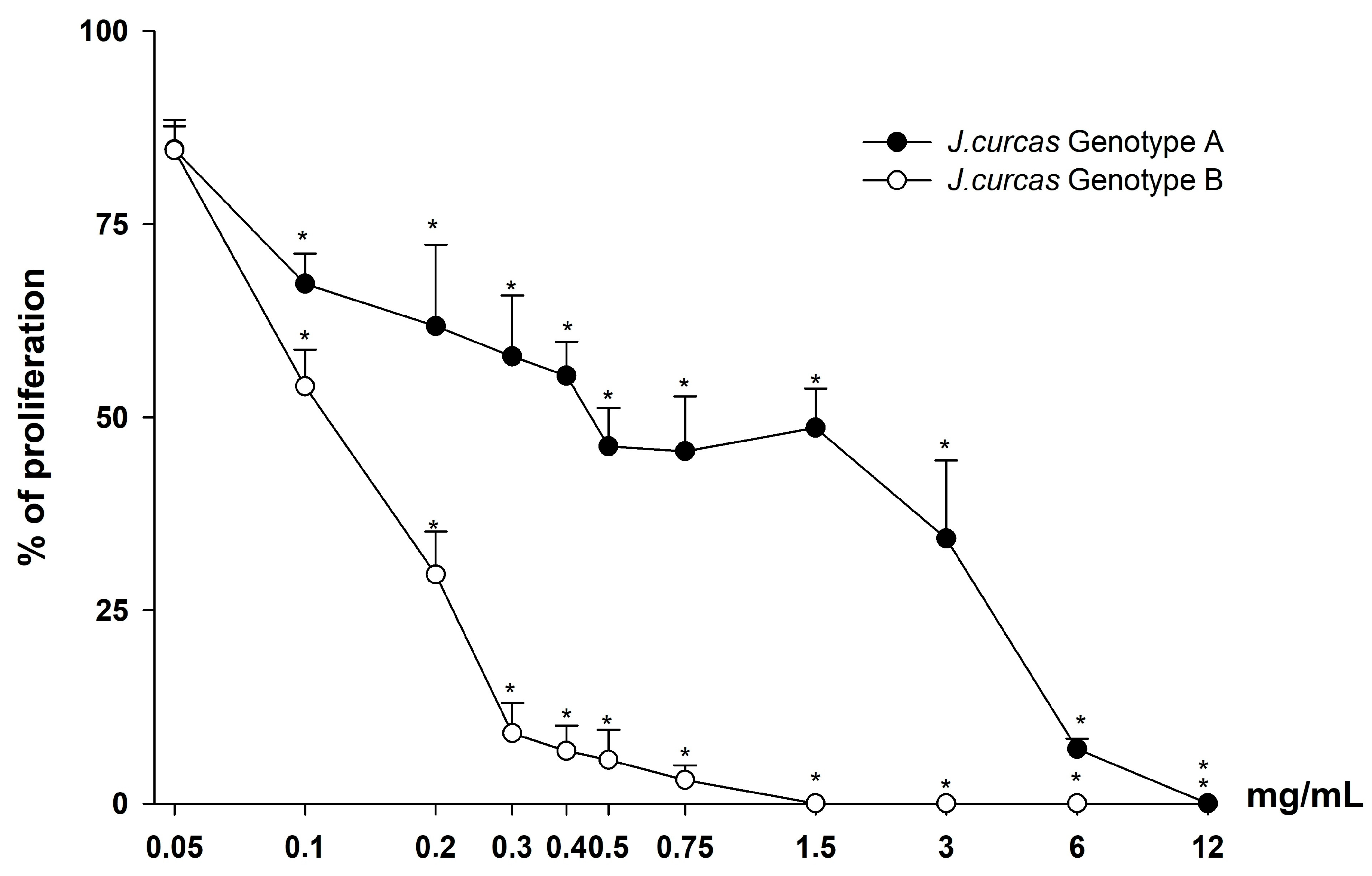
3.3. Antiproliferative Activity
3.3.1. MTT Assay and Trypan Blue Exclusion Test
3.3.2. NR Assay
3.3.3. Chromatin Condensation Test
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heller, J. Physic Nut, Jatropha curcas L., Promoting the Conservation and Use of Underutilized and Neglected Crops (IPGRI). Biodivers. Int. 1996, 1, 66. [Google Scholar]
- Insanu, M.; Dimaki, C.; Wilkins, R.; Brooker, J.; van der Linde, P.; Kayser, O. Rational use of Jatropha curcas L. in food and medicine: From toxicity problems to safe applications. Phytochem. Rev. 2013, 12, 107–119. [Google Scholar] [CrossRef]
- Kulkarni, M.L.; Sreekar, H.; Keshavamurthy, K.S.; Shenoy, N. Jatropha curcas poisoning. Indian J. Pediatrics 2005, 72, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Lakhanpal, P. Jatropha curcas poisoning in pediatric patients, Mauritius. Internet J. Pediatr. Neonatol. 2007, 8, 1–6. [Google Scholar]
- Parawira, W. Biodiesel production from Jatropha curcas: A review. Sci. Res. Essays 2010, 5, 1796–1808. [Google Scholar]
- Shahinuzzaman, M.; Yaakob, Z.; Moniruzzaman, M. Medicinal and cosmetics soap production from Jatropha oil. J. Cosmet. Dermatol. 2016, 15, 185–193. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Saad, W.Z.; Omar, A.R.; Ahmad, S.; Kuan, B.; Zolkifli, N.A.; Hendra, R.; Ho, Y.W. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J. Med. Plant Res. 2011, 5, 49–57. [Google Scholar]
- Mujumdar, A.M.; Misar, A.V. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. J. Ethnopharmacol. 2014, 90, 11–15. [Google Scholar] [CrossRef]
- Papalia, T.; Barreca, D.; Panuccio, M.R. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. Jatropha gossypiifolia L. and its biologically active metabolites: A mini review. J. Ethnopharmacol. 2019, 234, 197–203. [Google Scholar] [CrossRef]
- Ahirrao, R.A.; Patel, M.R.; Pokal, D.M.; Patil, J.K.; Suryawanshi, H.P. Phytochemical screening of leaves of Jatropha curcas plant. Int. J. Res. Ayurveda Pharm. 2011, 2, 1324–1327. [Google Scholar]
- Basha, S.D.; Sujatha, M. Inter and intra-population variability of Jatropha curcas (L.) characterized by RAPD and ISSR markers and development of population-specific SCAR markers. Euphytica 2007, 156, 375–386. [Google Scholar] [CrossRef]
- Leal, A.A.; Mangolin, C.A.; do AmaralJúnior, A.T.; Gonçalves, L.S.A.; Scapim, C.A.; Mott, A.S.; Eloi, I.B.O.; Cordovés, V.; da Silva, M.F.P. Efficiency of RAPD versus SSR markers for determining genetic diversity among popcorn lines. Genet. Mol. Res. 2010, 9, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Martelli, G.; Sunseri, F.; Greco, I.; Sabina, M.R.; Porreca, P.; Levi, A. Strawberry cultivars genetic relationship using randomly amplified polymorfíc DNA (RAPD) analysis. Adv. Hortic. Sci. 1999, 13, 99–104. [Google Scholar]
- Bachem, C.W.; Van der Hoeven, R.S.; de Bruijn, S.M.; Vreugdenhil, D.; Zabeau, M.; Visser, R.G. Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: Analysis of gene expression during potato tuber development. Plant J. 1996, 9, 745–753. [Google Scholar] [CrossRef]
- Calabrone, L.; Milella, L.; Greco, I.; Martelli, G. Isolation of putative LAR (leucoanthocyanidinreductase) gene fragment differentially expressed in Jatropha curcas L. leaves. Emir. J. Food Agric. 2013, 25, 225–230. [Google Scholar] [CrossRef]
- Boess, F.; Kamber, M.; Romer, S.; Gasser, R.; Muller, D.; Albertini, S.; Suter, L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: Possible implications for toxicogenomics use of in vitro systems. Toxicol. Sci. 2003, 73, 386–402. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Ganbold, M.; Barker, J.; Ma, R.; Jones, L.; Carew, M. Cytotoxicity and bioavailability studies on a decoction of Oldenlandiadiffusa and its fractions separated by HPLC. J. Ethnopharmacol. 2010, 131, 396–403. [Google Scholar] [CrossRef]
- Vitalone, A.; Guizzetti, M.; Costa, L.G.; Tita, B. Extracts of various species of Epilobium inhibit proliferation of human prostate cells. J. Pharm. Pharmacol. 2003, 55, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays—The neutral red (NR) and tetrazolium MTT tests. Toxicol. Vitr. 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Jelski, W.; Szmitkowski, M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin. Chim. Acta 2008, 295, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.R.; Hilliou, F.; Duarte, P.; Pereira, L.G.; Almeida, I.; Leech, M.; Sottomayor, M. Molecular Cloning and Characterization of a Vacuolar Class III Peroxidase Involved in the Metabolism of Anticancer Alkaloids in Catharanthusroseus. Plant Physiol. 2008, 146, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Balaji, R.; Rekha, N.; Deecaraman, M.; Manikandan, L. Antimetastatic and antiproliferative activity of methanolic fraction of Jatropha curcas against B16F10 melanoma induced lung metastasis in C57BL/6 mice. Afr. J. Pharm. Pharmacol. 2009, 3, 547–555. [Google Scholar]
- Lalou, C.; Basak, A.; Mishra, P.; Mohanta, B.C.; Banik, R.; Dinda, B.; Khatib, A.M. Inhibition of tumor cells proliferation and migration by the flavonoid furin inhibitor isolated from Oroxylumindicum. Curr. Med. Chem. 2013, 20, 583–591. [Google Scholar]
- Li, Y.L.; Ganc, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huanga, Y.P.; Liu, Y.W.; Liu, J.W. A flavonoid glycoside isolated from Smilaxchina L. rhizome. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Nishshanka, U.; Jayasuriya, H.; Chattopadhaya, C.; Kijak, P.J.; Chu, P.S.; Reimschuessel, R.; Tkachenko, A.; Ceric, O.; De Alwis, H.G. Screening for toxic phorbol esters in jerky pet treat products using LC-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1020, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Montes Osorio, L.R.; Torres Salvador, A.F.; Jongschaap, R.E.E.; Perez, C.A.A.; Sandoval, J.E.B.; Trindade, L.M.; Visser, R.G.F.; van Loo, E.N. High level of molecular and phenotypic biodiversity in Jatropha curcas from Central America compared to Africa, Asia and South America. BMC Plant Biol. 2014, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.G.; Hadi, S.I.I.A.; Costa Alves, G.S.; Mendonça, S.; De Siqueira, F.G.; Miller, R.N.G. Current Strategies for the Detoxification of Jatropha curcas Seed Cake: A Review. J. Agric. Food Chem. 2018, 66, 2510–2522. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Palanisamy, K.; Mulpuri, S. Jatropha: Phytochemistry, Pharmacology, and Toxicology. In Jatropha, Challenges for a New Energy Crop; Mulpuri, S., Carels, N., Bahadur, B., Eds.; Springer: Singapore, 2019; pp. 415–435. [Google Scholar]



| Primer | Sequence Primer | Bases | %GC |
|---|---|---|---|
| MG 5 | 5′-CGGCGTTACG-3′ | 10 | 70 |
| MG 6 | 5′-AACGGGCACC-3′ | 10 | 70 |
| MG 7 | 5′-AATAACCGCC-3′ | 10 | 50 |
| MG 8 | 5′-GGGGGCCTCA-3′ | 10 | 80 |
| MG 10 | 5′-CCGCCCCACT-3′ | 10 | 80 |
| MG 11 | 5′-AGGAGCTGCC-3′ | 10 | 70 |
| MG 34 | 5′-ATTGGGCGAT-3′ | 10 | 50 |
| MG 60 | 5′-AAGCCTCCCC-3′ | 10 | 70 |
| MG 61 | 5′-GGGCCTCTAT-3′ | 10 | 60 |
| MG 68 | 5′-GAGTAAGCGG-3′ | 10 | 60 |
| MG 82 | 5′-CTGGGAGTGG-3′ | 10 | 70 |
| MG 101 | 5′-GCCCCTTTAC-3′ | 10 | 60 |
| MG 103 | 5′-GTACATGGGC-3′ | 10 | 60 |
| MG 104 | 5′-GTCGCCTGAG-3′ | 10 | 70 |
| MG 105 | 5′-CCCTAATCAG-3′ | 10 | 50 |
| MG 108 | 5′-CCGGTTCCAG-3′ | 10 | 70 |
| MG 111 | 5′-GGGCGAGTGC-3′ | 10 | 80 |
| MG 112 | 5′-CCCCTCGAAT-3′ | 10 | 60 |
| MG 113 | 5′-ACGGGCGCTC-3′ | 10 | 80 |
| MG 114 | 5′-GCCCCTCGTC-3′ | 10 | 80 |
| MG 115 | 5′-CGGACCGCGT-3′ | 10 | 80 |
| MG 117 | 5′-CACTGCTGTC -3′ | 10 | 60 |
| MG 118 | 5′-CGGATATACCG-3′ | 10 | 60 |
| MG 119 | 5′-GCATGGTAGC-3′ | 10 | 60 |
| MG 120 | 5′-ATCGTCCAAC-3′ | 10 | 50 |
| MG 121 | 5′-ACCGTGCGTC-3′ | 10 | 70 |
| MG 131 | 5′-TGCTGGTCCA-3′ | 10 | 60 |
| MG 132 | 5′-ACCGGACACT-3′ | 10 | 60 |
| MG 137 | 5′-CGATATCCGG-3′ | 10 | 60 |
| MG 139 | 5′-CAGGCCCTTC-3′ | 10 | 70 |
| RS 1003 | 5′-AGCGCCGACAGGTGC-3′ | 15 | 73 |
| RS 1005 | 5′-TGACCCCTCATGACG-3′ | 15 | 60 |
| RS 1006 | 5′-CCCGGGATATACCGC-3′ | 15 | 67 |
| RS 1007 | 5′-TATATACGCCTATAG-3′ | 15 | 33 |
| RS 1008 | 5′-CTTTTCGCTGGGAGA-3′ | 15 | 53 |
| RS 1009 | 5′-AAATATATGCCCCTA-3′ | 15 | 33 |
| RS 1012 | 5′-TAAGGCCCACCTCCG-3′ | 15 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrone, L.; Martelli, G.; Mazzanti, G.; Vitalone, A. Genomic Study, Phytochemical Characterization, and Antiproliferative Activity of Two Different Genotypes of Jatropha curcas L. Obtained by a Breeding Program. Appl. Sci. 2019, 9, 4373. https://doi.org/10.3390/app9204373
Calabrone L, Martelli G, Mazzanti G, Vitalone A. Genomic Study, Phytochemical Characterization, and Antiproliferative Activity of Two Different Genotypes of Jatropha curcas L. Obtained by a Breeding Program. Applied Sciences. 2019; 9(20):4373. https://doi.org/10.3390/app9204373
Chicago/Turabian StyleCalabrone, Luana, Giuseppe Martelli, Gabriela Mazzanti, and Annabella Vitalone. 2019. "Genomic Study, Phytochemical Characterization, and Antiproliferative Activity of Two Different Genotypes of Jatropha curcas L. Obtained by a Breeding Program" Applied Sciences 9, no. 20: 4373. https://doi.org/10.3390/app9204373
APA StyleCalabrone, L., Martelli, G., Mazzanti, G., & Vitalone, A. (2019). Genomic Study, Phytochemical Characterization, and Antiproliferative Activity of Two Different Genotypes of Jatropha curcas L. Obtained by a Breeding Program. Applied Sciences, 9(20), 4373. https://doi.org/10.3390/app9204373






