Effects of an Acetic Acid and Acetone Mixture on the Characteristics and Scaffold–Cell Interaction of Electrospun Polycaprolactone Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
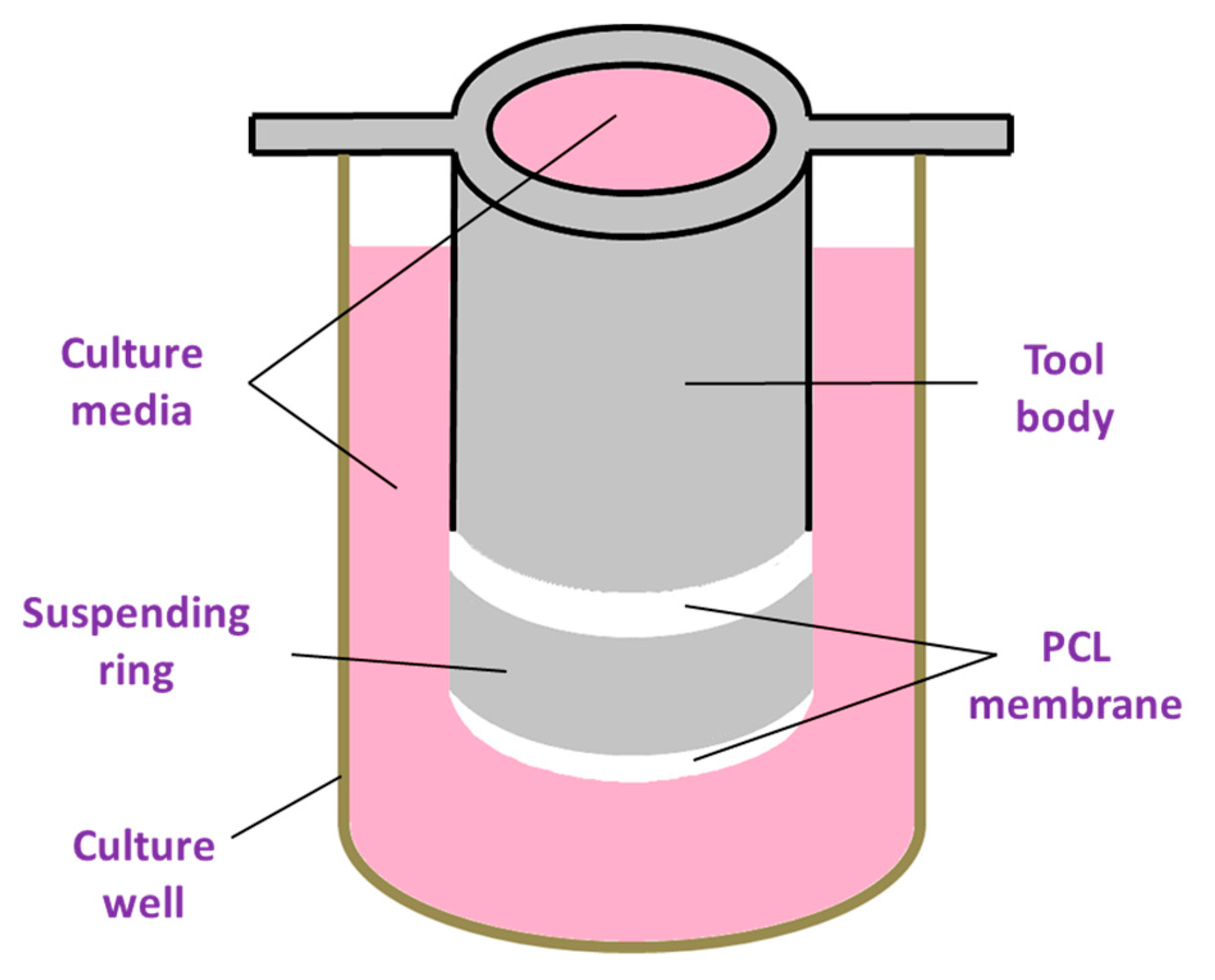
2.2.1. Fabrication of Electrospun Membranes
2.2.2. Sample Characterization
2.2.3. Cell Attachment and Proliferation Assays
3. Results and Discussion
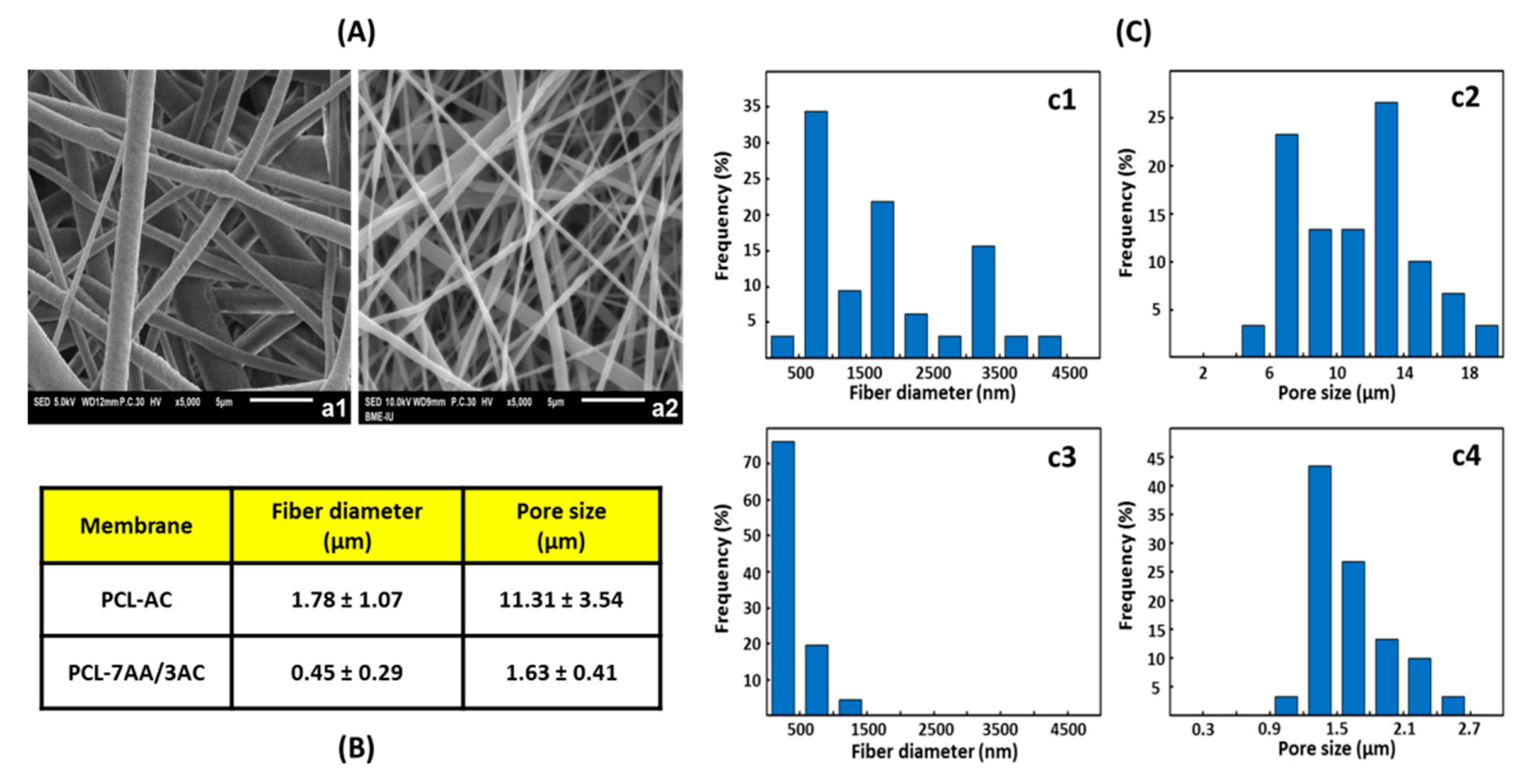
3.1. Surface Morphology
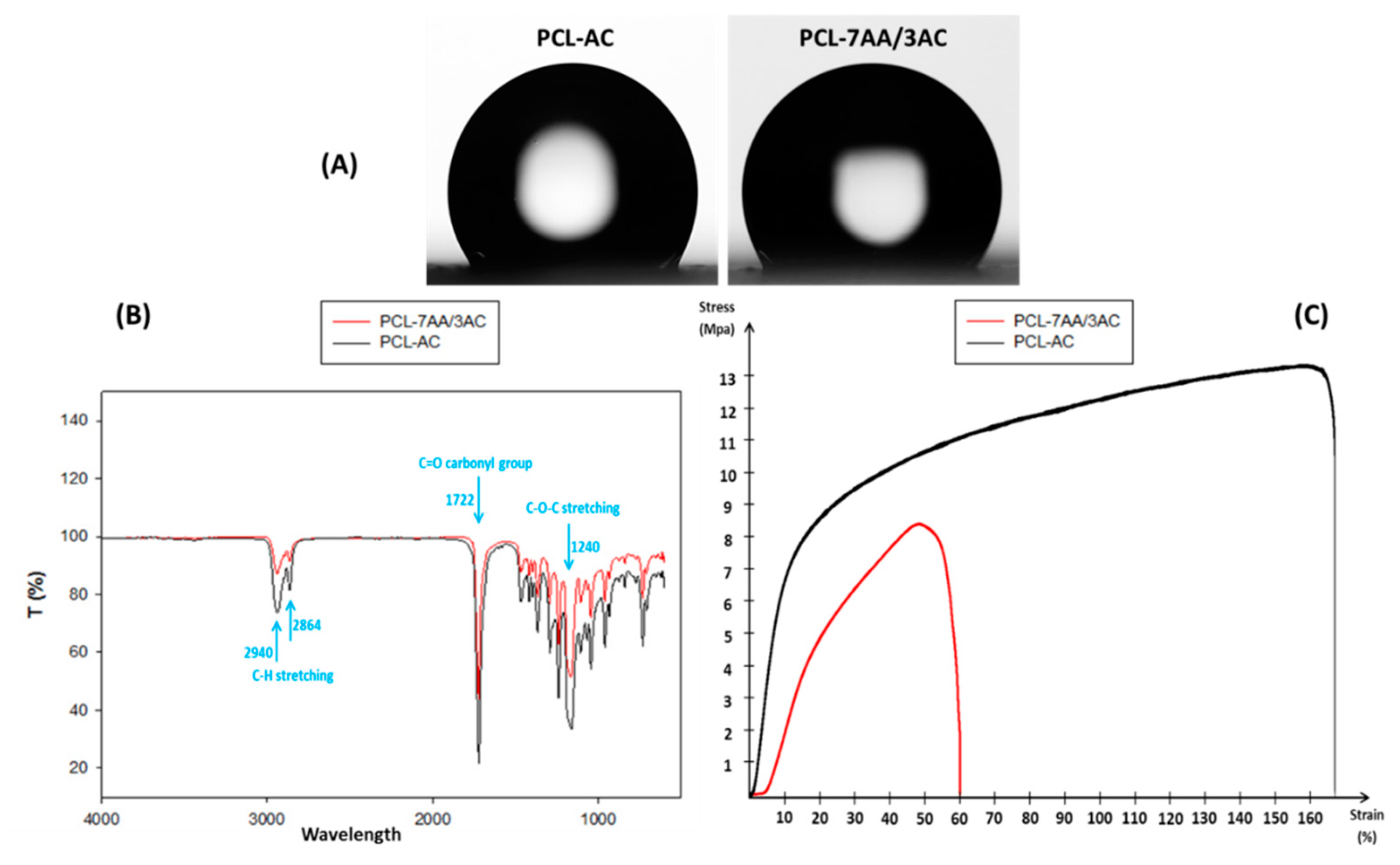
3.2. Wettability, Chemical, and Physical Properties
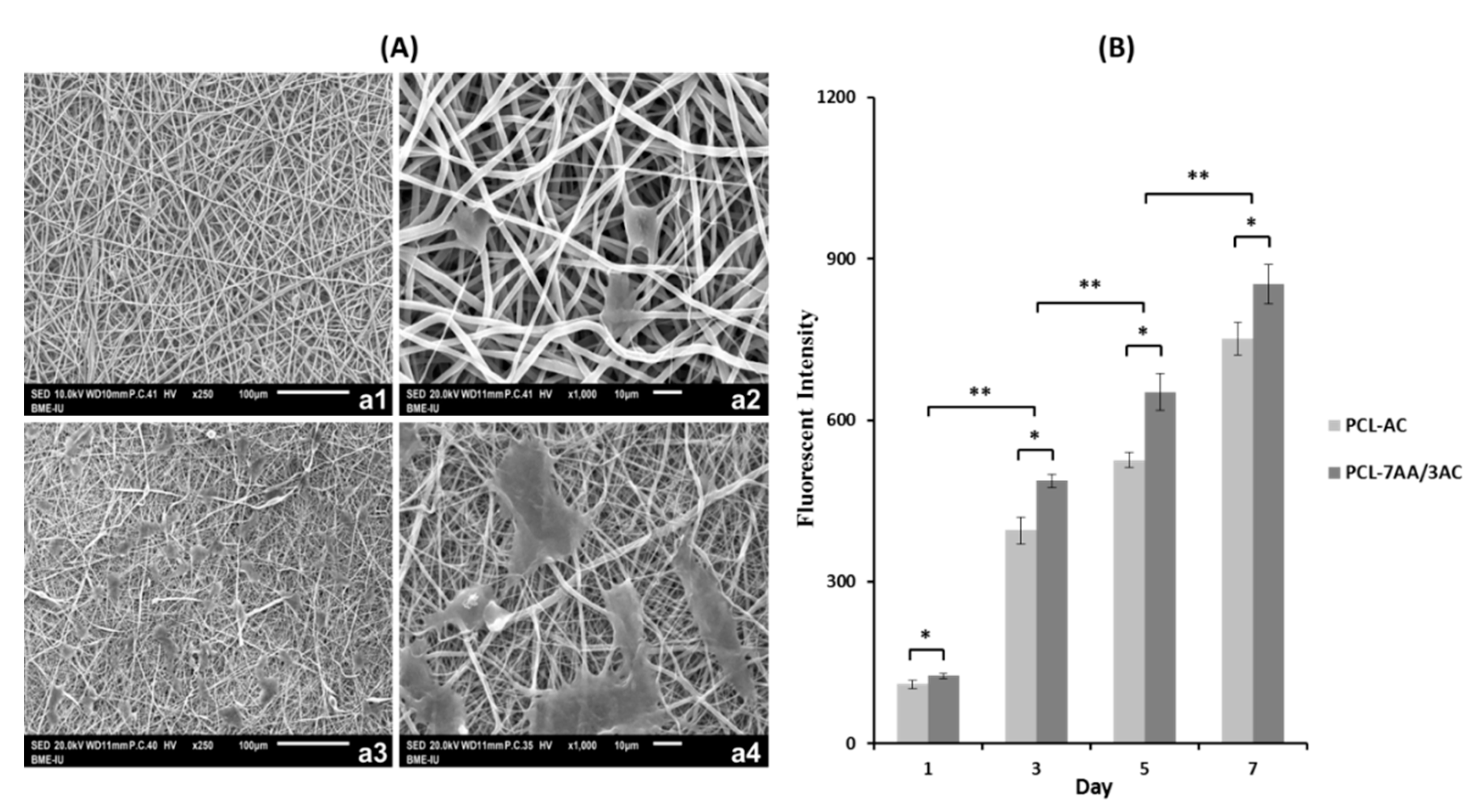
3.3. In Vitro Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Minh, H.H.; Hiep, N.T.; Hai, N.D.; Van Toi, V. Fabrication of Polycaprolactone/Polyurethane Loading Conjugated Linoleic Acid and Its Antiplatelet Adhesion. Int. J. Biomater. 2017, 2017, 5690625. [Google Scholar] [CrossRef]
- Thanh, N.T.; Hieu, M.H.; Phuong, N.T.M.; Thuan, T.D.B.; Thu, H.N.T.; Thai, V.P.; Minh, T.D.; Dai, H.N.; Vo, V.T.; Thi, H.N.; et al. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng. 2018, 91, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Meek, M.E.; Long, G. N,N-dimethylformamide: Hazard characterization and exposure–response analysis. J. Environ. Sci. Health Part C 2001, 19, 161–187. [Google Scholar]
- Fowles, J.; Boatman, R.; Bootman, J.; Lewis, C.; Morgott, D.; Rushton, E.; Van Rooij, J.; Banton, M. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Ekram, B.; Abdel-Hady, B.M.; El-kady, A.M.; Amr, S.M.; Waley, A.I.; Guirguis, O.W. Optimum parameters for the production of nano-scale electrospun polycaprolactone to be used as a biomedical material. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045018. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Schoenmaker, B.; Kalaoglu, Ö.I.; De Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Clogging-Free Electrospinning of Polycaprolactone Using Acetic Acid/Acetone Mixture. Polym. Plast. Technol. Eng. 2016, 55, 518–529. [Google Scholar] [CrossRef]
- Cooper, A.; Bhattarai, N.; Zhang, M. Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr. Polym. 2011, 85, 149–156. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Hackett, J.M.; Dang, T.T.; Tsai, E.C.; Cao, X. Electrospun Biocomposite Polycaprolactone/Collagen Tubes as Scaffolds for Neural Stem Cell Differentiation. Materials 2010, 3, 3714–3728. [Google Scholar] [CrossRef]
- Enis, I.Y.; Vojtech, J.; Sadikoglu, T.G. Alternative solvent systems for polycaprolactone nanowebs via electrospinning. J. Ind. Text. 2017, 47, 57–70. [Google Scholar] [CrossRef]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrost. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- Pan, Y.; Zeng, L. Simulation and Validation of Droplet Generation Process for Revealing Three Design Constraints in Electrohydrodynamic Jet Printing. Micromachines (Basel) 2019, 10, 94. [Google Scholar] [CrossRef]
- Ghorani, B.; Russell, S.J.; Goswami, P. Controlled Morphology and Mechanical Characterisation of Electrospun Cellulose Acetate Fibre Webs. Int. J. Polym. Sci. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar]
- Asvar, Z.; Mirzaei, E.; Azarpira, N.; Geramizadeh, B.; Fadaie, M. Evaluation of electrospinning parameters on the tensile strength and suture retention strength of polycaprolactone nanofibrous scaffolds through surface response methodology. J. Mech. Behav. Biomed. Mater. 2017, 75, 369–378. [Google Scholar] [CrossRef]
- Kancheva, M.; Toncheva, A.; Manolova, N.; Rashkov, I. Enhancing the mechanical properties of electrospun polyester mats by heat treatment. Express Polym. Lett. 2015, 9, 49–65. [Google Scholar] [CrossRef]
- Hernández, A.R.; Contreras, O.C.; Acevedo, J.C.; Moreno, L.G.N. Poly(ε-caprolactone) Degradation Under Acidic and Alkaline Conditions. Am. J. Polym. Sci. 2013, 3, 70–75. [Google Scholar]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Shrivastava, A. 2—Polymerization. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 17–48. [Google Scholar]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Shafei, S.; Foroughi, J.; Chen, Z.; Wong, C.S.; Naebe, M. Short Oxygen Plasma Treatment Leading to Long-Term Hydrophilicity of Conductive PCL-PPy Nanofiber Scaffolds. Polymers 2017, 9, 614. [Google Scholar] [CrossRef]
- Elsayed, Y.; Lekakou, C. 2—Designing and modeling pore size distribution in tissue scaffolds. In Characterisation and Design of Tissue Scaffolds; Tomlins, P., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 23–43. [Google Scholar]
- Vogler, M.; Vogel, S.; Krull, S.; Farhat, K.; Leisering, P.; Lutz, S.; Wuertz, C.M.; Katschinski, D.M.; Zieseniss, A. Hypoxia modulates fibroblastic architecture, adhesion and migration: A role for HIF-1α in cofilin regulation and cytoplasmic actin distribution. PLoS ONE 2013, 8, e69128. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-G.; Park, J.H.; Lee, J.B.; Oh, H.H.; Park, W.H.; Cho, D.; Kwon, O.H. Growth behavior of endothelial cells according to electrospun poly(D,L-lactic-co-glycolic acid) fiber diameter as a tissue engineering scaffold. Tissue Eng. Regen. Med. 2016, 13, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Cicciu, M. Real Opportunity for the Present and a Forward Step for the Future of Bone Tissue Engineering. J. Craniofac. Surg. 2017, 28, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. (Berlin Ger. 1985) 2016, 48, 131–147. [Google Scholar]
- Semnani, D.; Naghashzargar, E.; Hadjianfar, M.; Dehghan Manshadi, F.; Mohammadi, S.; Karbasi, S.; Effaty, F. Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. Int. J. Polym. Mater. Polym. Biomat. 2017, 66, 149–157. [Google Scholar] [CrossRef]
- Mohseni, M.; Castro, N.J.; Dang, H.P.; Nguyen, T.D.; Ho, H.M.; Tran, M.P.N.; Nguyen, T.H.; Tran, P.A. 13—Adipose tissue regeneration: Scaffold—Biomaterial strategies and translational perspectives. In Biomaterials in Translational Medicine; Yang, L., Bhaduri, S.B., Webster, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 291–330. [Google Scholar]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Han, F.; Zhang, P.; Sun, Y.; Lin, C.; Zhao, P.; Chen, J. Hydroxyapatite-doped polycaprolactone nanofiber membrane improves tendon-bone interface healing for anterior cruciate ligament reconstruction. Int. J. Nanomed. 2015, 10, 7333–7343. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, M.H.; Do, T.B.-T.; Dang, N.N.-T.; Le, A.N.-M.; Ta, H.T.-K.; Vo, T.V.; Nguyen, H.T. Effects of an Acetic Acid and Acetone Mixture on the Characteristics and Scaffold–Cell Interaction of Electrospun Polycaprolactone Membranes. Appl. Sci. 2019, 9, 4350. https://doi.org/10.3390/app9204350
Ho MH, Do TB-T, Dang NN-T, Le AN-M, Ta HT-K, Vo TV, Nguyen HT. Effects of an Acetic Acid and Acetone Mixture on the Characteristics and Scaffold–Cell Interaction of Electrospun Polycaprolactone Membranes. Applied Sciences. 2019; 9(20):4350. https://doi.org/10.3390/app9204350
Chicago/Turabian StyleHo, Minh Hieu, Thien Bui-Thuan Do, Nhi Ngoc-Thao Dang, An Nguyen-My Le, Hanh Thi-Kieu Ta, Toi Van Vo, and Hiep Thi Nguyen. 2019. "Effects of an Acetic Acid and Acetone Mixture on the Characteristics and Scaffold–Cell Interaction of Electrospun Polycaprolactone Membranes" Applied Sciences 9, no. 20: 4350. https://doi.org/10.3390/app9204350
APA StyleHo, M. H., Do, T. B.-T., Dang, N. N.-T., Le, A. N.-M., Ta, H. T.-K., Vo, T. V., & Nguyen, H. T. (2019). Effects of an Acetic Acid and Acetone Mixture on the Characteristics and Scaffold–Cell Interaction of Electrospun Polycaprolactone Membranes. Applied Sciences, 9(20), 4350. https://doi.org/10.3390/app9204350



