Magnetic Properties of NiZn Ferrite Nanofibers Prepared by Electrospinning
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of NiZn Ferrite Nanofibers via Electrospinning
2.2. Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chu, Y.-Q.; Fu, Z.-W.; Qin, Q.-Z. Cobalt ferrite thin films as anode material for lithium ion batteries. Electrochim. Acta 2004, 49, 4915–4921. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Shao, H. High capacity ZnFe2O4 anode material for lithium ion batteries. Electrochim. Acta 2011, 56, 9433–9438. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.A.; de Souza, E.F.; de Carvalho, M.C.; Martins, R.L.; Schmal, M. Cobalt ferrite nanoparticles for the preferential oxidation of CO. Appl. Catal. A Gen. 2016, 519, 139–145. [Google Scholar] [CrossRef]
- Wan, L.S. A Study into Photocatalytic Degradation of Methylene Blue and Glycerol Aqueous Solution over Copper Ferrite Catalyst. Ph.D. Thesis, UMP, Gambang, Malaysia, 2015. [Google Scholar]
- Hankiewicz, J.H.; Alghamdi, N.; Hammelev, N.M.; Anderson, N.R.; Camley, R.E.; Stupic, K.; Przybylski, M.; Zukrowski, J.; Celinski, Z.J. Zinc doped copper ferrite particles as temperature sensors for magnetic resonance imaging. Aip Adv. 2017, 7, 056703. [Google Scholar] [CrossRef]
- Dumitrescu, A.; Lisa, G.; Iordan, A.; Tudorache, F.; Petrila, I.; Borhan, A.; Palamaru, M.; Mihailescu, C.; Leontie, L.; Munteanu, C. Ni ferrite highly organized as humidity sensors. Mater. Chem. Phys. 2015, 156, 170–179. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Lai, M.; Sang, T. Preparation and microwave absorption mechanisms of the NiZn ferrite nanofibers. J. Alloys Compd. 2015, 627, 367–373. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Xiao, S.; Chen, G. The cobalt zinc spinel ferrite nanofiber: Lightweight and efficient microwave absorber. J. Am. Ceram. Soc. 2014, 97, 1363–1366. [Google Scholar] [CrossRef]
- Wu, Y.; Han, M.; Deng, L. Enhancing the microwave absorption properties of Fe–Cu–Nb–Si–B nanocomposite flakes by coating with spinel ferrite NiFe2O4. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Duan, H.-Z.; Zhou, F.-L.; Cheng, X.; Chen, G.-H.; Li, Q.-L. Preparation of hollow microspheres of Ce3+ doped NiCo ferrite with high microwave absorbing performance. J. Magn. Magn. Mater. 2017, 424, 467–471. [Google Scholar] [CrossRef]
- Ren, X.; Xu, G. Electromagnetic and microwave absorbing properties of NiCoZn-ferrites doped with La3+. J. Magn. Magn. Mater. 2014, 354, 44–48. [Google Scholar] [CrossRef]
- Ghodake, J.; Kambale, R.C.; Shinde, T.; Maskar, P.; Suryavanshi, S. Magnetic and microwave absorbing properties of Co2+ substituted nickel–zinc ferrites with the emphasis on initial permeability studies. J. Magn. Magn. Mater. 2016, 401, 938–942. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Rao, W.; Sang, T.; Song, B.; Wong, C. Tunable electromagnetic properties and enhanced microwave absorption ability of flaky graphite/cobalt zinc ferrite composites. J. Alloys Compd. 2016, 662, 409–414. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Lv, Q.; Shen, Z.-M. Fabrication and microwave absorbing properties of Ni–Zn spinel ferrites. J. Alloys Compd. 2009, 480, 634–638. [Google Scholar] [CrossRef]
- Yu, K.; Chen, J. Enhancing solar cell efficiencies through 1-D nanostructures. Nanoscale Res. Lett. 2009, 4, 1. [Google Scholar] [CrossRef]
- Dharani, S.; Mulmudi, H.K.; Yantara, N.; Trang, P.T.T.; Park, N.G.; Graetzel, M.; Mhaisalkar, S.; Mathews, N.; Boix, P.P. High efficiency electrospun TiO2 nanofiber based hybrid organic–inorganic perovskite solar cell. Nanoscale 2014, 6, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Li, X.; Wang, C.; Zhang, M.; Liu, Y. Electrospun nanofibers of p-type NiO/n-type ZnO heterojunctions with enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 2915–2923. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, S.; Lim, S.K.; Park, H. Photocatalytic comparison of TiO2 nanoparticles and electrospun TiO2 nanofibers: Effects of mesoporosity and interparticle charge transfer. J. Phys. Chem. C 2010, 114, 16475–16480. [Google Scholar] [CrossRef]
- Chuangchote, S.; Jitputti, J.; Sagawa, T.; Yoshikawa, S. Photocatalytic activity for hydrogen evolution of electrospun TiO2 nanofibers. ACS Appl. Mater. Interfaces 2009, 1, 1140–1143. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Wang, B.; Wang, J.-N. Magnetic and microwave absorbing properties of electrospun Ba(1−x)LaxFe12O19 nanofibers. J. Magn. Magn. Mater. 2012, 324, 1305–1311. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Yang, Y.; Kang, F.; Gu, J. Fe3O4/carbon composite nanofiber absorber with enhanced microwave absorption performance. Mater. Sci. Eng. B 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Han, R.; Li, W.; Pan, W.; Zhu, M.; Zhou, D.; Li, F.-S. 1D magnetic materials of Fe3O4 and Fe with high performance of microwave absorption fabricated by electrospinning method. Sci. Rep. 2014, 4, 7493. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, H.; Cheng, B.; Zhao, X.; Zhang, Q. Preparation and photocatalytic activity of mesoporous anatase TiO2 nanofibers by a hydrothermal method. J. Photochem. Photobiol. A Chem. 2006, 182, 121–127. [Google Scholar] [CrossRef]
- Yu, J.; Kudo, A. Hydrothermal synthesis of nanofibrous bismuth vanadate. Chem. Lett. 2005, 34, 850–851. [Google Scholar] [CrossRef]
- Cao, G.; Liu, D. Template-based synthesis of nanorod, nanowire, and nanotube arrays. Adv. Colloid Interface Sci. 2008, 136, 45–64. [Google Scholar] [CrossRef]
- Kim, W.-T.; Kim, I.-H.; Choi, W.-Y. Fabrication of TiO2 nanotube arrays and their application to a gas sensor. J. Nanosci. Nanotechnol. 2015, 15, 8161–8165. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Sonsupap, S.; Kidkhunthod, P.; Chanlek, N.; Pinitsoontorn, S.; Maensiri, S. Fabrication, structure, and magnetic properties of electrospun Ce0.96Fe0.04O2 nanofibers. Appl. Surf. Sci. 2016, 380, 16–22. [Google Scholar] [CrossRef]
- Saensuk, O.; Phokha, S.; Bootchanont, A.; Maensiri, S.; Swatsitang, E. Fabrication and magnetic properties of NiFe2O4 nanofibers obtained by electrospinning. Ceram. Int. 2015, 41, 8133–8141. [Google Scholar] [CrossRef]
- Ponhan, W.; Kadtajun, S.; Khetchompoo, I.; Chinnasa, P. Fabrication and magnetic properties of Co0.05LaTi0.95O3 nanofibers. J. Phys. Conf. Ser. 2018, 1144, 012066. [Google Scholar] [CrossRef]
- Wang, C.; Shen, Y.; Wang, X.; Zhang, H.; Xie, A. Synthesis of novel NiZn-ferrite/Polyaniline nanocomposites and their microwave absorption properties. Mater. Sci. Semicond. Process. 2013, 16, 77–82. [Google Scholar] [CrossRef]
- Ali, N.N.; Atassi, Y.; Salloum, A.; Charba, A.; Malki, A.; Jafarian, M. Comparative study of microwave absorption characteristics of (Polyaniline/NiZn ferrite) nanocomposites with different ferrite percentages. Mater. Chem. Phys. 2018, 211, 79–87. [Google Scholar] [CrossRef]
- Streckova, M.; Mudra, E.; Sebek, M.; Sopcak, T.; Dusza, J.; Kovac, J. Preparation and Investigations of Ni0.2Zn0.8Fe2O4 Ferrite Nanofiber Membranes by Needleless Electrospinning Method. Acta Phys. Pol. A. 2017, 131. [Google Scholar]
- Song, X.; Liu, W.; Wang, J.; Xu, S.; Liu, B.; Liu, J.; Ma, Y. Microstructural differences between electrospun alumina borate nanofibers prepared by solutions with different PVP contents. Ceram. Int. 2017, 43, 9831–9837. [Google Scholar] [CrossRef]
- Chen, X.; Feng, C.; Wu, Z.L.; Yang, F.; Liu, Y.; Jiang, S.; Li, M.H.; Yu, G.H. Interfacial oxygen migration and its effect on the magnetic anisotropy in Pt/Co/MgO/Pt films. Appl. Phys. Lett. 2014, 104, 052413. [Google Scholar] [CrossRef]
- Kumar, L.; Kar, M. Influence of Al3+ ion concentration on the crystal structure and magnetic anisotropy of nanocrystalline spinel cobalt ferrite. J. Magn. Magn. Mater. 2011, 323, 2042–2048. [Google Scholar] [CrossRef]
- Jaffari, G.H.; Rumaiz, A.; Woicik, J.; Shah, S.I. Influence of oxygen vacancies on the electronic structure and magnetic properties of NiFe2O4 thin films. J. Appl. Phys. 2012, 111, 093906. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, G.; Zheng, M.; Chang, X.; Li, S.; Zhang, Y. Synthesis and magnetic properties of crystalline mesoporous CoFe2O4 with large specific surface area. J. Mater. Chem. 2010, 20, 945–952. [Google Scholar] [CrossRef]
- Gong, W.; Li, H.; Zhao, Z.; Chen, J. Ultrafine particles of Fe, Co, and Ni ferromagnetic metals. J. Appl. Phys. 1991, 69, 5119–5121. [Google Scholar] [CrossRef]
- Herranz, G.; Ranchal, R.; Bibes, M.; Jaffres, H.; Jacquet, E.; Maurice, J.-L.; Bouzehouane, K.; Wyczisk, F.; Tafra, E.; Basletic, M. Co-doped (La, Sr)TiO3−δ: A high Curie temperature diluted magnetic system with large spin polarization. Phys. Rev. Lett. 2006, 96, 027207. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Shen, X.Q.; Song, F.Z.; Liu, M.Q. Fabrication and magnetic properties of Ni0.5Zn0.5Fe2O4 nanofibres by electrospinning. Chin. Phys. B 2009, 18, 4960. [Google Scholar]
- Fu, J.; Zhang, J.; Peng, Y.; Zhao, C.; He, Y.; Zhang, Z.; Pan, X.; Mellors, N.J.; Xie, E. Wire-in-tube structure fabricated by single capillary electrospinning via nanoscale Kirkendall effect: The case of nickel–zinc ferrite. Nanoscale 2013, 5, 12551–12557. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.A.; Cahill, C.L.; Carpenter, E.E.; Calvin, S.; Swaminathan, R.; McHenry, M.E.; Harris, V.G. Magnetic and structural properties of nickel zinc ferrite nanoparticles synthesized at room temperature. J. Appl. Phys. 2004, 95, 6392–6395. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Bashiri, M.; Moeen, S.J. Synthesis of nickel–zinc ferrite magnetic nanoparticle and dye degradation using photocatalytic ozonation. Mater. Res. Bull. 2012, 47, 4403–4408. [Google Scholar] [CrossRef]


| Precursors | Ni(NO3)2·6H2O (g) | Zn(NO3)2·6H2O (g) | Fe(NO3)2·9H2O (g) | PVP (g) | DMF (g) |
|---|---|---|---|---|---|
| Precursor A | 1.45 | 1.48 | 4.04 | 18 | 100 |
| Precursor B | 20 | ||||
| Precursor C | 22 |
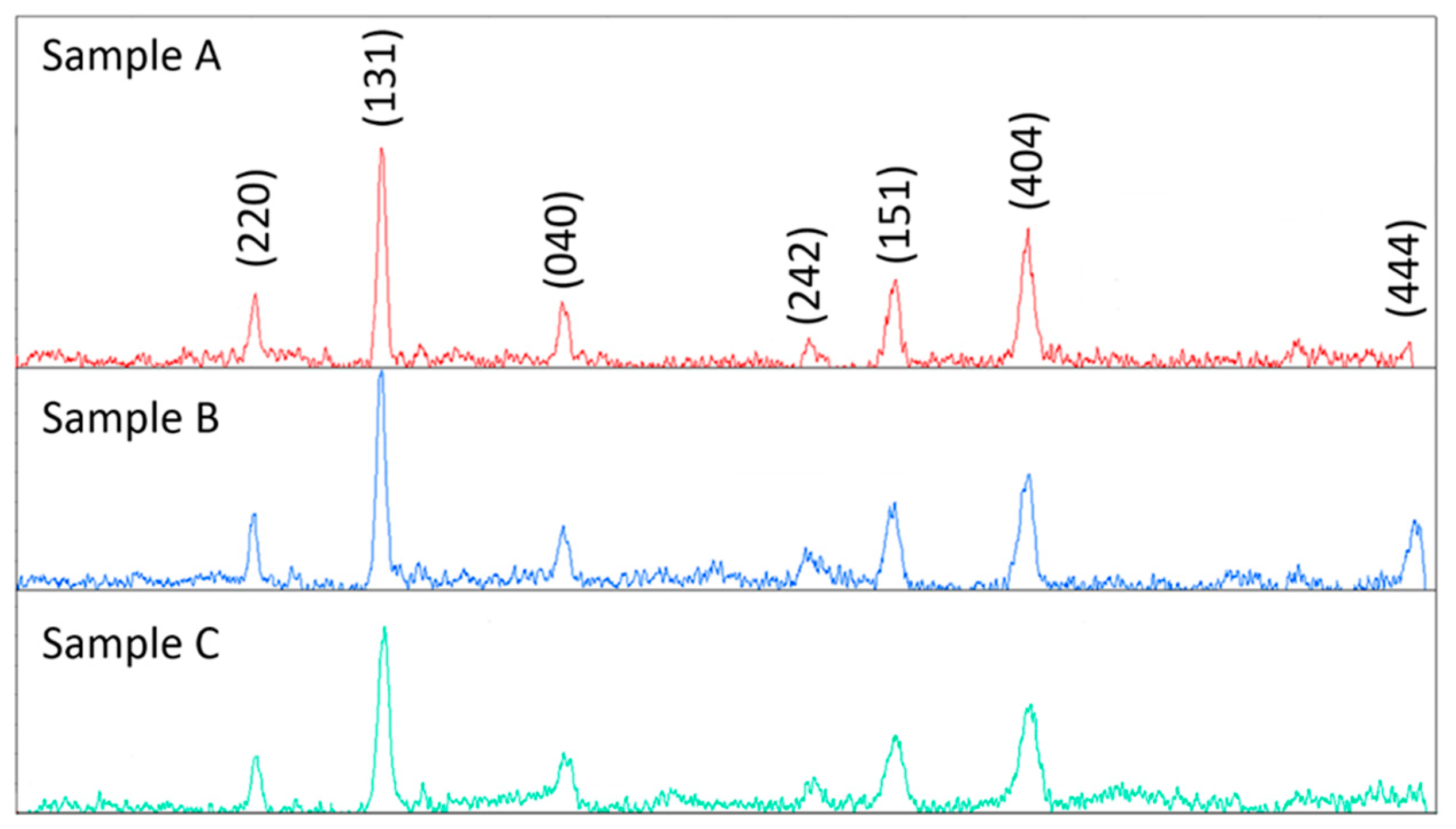
| Samples | Plane (hkl) | 2θ (°) | FWHM (°) | Average Crystallite Size (nm) |
|---|---|---|---|---|
| Sample A | (131) | 35.25 | 0.33 | 20 ± 6 |
| (151) | 56.72 | 0.58 | ||
| (404) | 62.47 | 0.56 | ||
| Sample B | (131) | 35.60 | 0.44 | 16 ± 3 |
| (151) | 56.76 | 0.69 | ||
| (404) | 62.82 | 0.68 | ||
| Sample C | (131) | 35.63 | 0.58 | 13 ± 2 |
| (151) | 56.78 | 0.87 | ||
| (404) | 62.81 | 0.82 |
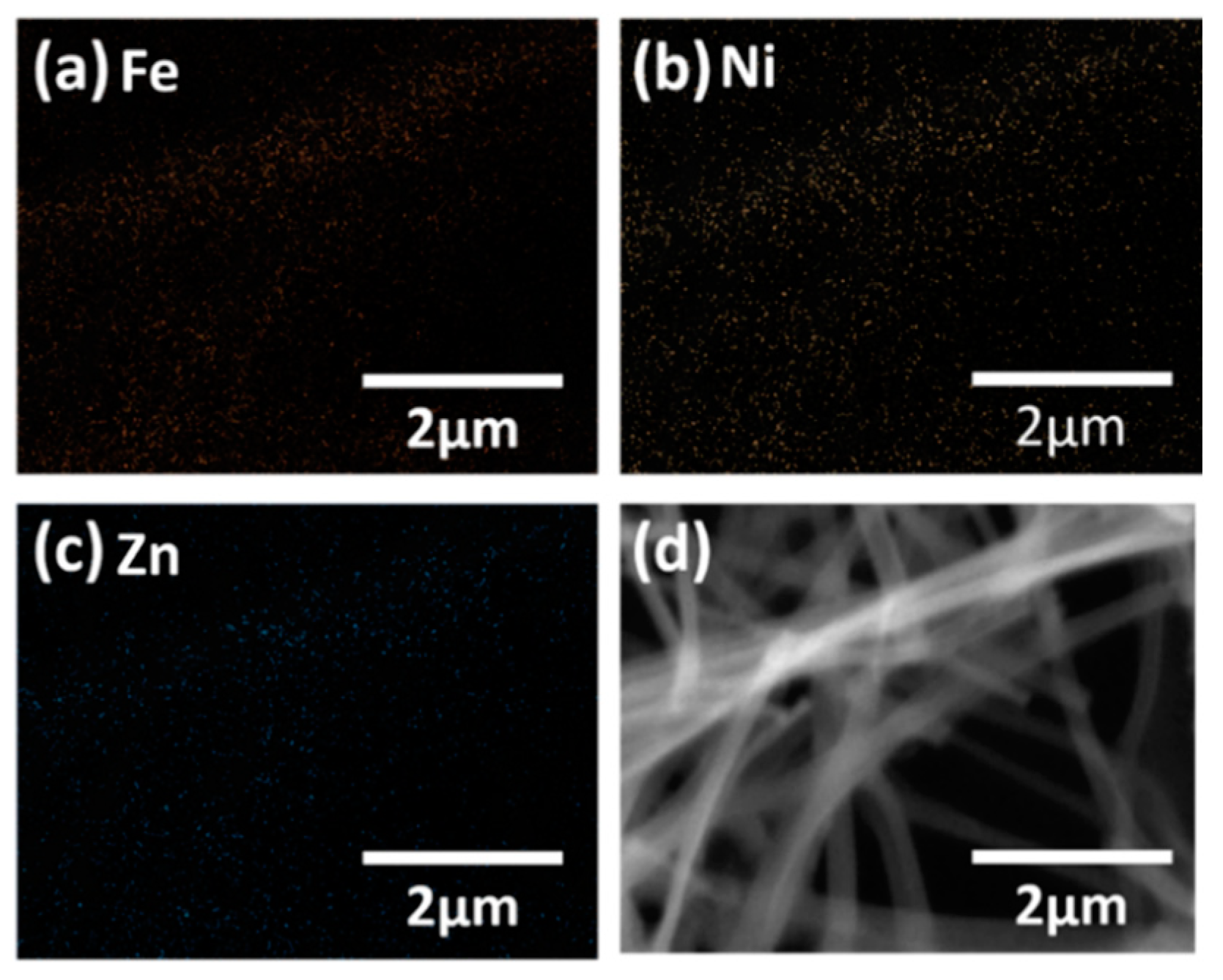
| Element | Weight Percent (%) | Atomic Percent (%) | Net Intensity | Error (%) |
|---|---|---|---|---|
| Fe | 45.86 | 28.07 | 270.80 | 1.93 |
| Ni | 12.59 | 7.33 | 55.70 | 3.08 |
| Zn | 14.98 | 7.83 | 49.50 | 3.08 |
| O | 26.57 | 56.77 | 0 | 0 |
| Ni0.5Zn0.5Fe2O4 Nanofibers | ||||
|---|---|---|---|---|
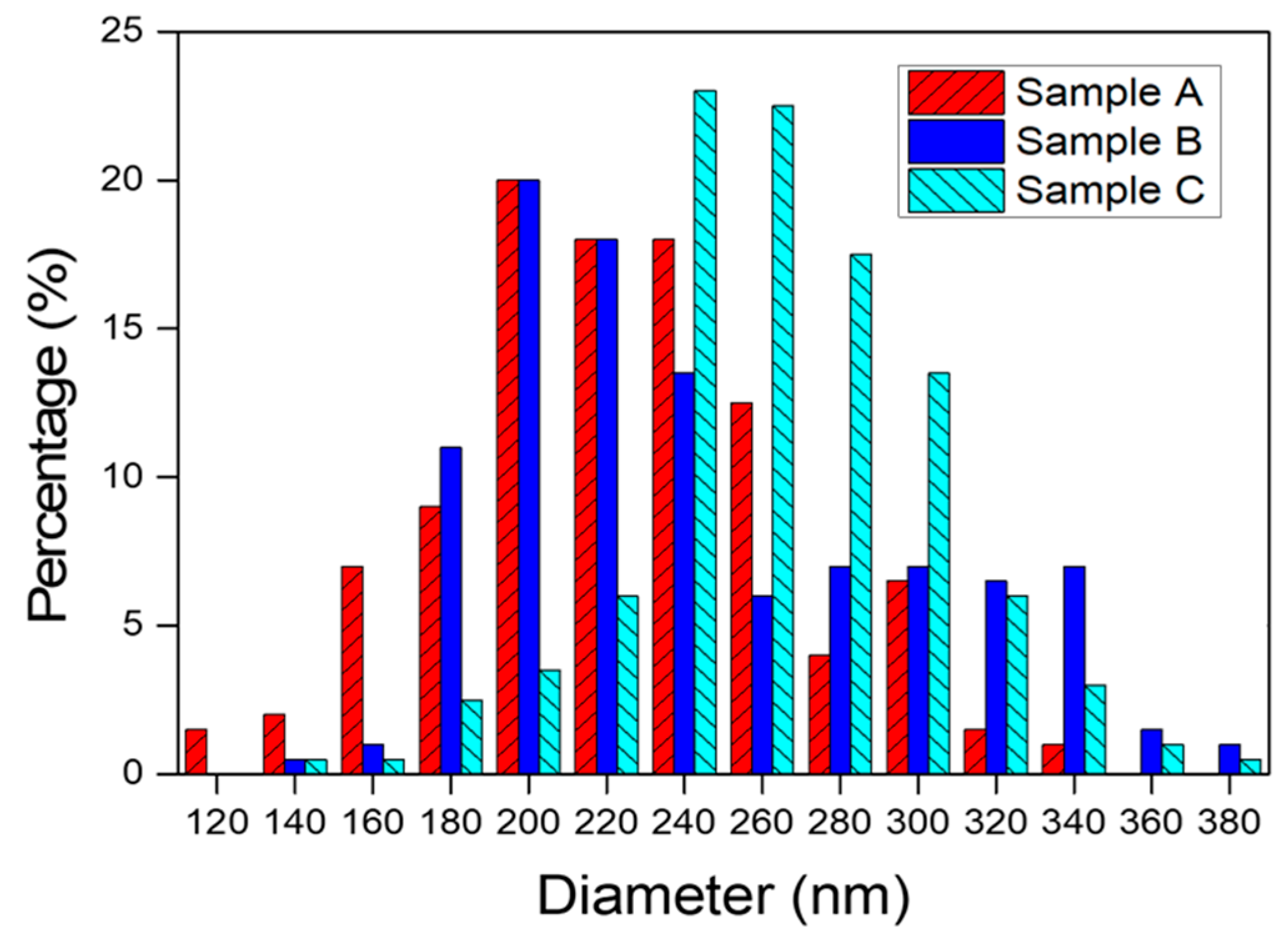
| Samples | Maximum Diameter (nm) | Minimum Diameter (nm) | Average Diameter (nm) | Standard Deviation (nm) |
| Sample A | 355.02 | 112.9 | 224.26 | 45.42 |
| Sample B | 391.68 | 139.66 | 245.48 | 55.78 |
| Sample C | 402.52 | 157.54 | 266.42 | 39.87 |
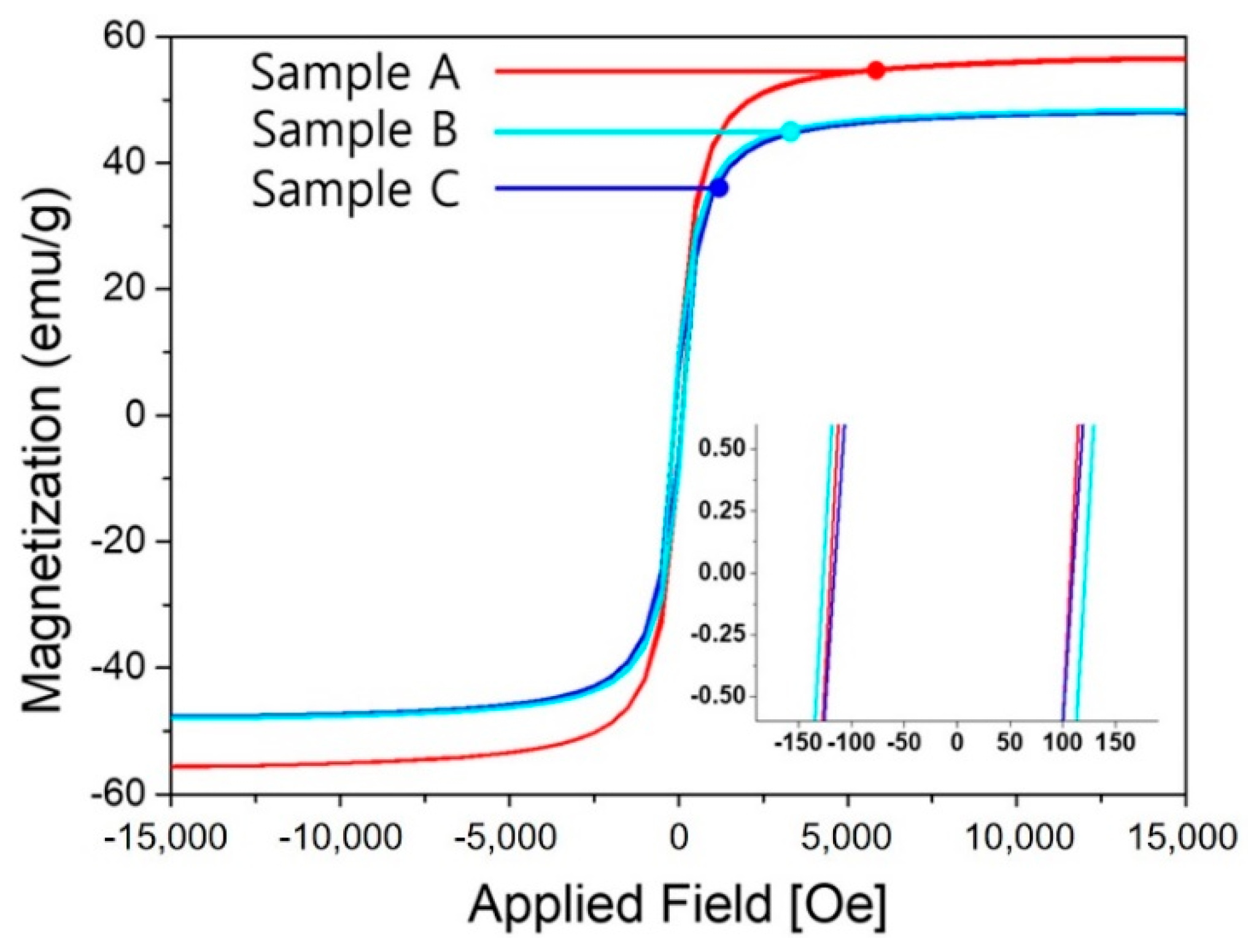
| Samples | Saturation Magnetization (emu/g) | Coercivity (Oe) | Remnant Magnetization (emu/g) |
|---|---|---|---|
| Sample A | 56.65 | 153.12 | 9.82 |
| Sample B | 48.09 | 150.41 | 7.37 |
| Sample C | 48.29 | 169.45 | 9.32 |
| NiZn ferrite Nanofibers [8,46,47] | 37–90.2 | 43–175.3 | - |
| NiZn ferrite Nanoparticles [48,49] | 23.95–56 | 16–200 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, K.-H.; Kim, W.-T.; Song, T.-H.; Choi, W.-Y. Magnetic Properties of NiZn Ferrite Nanofibers Prepared by Electrospinning. Appl. Sci. 2019, 9, 4297. https://doi.org/10.3390/app9204297
Na K-H, Kim W-T, Song T-H, Choi W-Y. Magnetic Properties of NiZn Ferrite Nanofibers Prepared by Electrospinning. Applied Sciences. 2019; 9(20):4297. https://doi.org/10.3390/app9204297
Chicago/Turabian StyleNa, Kyeong-Han, Wan-Tae Kim, Tae-Hyeob Song, and Won-Youl Choi. 2019. "Magnetic Properties of NiZn Ferrite Nanofibers Prepared by Electrospinning" Applied Sciences 9, no. 20: 4297. https://doi.org/10.3390/app9204297
APA StyleNa, K.-H., Kim, W.-T., Song, T.-H., & Choi, W.-Y. (2019). Magnetic Properties of NiZn Ferrite Nanofibers Prepared by Electrospinning. Applied Sciences, 9(20), 4297. https://doi.org/10.3390/app9204297






