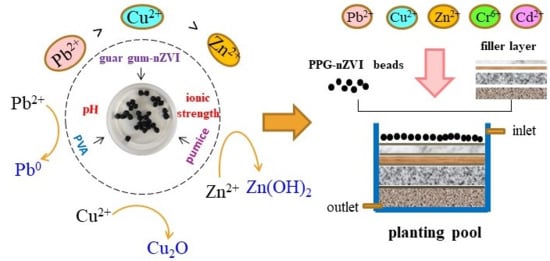
Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sorption Experiments
2.3. Characterization
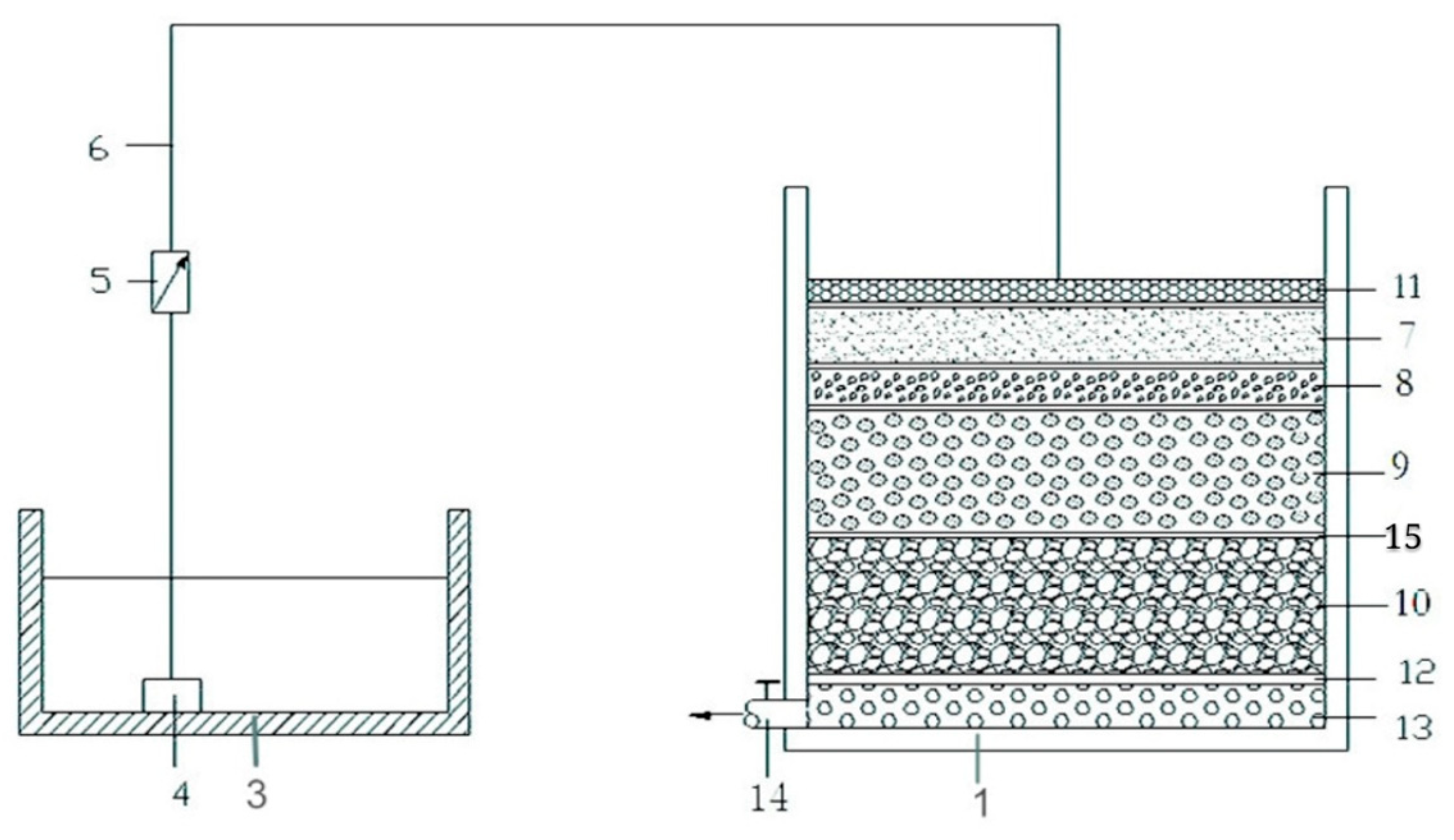
2.4. Application of PPG-nZVI Beads in Simulated Stormwater Infiltration Facility
3. Results and Discussion
3.1. Sorption Kinetics
3.2. Sorption Isotherms
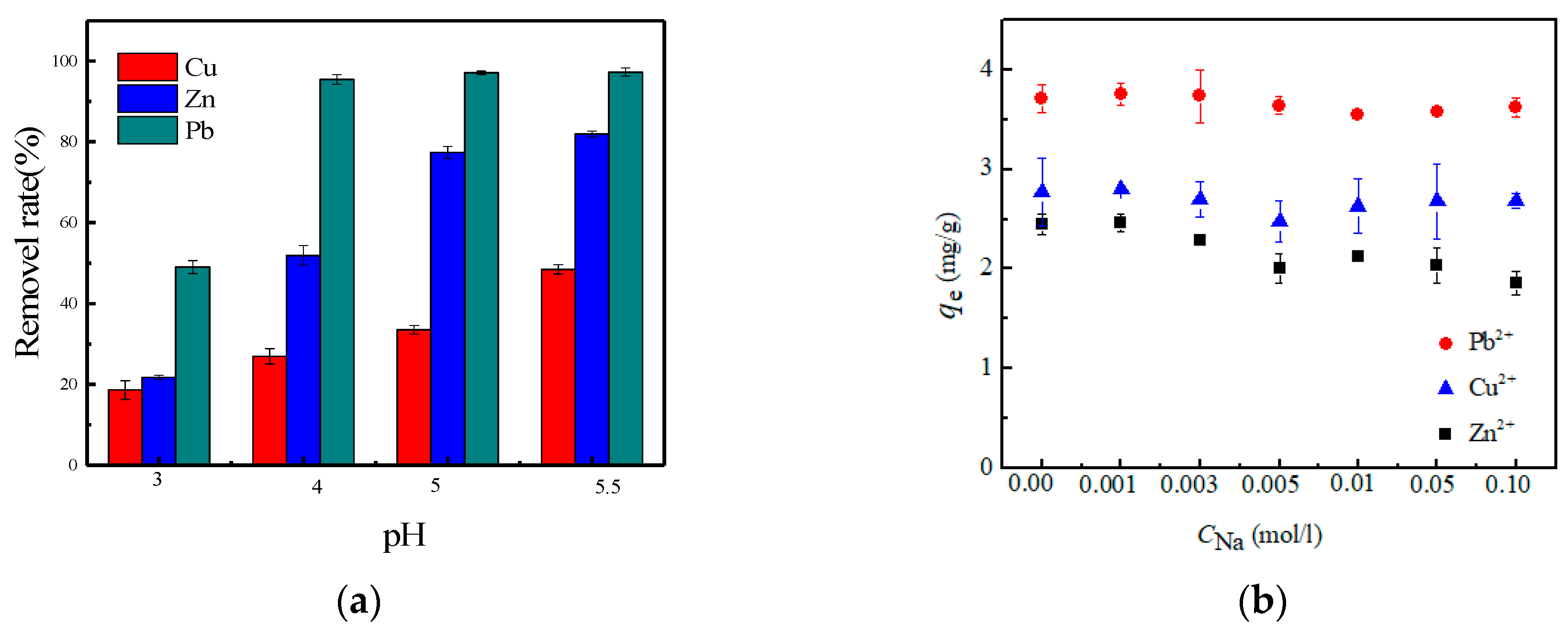
3.3. Effect of pH and Ionic Strength on Sorption
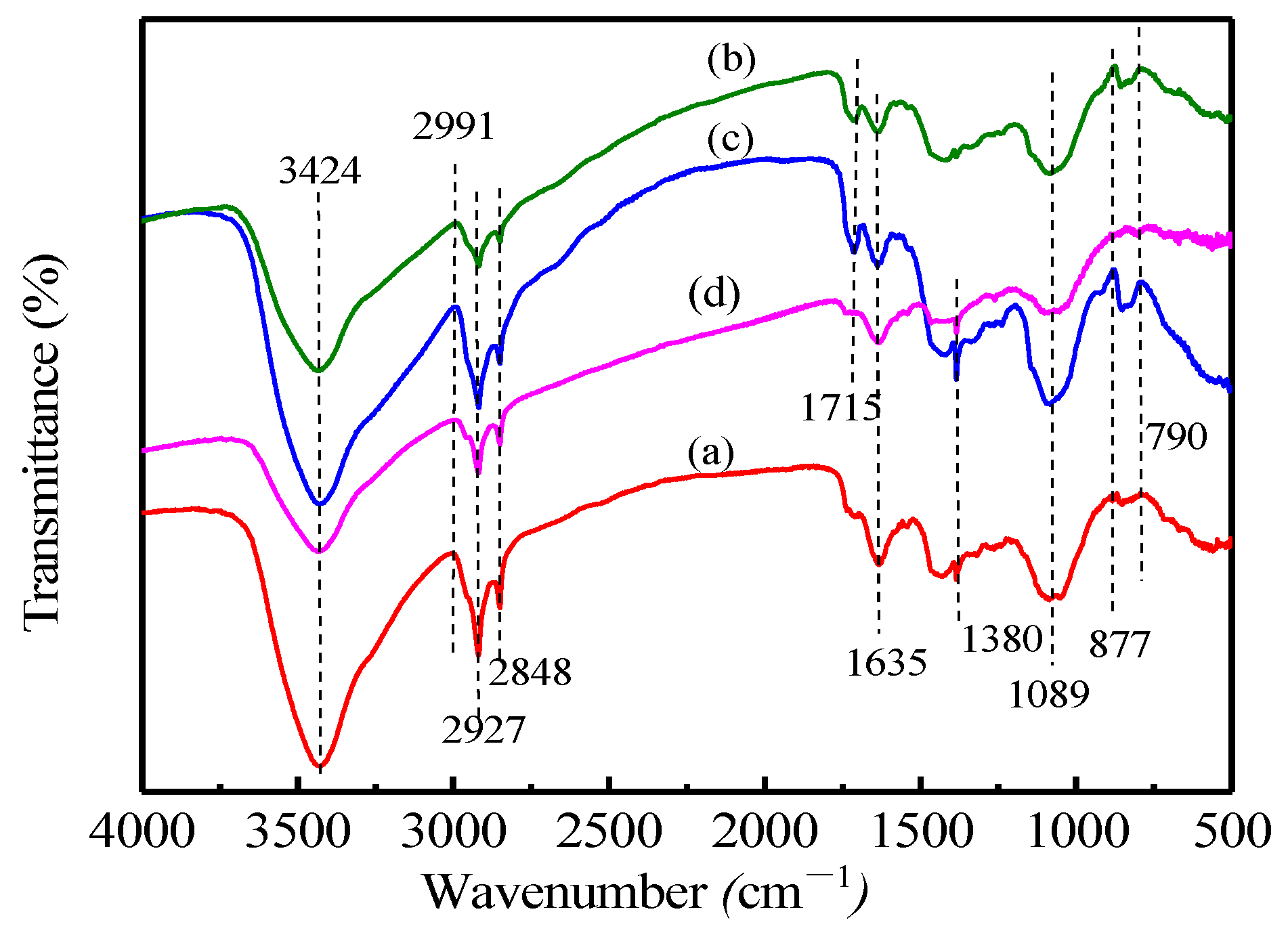
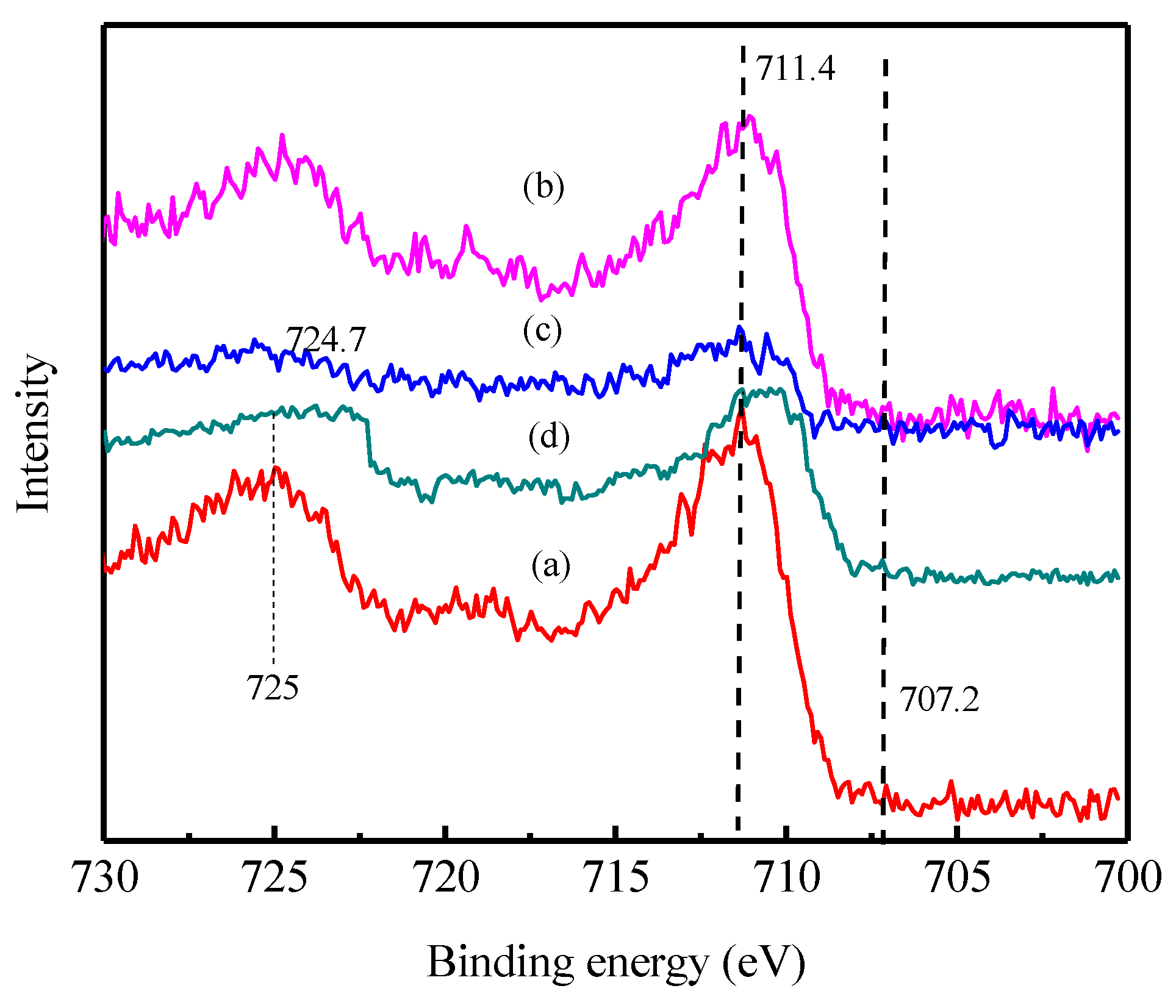
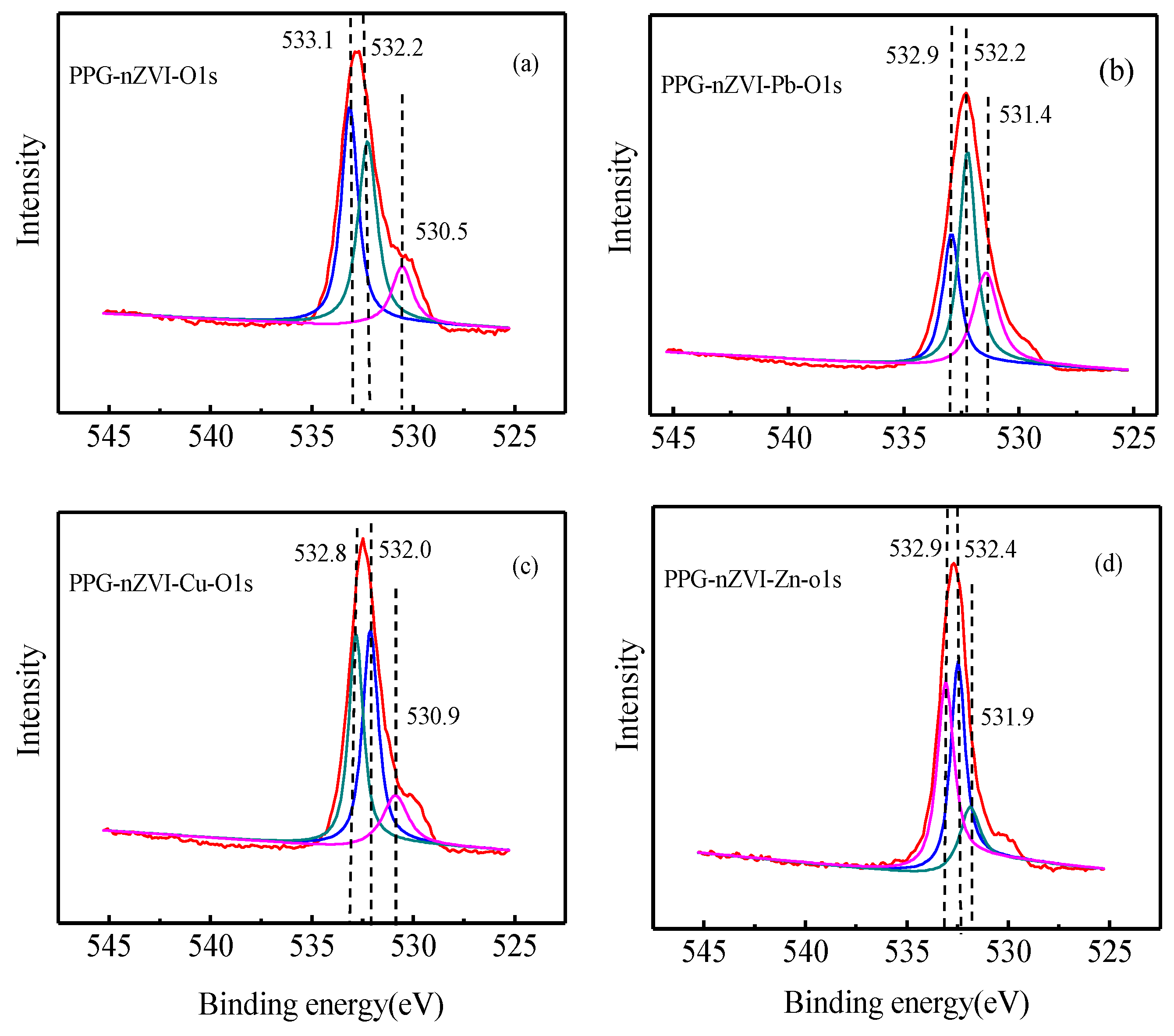
3.4. Characterization before and after Sorption
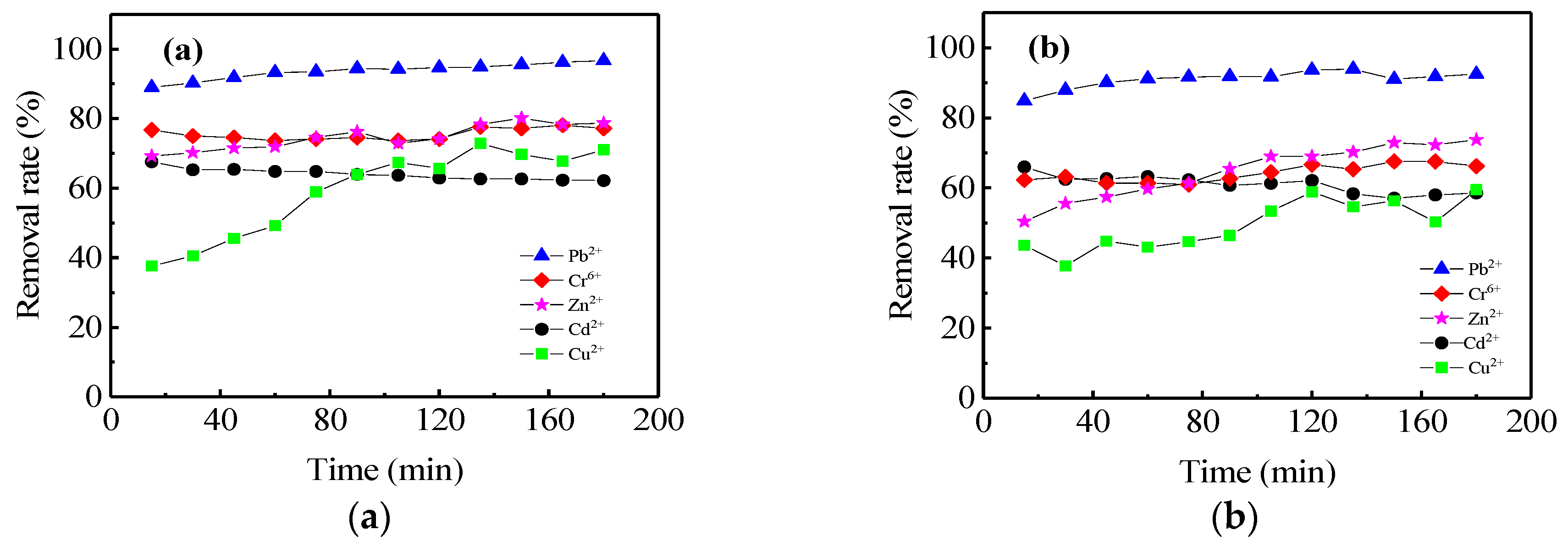
3.5. Efficient Removal of Heavy Metals in Stormwater Infiltration Facility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hizal, J.; Apak, R. Modeling of copper(II) and lead(II) adsorption on kaolinite-based clay minerals individually and in the presence of humic acid. J. Colloid Interface Sci. 2006, 295, 1–13. [Google Scholar] [CrossRef]
- Zou, Y.D.; Wang, X.X.; Khan, A.; Wang, P.Y.; Liu, Y.H.; Alsaedi, A.; Hayat, T.; Wang, X.K. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Wu, N.; Wei, H.H.; Zhang, L.Z. Efficient Removal of Heavy Metal Ions with Biopolymer Template Synthesized Mesoporous Titania Beads of Hundreds of Micrometers Size. Environ. Sci. Technol. 2012, 46, 419–425. [Google Scholar] [CrossRef]
- Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-Shahat, M.F. Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Su, Y.M.; Zhou, X.F.; Dai, C.M.; Keller, A.A. A new insight on the core-shell structure of zerovalent iron nanoparticles and its application for Pb(II) sequestration. J. Hazard. Mater. 2013, 263, 685–693. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J. Hazard Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Haiyan, L.; Yu, G.; Xiaoran, Z. High efficient removal of lead from aqueous solution by preparation of novel PPG-nZVI beads as sorbents. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 513, 306–314. [Google Scholar] [CrossRef]
- Geng, B.; Jin, Z.; Li, T.; Qi, X. Preparation of chitosan-stabilized Fe(0) nanoparticles for removal of hexavalent chromium in water. Sci. Total Environ. 2009, 407, 4994–5000. [Google Scholar] [CrossRef]
- Chung, H.-K.; Kim, W.-H.; Park, J.; Cho, J.; Jeong, T.-Y.; Park, P.-K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Yan, W.; Herzing, A.A.; Kiely, C.J.; Zhang, W.X. Nanoscale zero-valent iron (nZVI): aspects of the core-shell structure and reactions with inorganic species in water. J. Contam. Hydrol. 2010, 118, 96–104. [Google Scholar] [CrossRef]
- Chen, C.L.; Tian, T.; Wang, M.K.; Wang, G. Release of Pb in soils washed with various extractants. Geoderma 2016, 275, 74–81. [Google Scholar] [CrossRef]
- Xuesong, W.; Zhipeng, L.; Huahua, M.; Wen, H.; Hongliang, S. Kinetics of Pb (II) adsorption on black carbon derived from wheat residue. Chem. Eng. J. 2011, 166, 986–993. [Google Scholar] [CrossRef]
- Choy, K.K.; McKay, G. Sorption of metal ions from aqueous solution using bone char. Environ. Int. 2005, 31, 845–854. [Google Scholar] [CrossRef]
- Jun-xia, Y.; Li-yan, W.; Ru-an, C.; Yue-fei, Z.; Zhi-gao, X.; Jia, G. Adsorption of Pb2+, Cd2+, Cu2+, and Zn2+ from aqueous solution by modified sugarcane bagasse. Res. Chem. Intermed. 2013, 41, 1525–1541. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.; Wang, Z.L. Stabilized chitosan/Fe-0-nanoparticle beads to remove heavy metals from polluted sediments. Water Sci. Technol. 2016, 73, 1090–1097. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Fu, R.B.; Yang, Y.P.; Xu, Z.; Zhang, X.; Guo, X.P.; Bi, D.S. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Understanding the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle Bridging: A review. Sep. Purif. Technol. 2019, 231. [Google Scholar] [CrossRef]
- Soh-Fong, L.; Yu-Ming, Z.; Shuai-Wen, Z.; Chen, J.P. Characterization of Copper Adsorption onto an Alginate Encapsulated Magnetic Sorbent by a Combined. Environ. Sci. Technol. 2008, 42, 2551–2556. [Google Scholar]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Yuan-Pang, S.; Xiao-Qin, L.; Wei-Xian, Z.; Paul, W.H. A method for the preparation of stable dispersion of zero-valent iron nanoparticles. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 308, 60–66. [Google Scholar] [CrossRef]
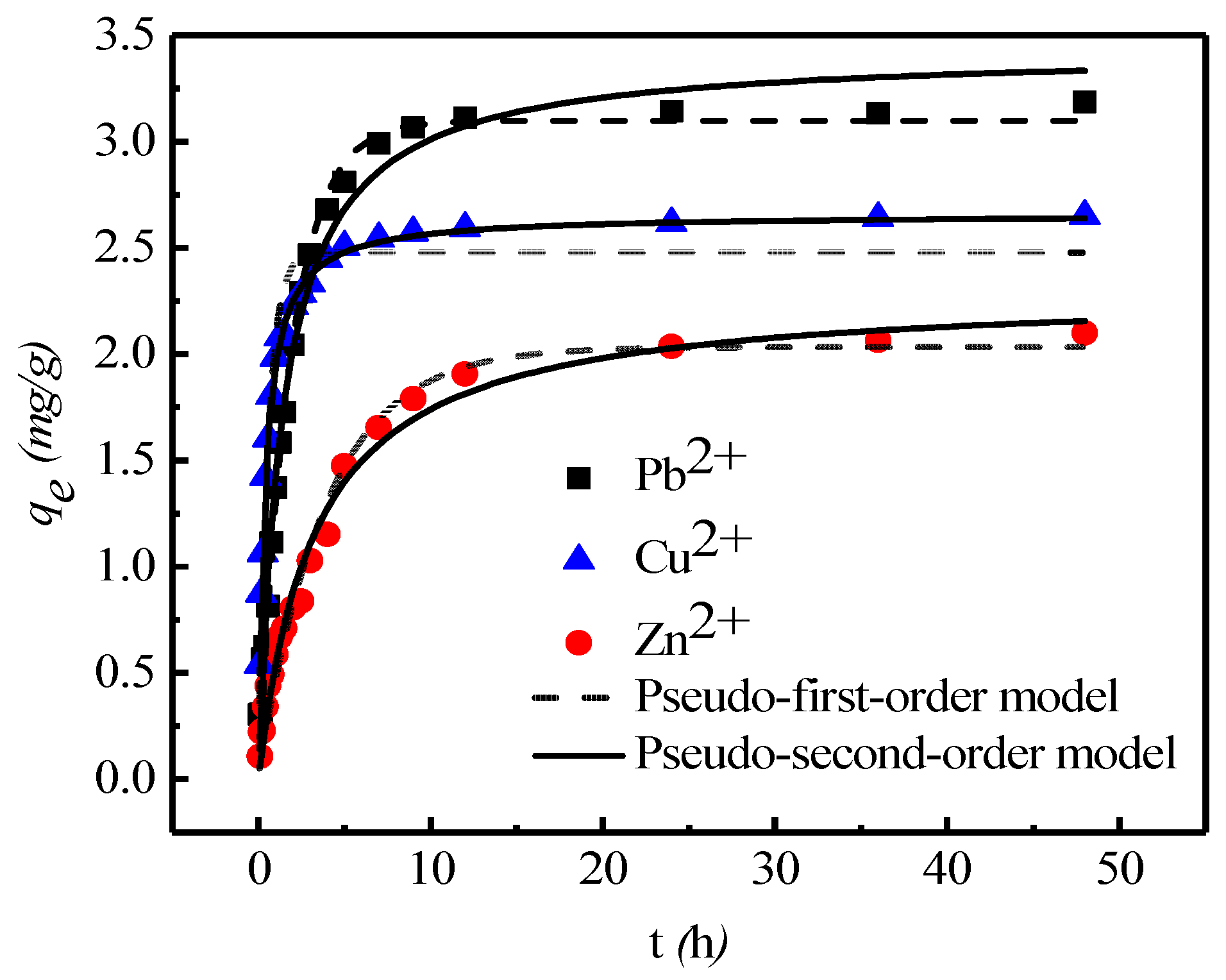
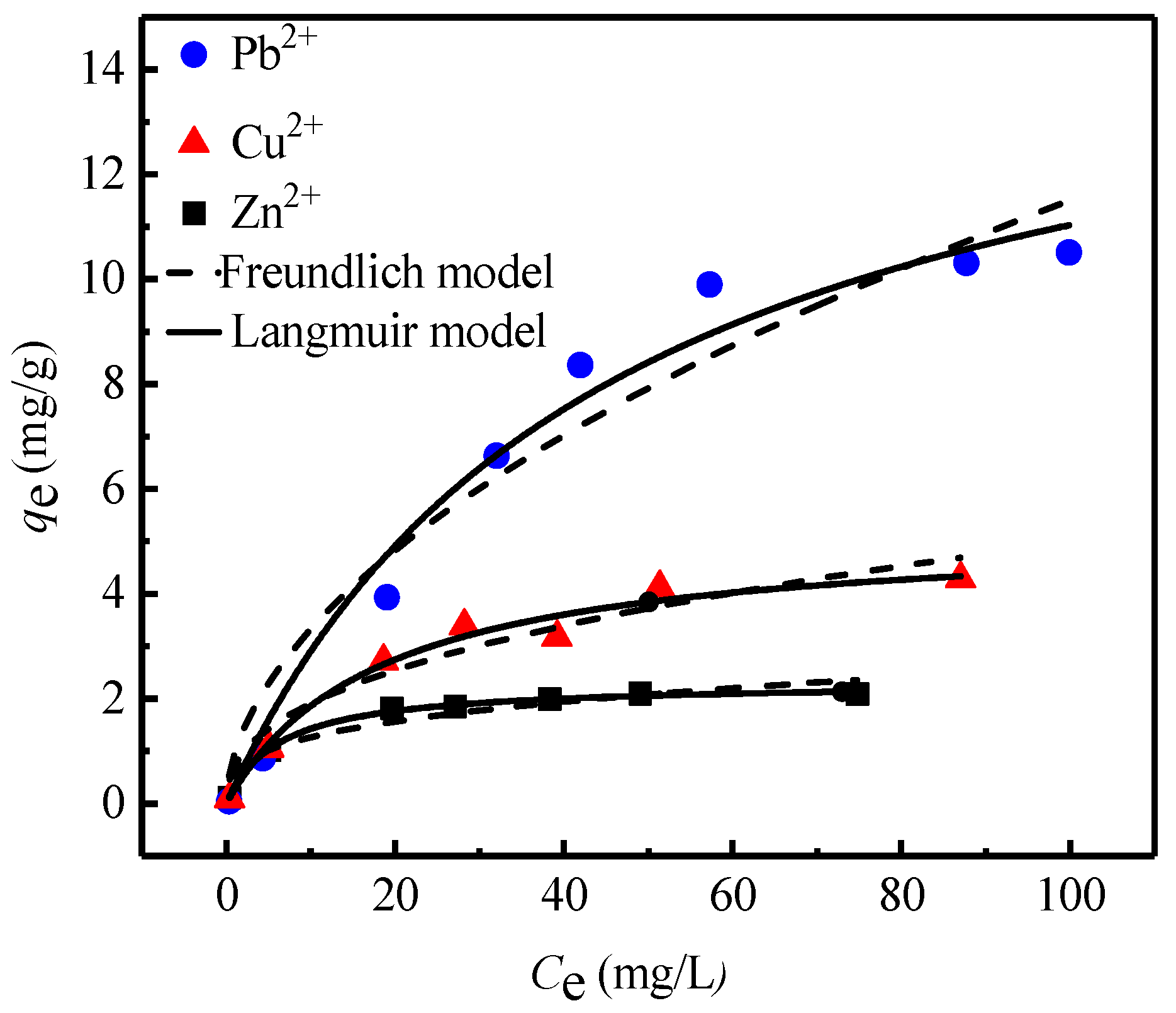
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|
| k1 (L/min) | qe (mg/g) | r2 | k2 (g/mg·min) | qe (mg/g) | r2 | |
| Pb2+ | 0.54 ± 0.01 | 3.10 ± 0.02 | 0.97 | 0.21 ± 0.01 | 3.43 ± 0.06 | 0.98 |
| Cu2+ | 1.83 ± 0.14 | 2.47 ± 0.04 | 0.94 | 1.03 ± 0.02 | 2.66 ± 0.01 | 0.99 |
| Zn2+ | 0.25 ± 0.01 | 2.03 ± 0.05 | 0.99 | 0.01 ± 0.01 | 2.30 ± 0.05 | 0.98 |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| b1 (L/min) | qm (mg·g−) | r2 | Kf (mg1−1/n·L1/n·g−1) | n (mg·L−1) | r2 | |
| Pb2+ | 0.02 ± 0.01 | 16.01 ± 1.84 | 0.98 | 0.72 ± 0.21 | 2.39 ± 0.43 | 0.93 |
| Cu2+ | 0.05 ± 0.01 | 5.23 ± 0.40 | 0.98 | 0.61 ± 0.15 | 3.23 ± 0.70 | 0.89 |
| Zn2+ | 0.16 ± 0.01 | 2.31 ± 0.04 | 0.99 | 0.96 ± 0.36 | 1.85 ± 0.31 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yan, L.; Liu, J.; Zhang, Z.; Tan, C. Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility. Appl. Sci. 2019, 9, 4213. https://doi.org/10.3390/app9204213
Zhang X, Yan L, Liu J, Zhang Z, Tan C. Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility. Applied Sciences. 2019; 9(20):4213. https://doi.org/10.3390/app9204213
Chicago/Turabian StyleZhang, Xiaoran, Lei Yan, Junfeng Liu, Ziyang Zhang, and Chaohong Tan. 2019. "Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility" Applied Sciences 9, no. 20: 4213. https://doi.org/10.3390/app9204213
APA StyleZhang, X., Yan, L., Liu, J., Zhang, Z., & Tan, C. (2019). Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility. Applied Sciences, 9(20), 4213. https://doi.org/10.3390/app9204213