Study on Lightweight and Strengthening Effect of Carbon Nanotube in Highly Ordered Nanoporous Nickel: A Molecular Dynamics Study
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
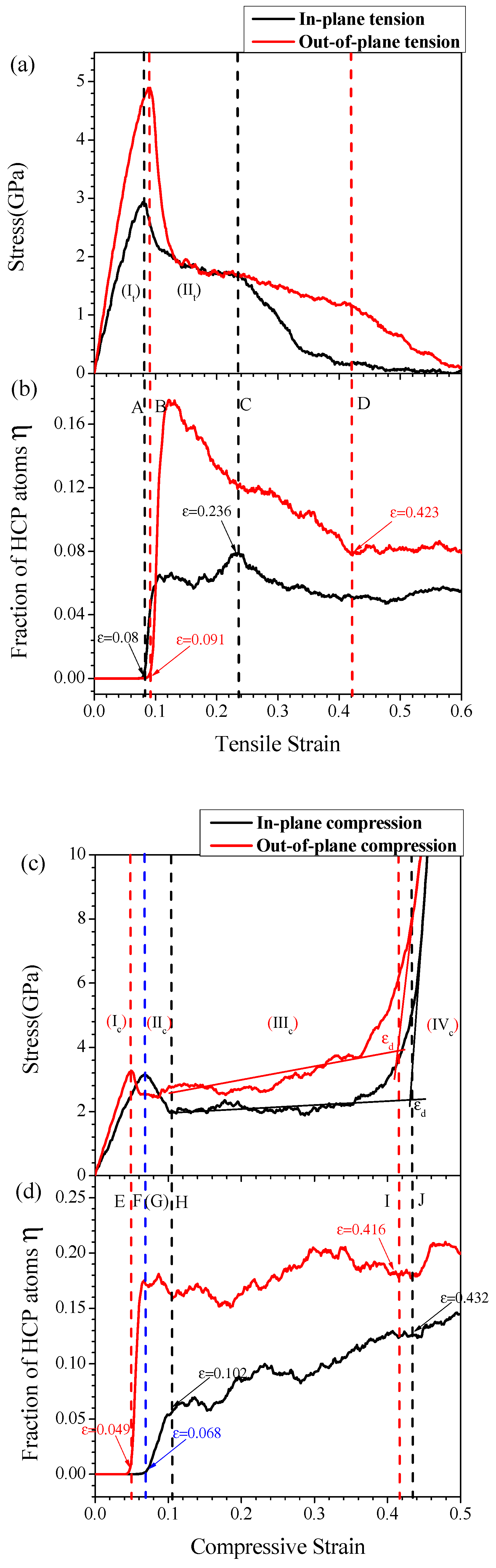
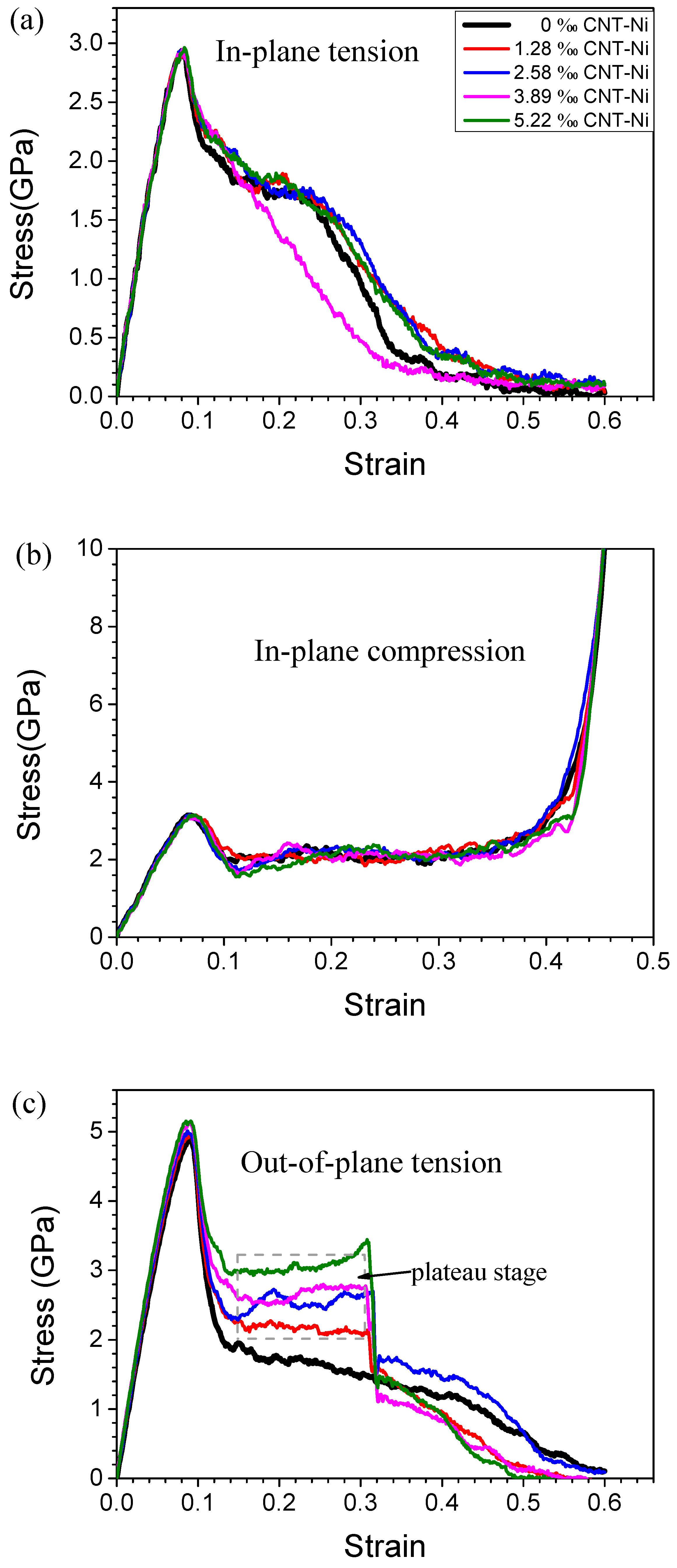
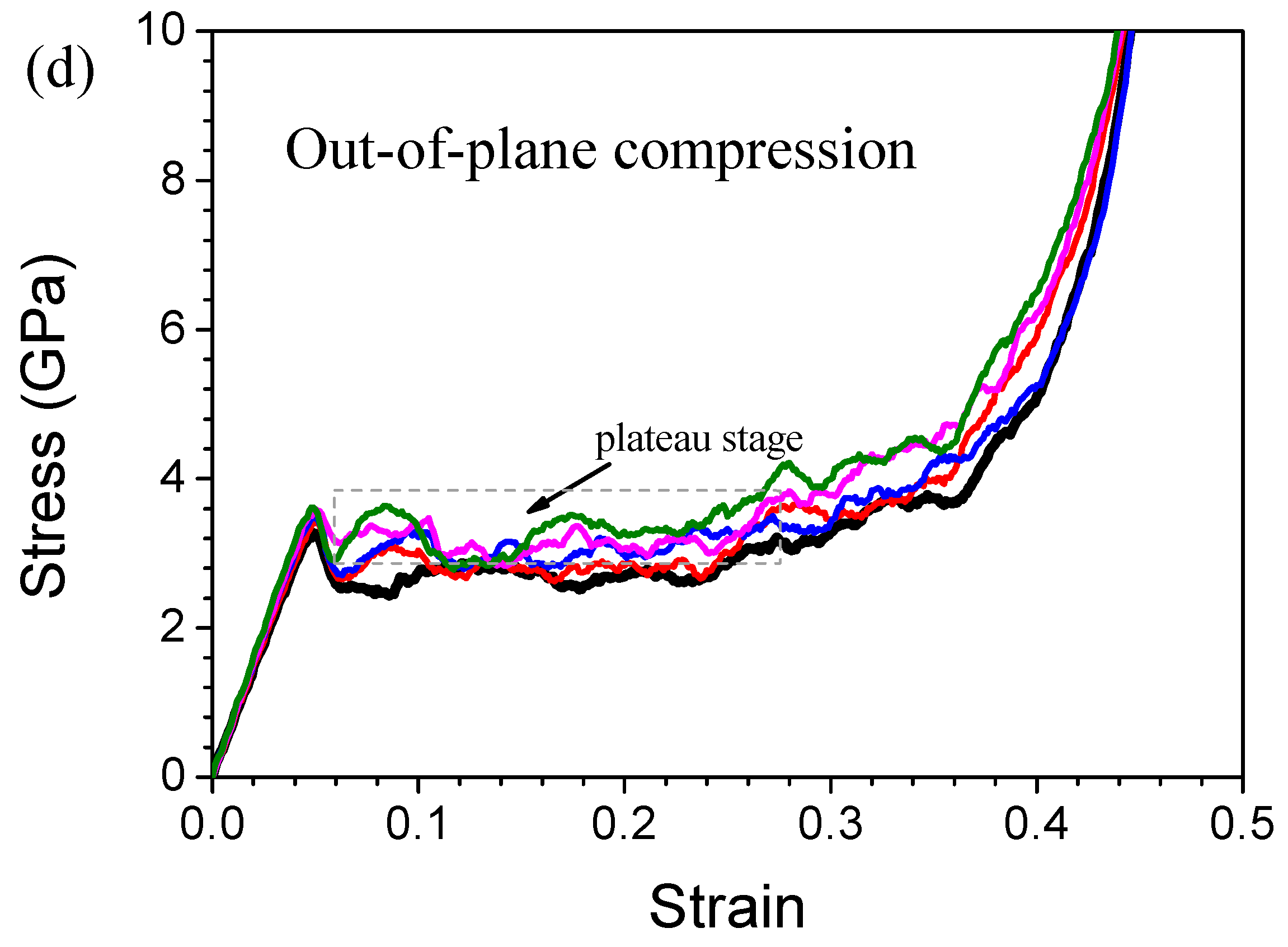
3.1. Stress-Strain Curves and Lattice Structure Evolution of HONNs
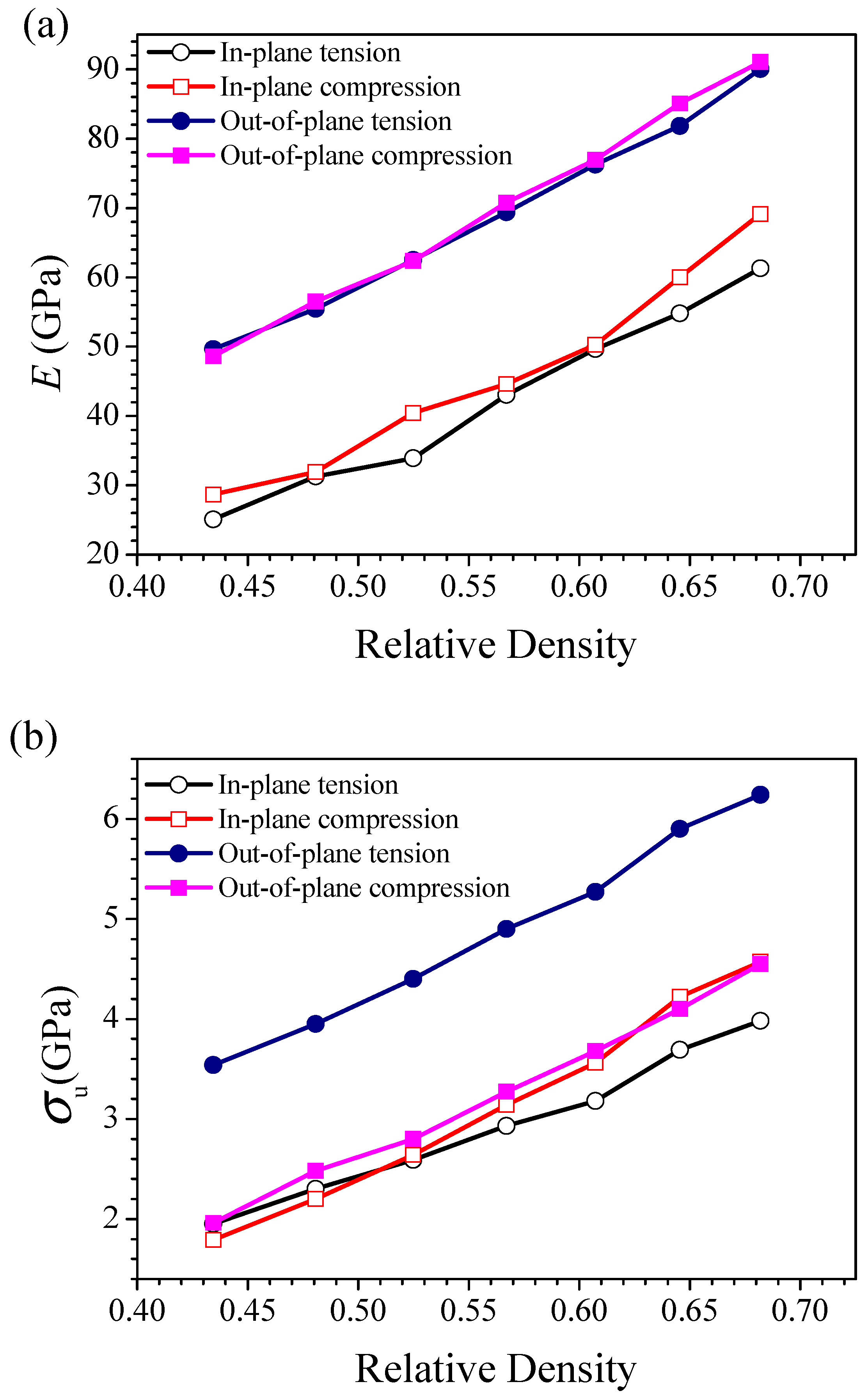
3.2. Mechanical Properties of HONNs
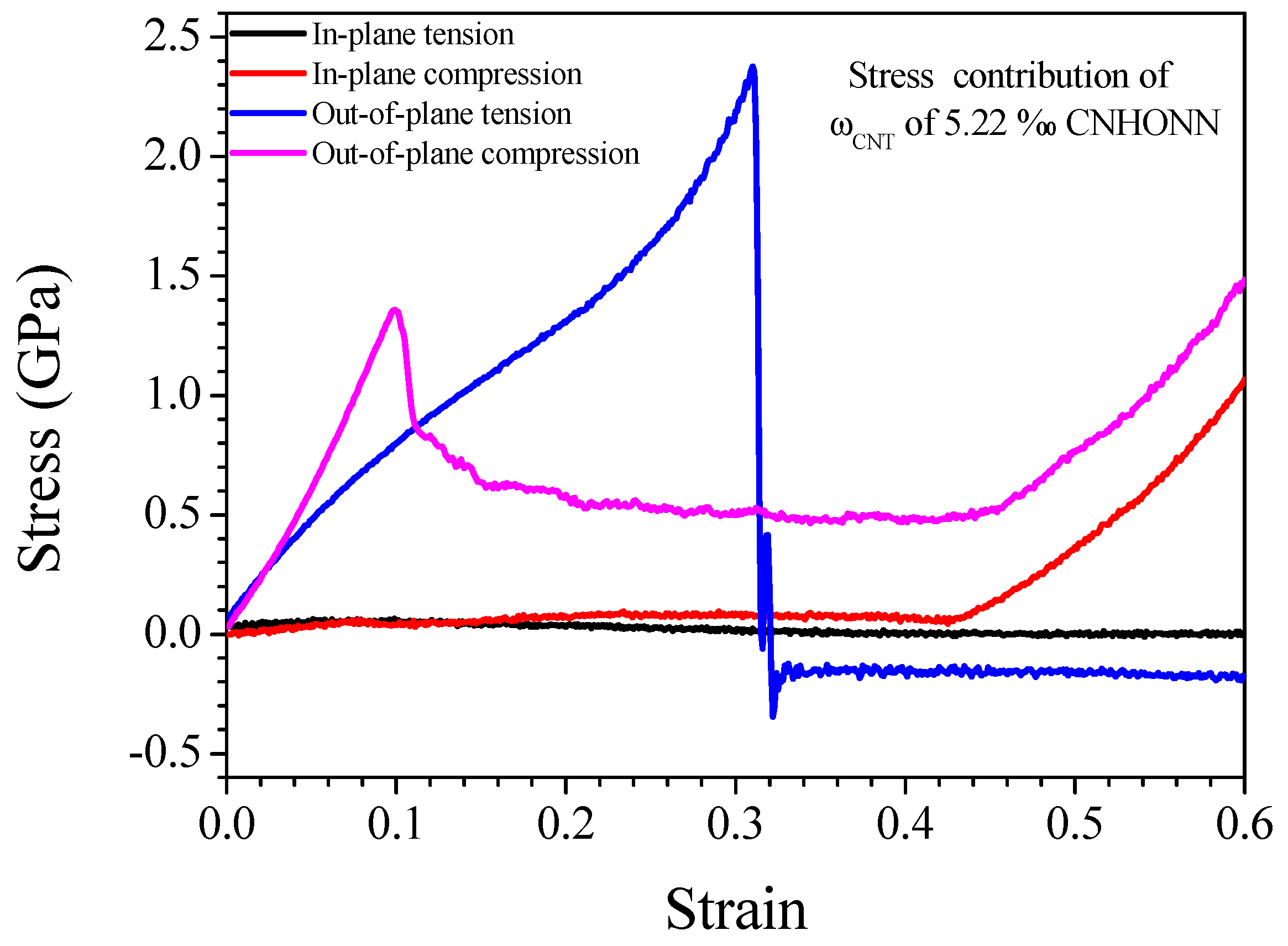
3.3. Effect of CNTs on Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2003. [Google Scholar]
- Ivers-Tiffee, E.; Weber, A.; Herbstritt, D. Materials and technologies for sofc-components. J. Eur. Ceram. Soc. 2001, 21, 1805–1811. [Google Scholar] [CrossRef]
- Radovic, M.; Lara-Curzio, E. Mechanical properties of tape cast nickel-based anode materials for solid oxide fuel cells before and after reduction in hydrogen. Acta Mater. 2004, 52, 5747–5756. [Google Scholar] [CrossRef]
- Martinez, A.; Gerdes, K.; Gemmen, R.; Poston, J. Thermodynamic analysis of interactions between ni-based solid oxide fuel cells (sofc) anodes and trace species in a survey of coal syngas. J. Power Sources 2010, 195, 5206–5212. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mukhopadhyay, J.; Sharma, A.D.; Basu, R.N. Effect of anode configuration on electrical properties and cell polarization in planar anode supported sofc. Solid State Ion. 2013, 233, 20–31. [Google Scholar] [CrossRef]
- Nelson, P.A.; Elliott, J.M.; Attard, G.S.; Owen, J.R. Mesoporous nickel/nickel oxide-a nanoarchitectured electrode. Chem. Mater. 2002, 14, 524–529. [Google Scholar] [CrossRef]
- Nelson, P.A.; Owen, J.R. A high-performance supercapacitor/battery hybrid incorporating templated mesoporous electrodes. J. Electrochem. Soc. 2003, 150, A1313. [Google Scholar] [CrossRef]
- Xu, M.; Li, T.; Yang, M.; Andersson, M. Solid oxide fuel cell interconnect design optimization considering the thermal stresses. Sci. Bull. 2016, 61, 1333–1344. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Treacy, M.J.; Ebbesen, T.; Gibson, J. Exceptionally high young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678. [Google Scholar] [CrossRef]
- Ebbesen, T.; Lezec, H.; Hiura, H.; Bennett, J.; Ghaemi, H.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, C.M.; Bao, S.J.; Bao, Q.L. Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J. Power Sources 2007, 170, 79–84. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J.; Zhou, W.; Zhu, J.; Huang, X.; Qi, X.; Zhang, H.; Yu, T. Cnt/ni hybrid nanostructured arrays: Synthesis and application as high-performance electrode materials for pseudocapacitors. Energy Environ. Sci. 2011, 4, 5000–5007. [Google Scholar] [CrossRef]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.; Criddle, C.S.; Cui, Y. Three-dimensional carbon nanotube-textile anode for high-performance microbial fuel cells. Nano Lett. 2011, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A.; Laurent, C.; Flahaut, E.; Rousset, A. Carbon nanotubes in novel ceramic matrix nanocomposites. Ceram. Int. 2000, 26, 677–683. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef]
- Curtin, W.A.; Sheldon, B.W. Cnt-reinforced ceramics and metals. Mater. Today 2004, 7, 44–49. [Google Scholar] [CrossRef]
- Frandsen, H.L.; Ramos, T.; Faes, A.; Pihlatie, M.; Brodersen, K. Optimization of the strength of sofc anode supports. J. Eur. Ceram. Soc. 2012, 32, 1041–1052. [Google Scholar] [CrossRef]
- Zeng, S.; Xu, M.; Parbey, J.; Yu, G.; Andersson, M.; Li, Q.; Li, B.; Li, T. Thermal stress analysis of a planar anode-supported solid oxide fuel cell: Effects of anode porosity. Int. J. Hydrogen Energy 2017, 42, 20239–20248. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Liu, M.; Chen, Q. Mechanical strength of carbon nanotube–nickel nanocomposites. Nanotechnology 2007, 18, 505704. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Neira, A.; Scharf, T.W.; Tiley, J.; Banerjee, R. Laser-deposited carbon nanotube reinforced nickel matrix composites. Scr. Mater. 2008, 59, 487–490. [Google Scholar] [CrossRef]
- Dai, P.-Q.; Xu, W.-C.; Huang, Q.-Y. Mechanical properties and microstructure of nanocrystalline nickel-carbon nanotube composites produced by electrodeposition. Mater. Sci. Eng. A 2008, 483–484, 172–174. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lim, B.K.; Tiley, J.; Banerjee, R.; Hong, S.H. Interface analysis of ultra-high strength carbon nanotube/nickel composites processed by molecular level mixing. Carbon 2013, 57, 282–287. [Google Scholar] [CrossRef]
- Borkar, T.; Hwang, J.; Hwang, J.Y.; Scharf, T.W.; Tiley, J.; Hong, S.H.; Banerjee, R. Strength versus ductility in carbon nanotube reinforced nickel matrix nanocomposites. J. Mater. Res. 2014, 29, 761–769. [Google Scholar] [CrossRef]
- Song, Q.S.; Aravindaraj, G.K.; Sultana, H.; Chan, S.L.I. Performance improvement of pasted nickel electrodes with multi-wall carbon nanotubes for rechargeable nickel batteries. Electrochim. Acta 2007, 53, 1890–1896. [Google Scholar] [CrossRef]
- Liu, X.; Gurel, V.; Morris, D.; Murray, D.W.; Zhitkovich, A.; Kane, A.B.; Hurt, R.H. Bioavailability of nickel in single-wall carbon nanotubes. Adv. Mater. 2007, 19, 2790–2796. [Google Scholar] [CrossRef]
- Chen, Y.S.; Huang, J.H. Arrayed cnt-ni nanocomposites grown directly on si substrate for amperometric detection of ethanol. Biosens. Bioelectron. 2010, 26, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.; Kim, S.H.; Lee, C.W.; Kim, H.; Choi, S.K.; Kim, S.H.; Kim, E.; Park, J.; Kim, H. Synthesis of carbon nanotube-nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens. Bioelectron. 2015, 63, 325–330. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, W.G.; Feng, X.Q.; Li, D.S.; Qin, Q.H.; Liu, X.B. In-plane compressive behavior of graphene-coated aluminum nano-honeycombs. Comput. Mater. Sci. 2019, 156, 396–403. [Google Scholar] [CrossRef]
- Qin, Z.; Qin, Q.H.; Feng, X.Q. Mechanical property of carbon nanotubes with intramolecular junctions: Molecular dynamics simulations. Phys. Lett. A 2008, 372, 6661–6666. [Google Scholar] [CrossRef]
- Esfarjani, K.; Gorjizadeh, N.; Nasrollahi, Z. Molecular dynamics of single wall carbon nanotube growth on nickel surface. Comput. Mater. Sci. 2006, 36, 117–120. [Google Scholar] [CrossRef]
- Shibuta, Y.; Maruyama, S. A molecular dynamics study of the effect of a substrate on catalytic metal clusters in nucleation process of single-walled carbon nanotubes. Chem. Phys. Lett. 2007, 437, 218–223. [Google Scholar] [CrossRef]
- Oguri, T.; Shimamura, K.; Shibuta, Y.; Shimojo, F.; Yamaguchi, S. Ab initio molecular dynamics simulation of the dissociation of ethanol on a nickel cluster: Understanding the initial stage of metal-catalyzed growth of carbon nanotubes. J. Phys. Chem. C 2013, 117, 9983–9990. [Google Scholar] [CrossRef]
- Fukuhara, S.; Shimojo, F.; Shibuta, Y. Conformation and catalytic activity of nickel—Carbon cluster for ethanol dissociation in carbon nanotube synthesis: Ab initio molecular dynamics simulation. Chem. Phys. Lett. 2017, 679, 164–171. [Google Scholar] [CrossRef]
- Song, H.Y.; Zha, X.W. Influence of nickel coating on the interfacial bonding characteristics of carbon nanotube–aluminum composites. Comput. Mater. Sci. 2010, 49, 899–903. [Google Scholar] [CrossRef]
- Song, H.Y.; Zha, X.W. Mechanical properties of nickel-coated single-walled carbon nanotubes and their embedded gold matrix composites. Phys. Lett. A 2010, 374, 1068–1072. [Google Scholar] [CrossRef]
- Zhou, X.; Song, S.; Li, L.; Zhang, R. Molecular dynamics simulation for mechanical properties of magnesium matrix composites reinforced with nickel-coated single-walled carbon nanotubes. J. Compos. Mater. 2015, 50, 191–200. [Google Scholar] [CrossRef]
- Duan, K.; Li, L.; Hu, Y.; Wang, X. Enhanced interfacial strength of carbon nanotube/copper nanocomposites via ni-coating: Molecular-dynamics insights. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 88, 259–264. [Google Scholar] [CrossRef]
- Rezaei, R.; Shariati, M.; Tavakoli-Anbaran, H.; Deng, C. Mechanical characteristics of cnt-reinforced metallic glass nanocomposites by molecular dynamics simulations. Comput. Mater. Sci. 2016, 119, 19–26. [Google Scholar] [CrossRef]
- Silvestre, N.; Faria, B.; Canongia Lopes, J.N. Compressive behavior of cnt-reinforced aluminum composites using molecular dynamics. Compos. Sci. Technol. 2014, 90, 16–24. [Google Scholar] [CrossRef]
- Choi, B.K.; Yoon, G.H.; Lee, S. Molecular dynamics studies of cnt-reinforced aluminum composites under uniaxial tensile loading. Compos. Part B Eng. 2016, 91, 119–125. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Sansoz, F.; Shen, M. Molecular dynamics simulation on temperature and stain rate-dependent tensile response and failure behavior of ni-coated cnt/mg composites. Appl. Phys. A 2018, 124, 506. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with ovito–the open visualization tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Foiles, S.; Baskes, M.; Daw, M.S. Embedded-atom-method functions for the fcc metals cu, ag, au, ni, pd, pt, and their alloys. Phys. Rev. B 1986, 33, 7983. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Boda, D.; Henderson, D. The effects of deviations from lorentz–berthelot rules on the properties of a simple mixture. Mol. Phys. 2008, 106, 2367–2370. [Google Scholar] [CrossRef]
- Kutana, A.; Giapis, K.P. Transient deformation regime in bending of single-walled carbon nanotubes. Phys. Rev. Lett. 2006, 97, 245501. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Huang, Y.; Jiang, H.; Ravichandran, G.; Gao, H.; Hwang, K.; Liu, B. A cohesive law for carbon nanotube/polymer interfaces based on the van der waals force. J. Mech. Phys. Solids 2006, 54, 2436–2452. [Google Scholar] [CrossRef]
- Yi, L.; Chang, T.; Feng, X.Q.; Zhang, Y.; Wang, J.; Huang, B. Giant energy absorption capacity of graphene-based carbon honeycombs. Carbon 2017, 118, 348–357. [Google Scholar] [CrossRef]
- Faken, D.; Jónsson, H. Systematic analysis of local atomic structure combined with 3d computer graphics. Comput. Mater. Sci. 1994, 2, 279–286. [Google Scholar] [CrossRef]
- Paul, A.; Ramamurty, U. Strain rate sensitivity of a closed-cell aluminum foam. Mater. Sci. Eng. A 2000, 281, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Ashby, M. The out-of-plane properties of honeycombs. Int. J. Mech. Sci. 1992, 34, 475–489. [Google Scholar] [CrossRef]
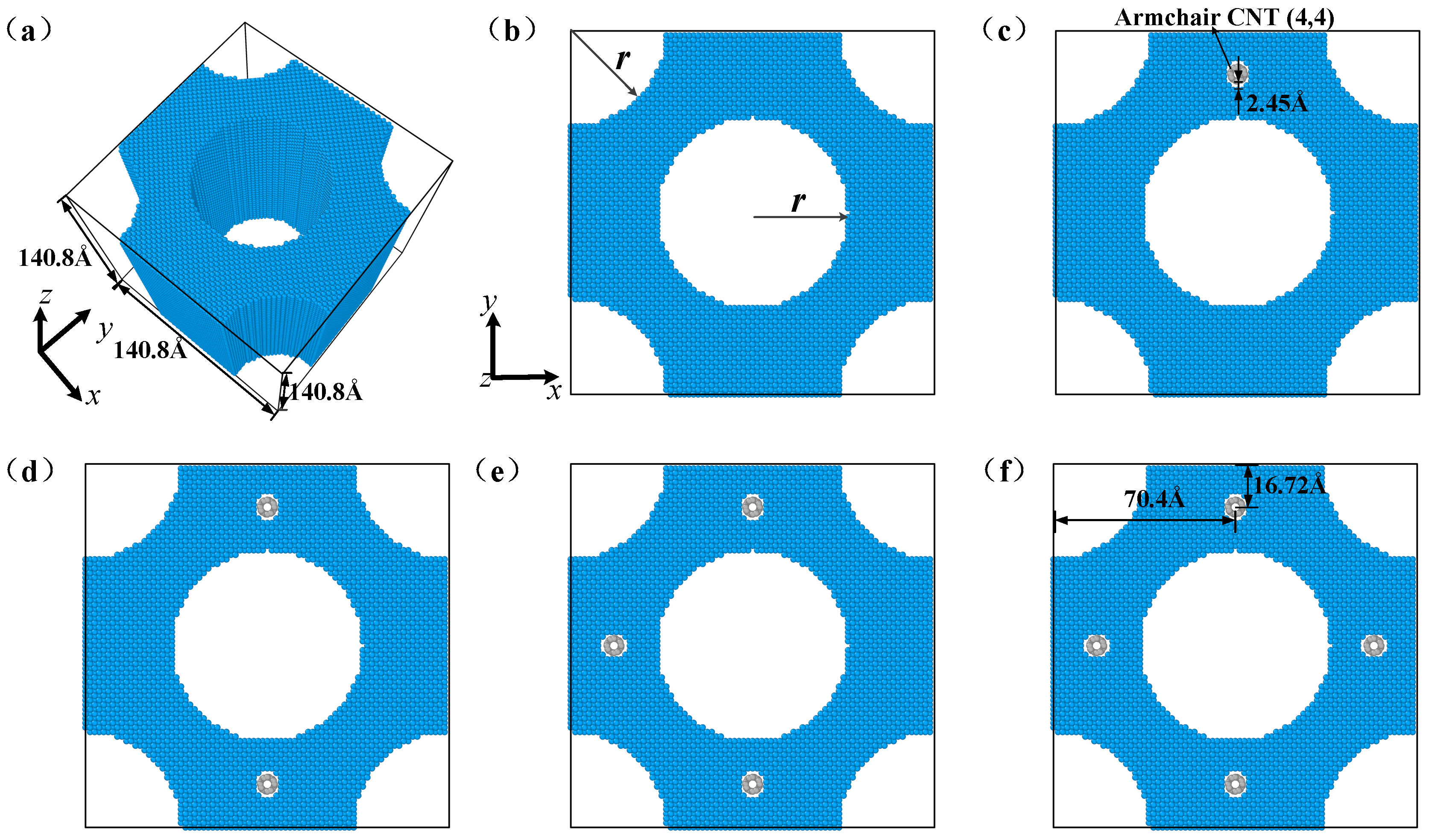


| Group | Radius of the Pore(Å) | Relative Density | Number of Atoms |
|---|---|---|---|
| 1 | 42.24 | 0.43 | 112,720 |
| 2 | 40.48 | 0.48 | 123,920 |
| 3 | 38.72 | 0.52 | 134,800 |
| 4 | 36.96 | 0.57 | 146,320 |
| 5 | 35.2 | 0.61 | 155,680 |
| 6 | 33.44 | 0.65 | 165,840 |
| 7 | 31.68 | 0.68 | 175,440 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Jiang, W.-G.; Li, D.-S.; Qin, Q.-H. Study on Lightweight and Strengthening Effect of Carbon Nanotube in Highly Ordered Nanoporous Nickel: A Molecular Dynamics Study. Appl. Sci. 2019, 9, 352. https://doi.org/10.3390/app9020352
Zhou Y, Jiang W-G, Li D-S, Qin Q-H. Study on Lightweight and Strengthening Effect of Carbon Nanotube in Highly Ordered Nanoporous Nickel: A Molecular Dynamics Study. Applied Sciences. 2019; 9(2):352. https://doi.org/10.3390/app9020352
Chicago/Turabian StyleZhou, Yu, Wu-Gui Jiang, Duo-Sheng Li, and Qing-Hua Qin. 2019. "Study on Lightweight and Strengthening Effect of Carbon Nanotube in Highly Ordered Nanoporous Nickel: A Molecular Dynamics Study" Applied Sciences 9, no. 2: 352. https://doi.org/10.3390/app9020352
APA StyleZhou, Y., Jiang, W.-G., Li, D.-S., & Qin, Q.-H. (2019). Study on Lightweight and Strengthening Effect of Carbon Nanotube in Highly Ordered Nanoporous Nickel: A Molecular Dynamics Study. Applied Sciences, 9(2), 352. https://doi.org/10.3390/app9020352







