Mechanism Research and Countermeasure Analysis of Yellow Plume during the Gas Turbine Start-Up Period
Abstract
:1. Introduction
2. Research Progress
2.1. International Research Progress
2.2. Research Progress in China
3. Study on the Mechanism of Yellow Plume
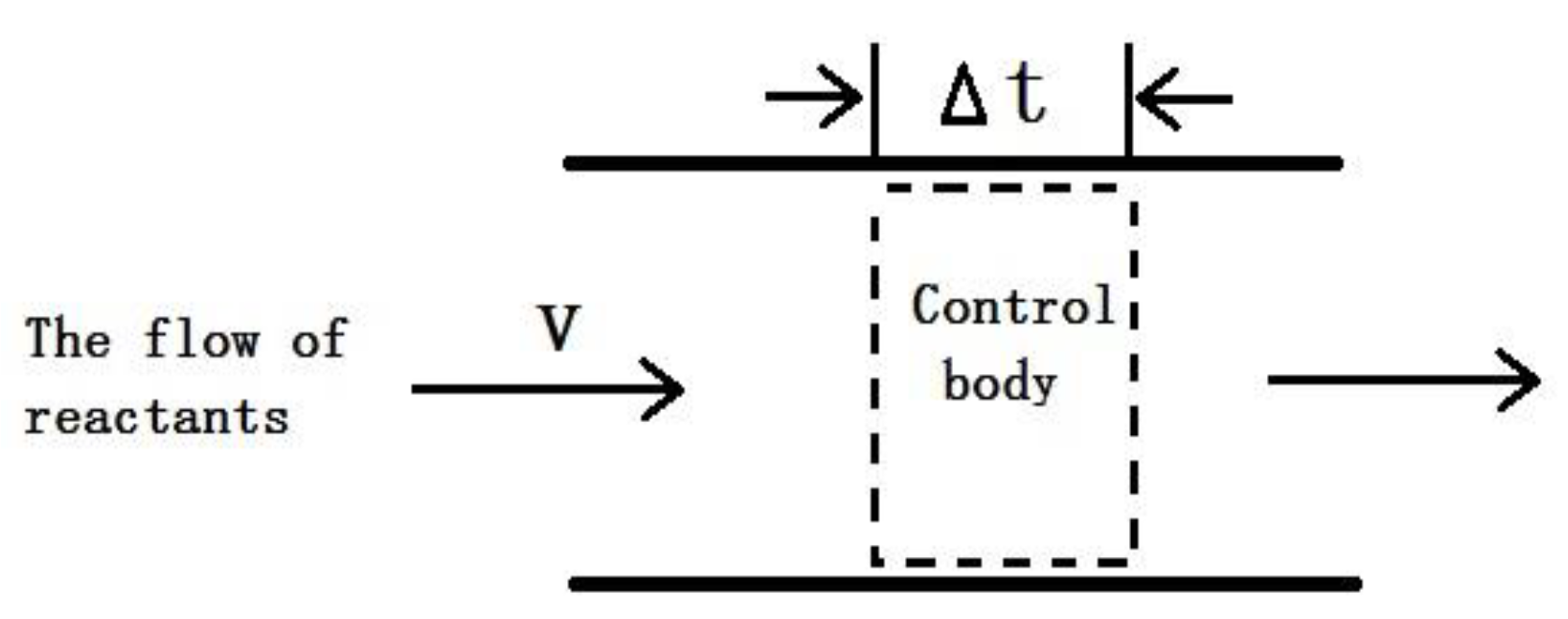
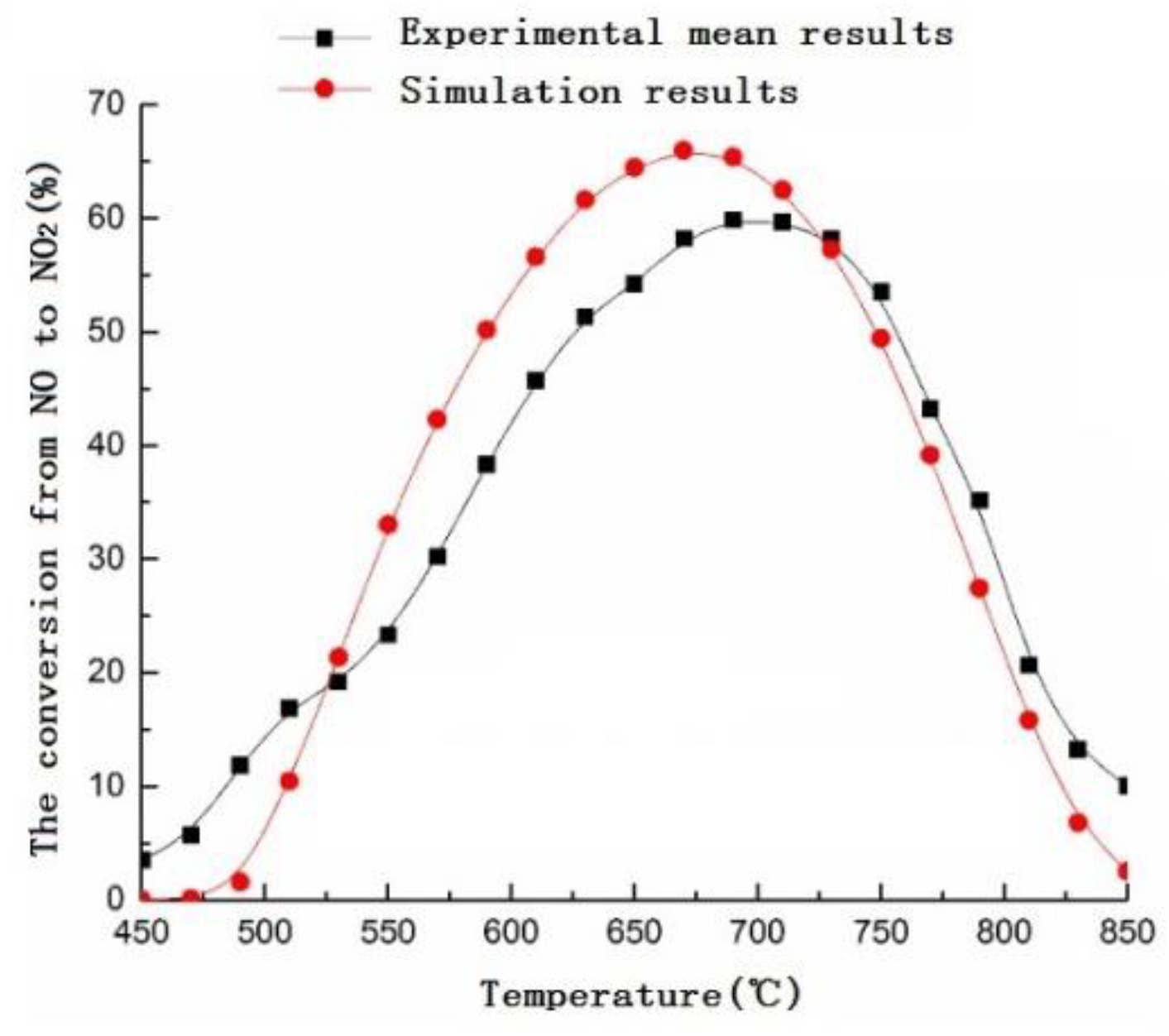
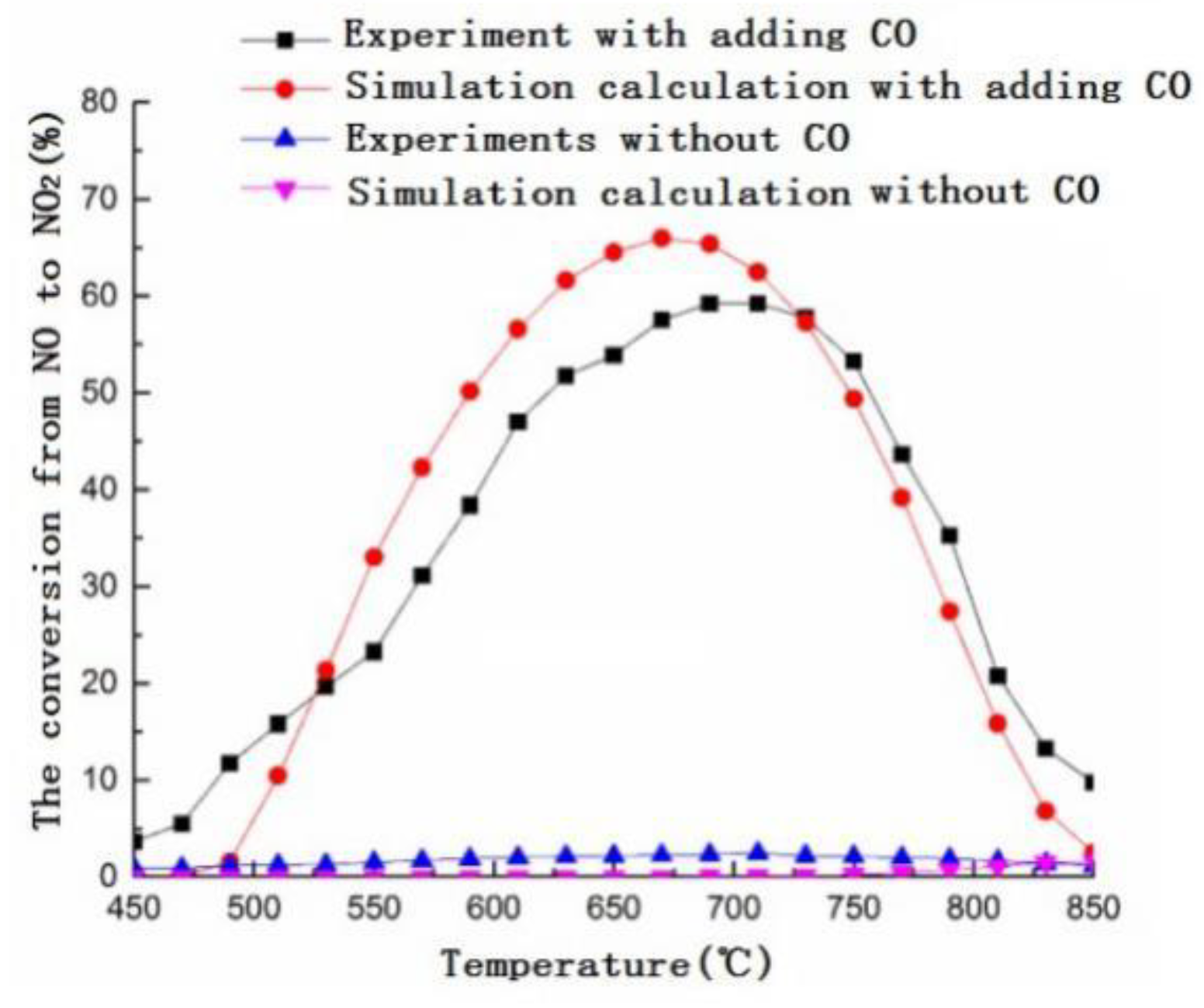
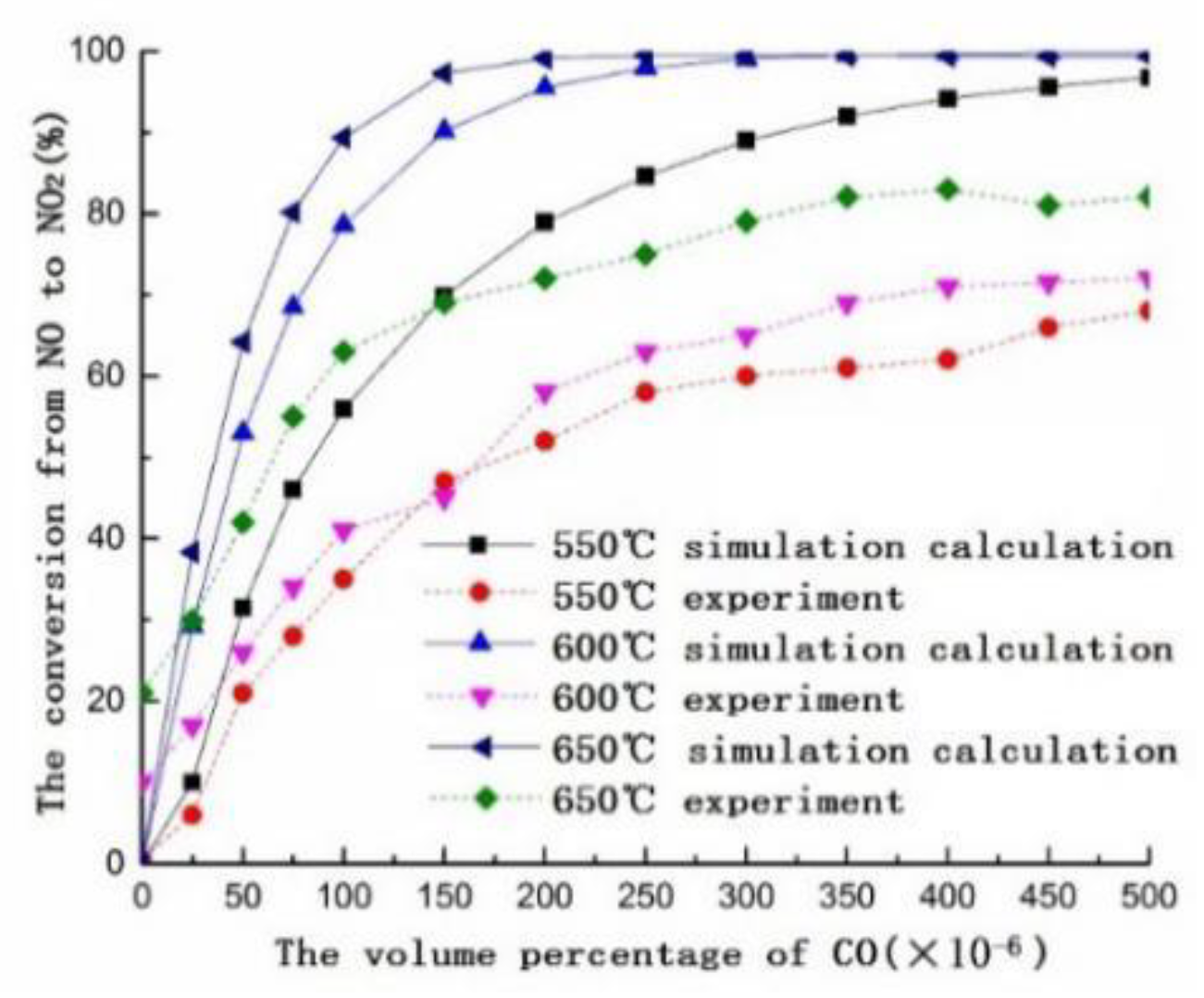
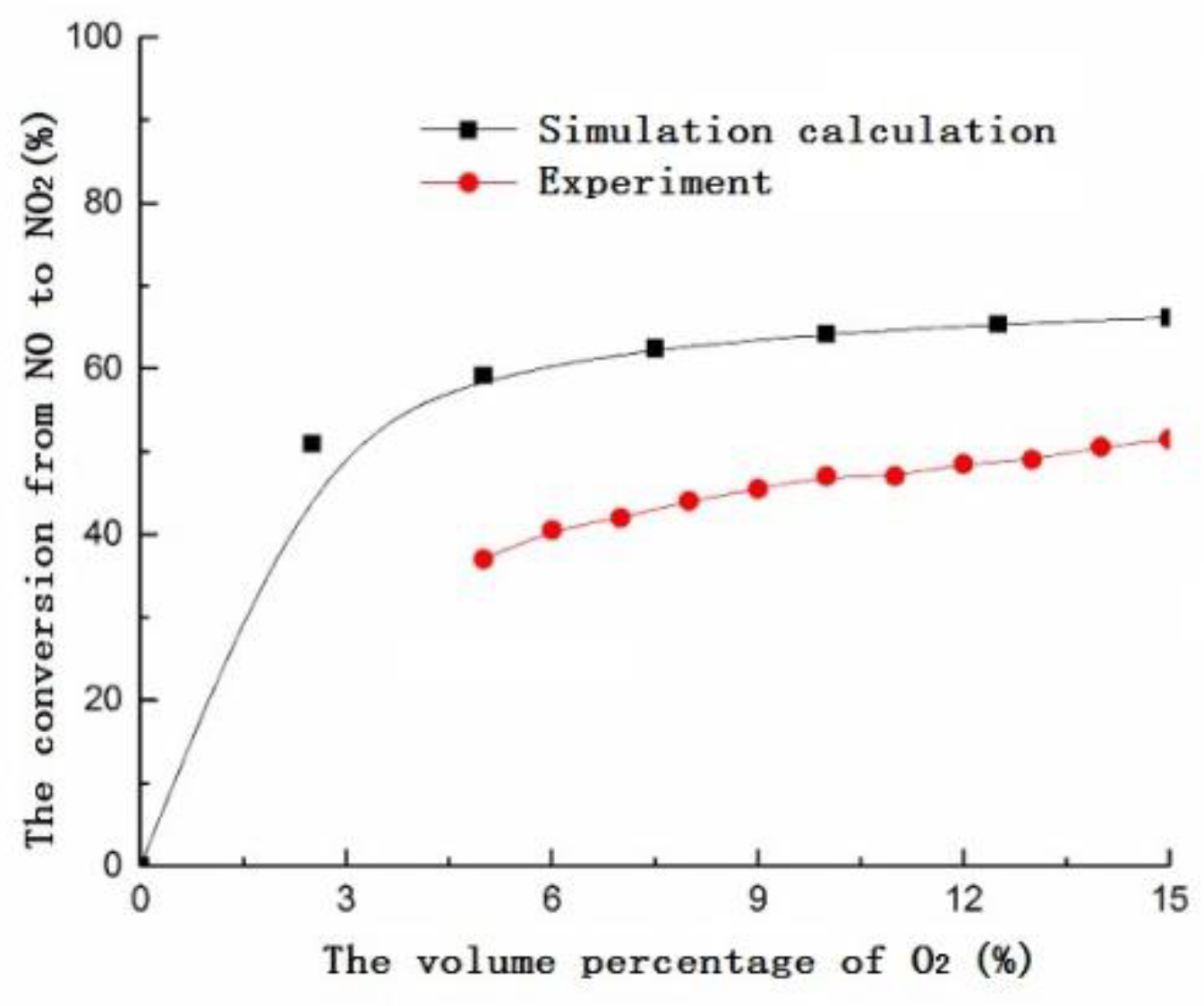
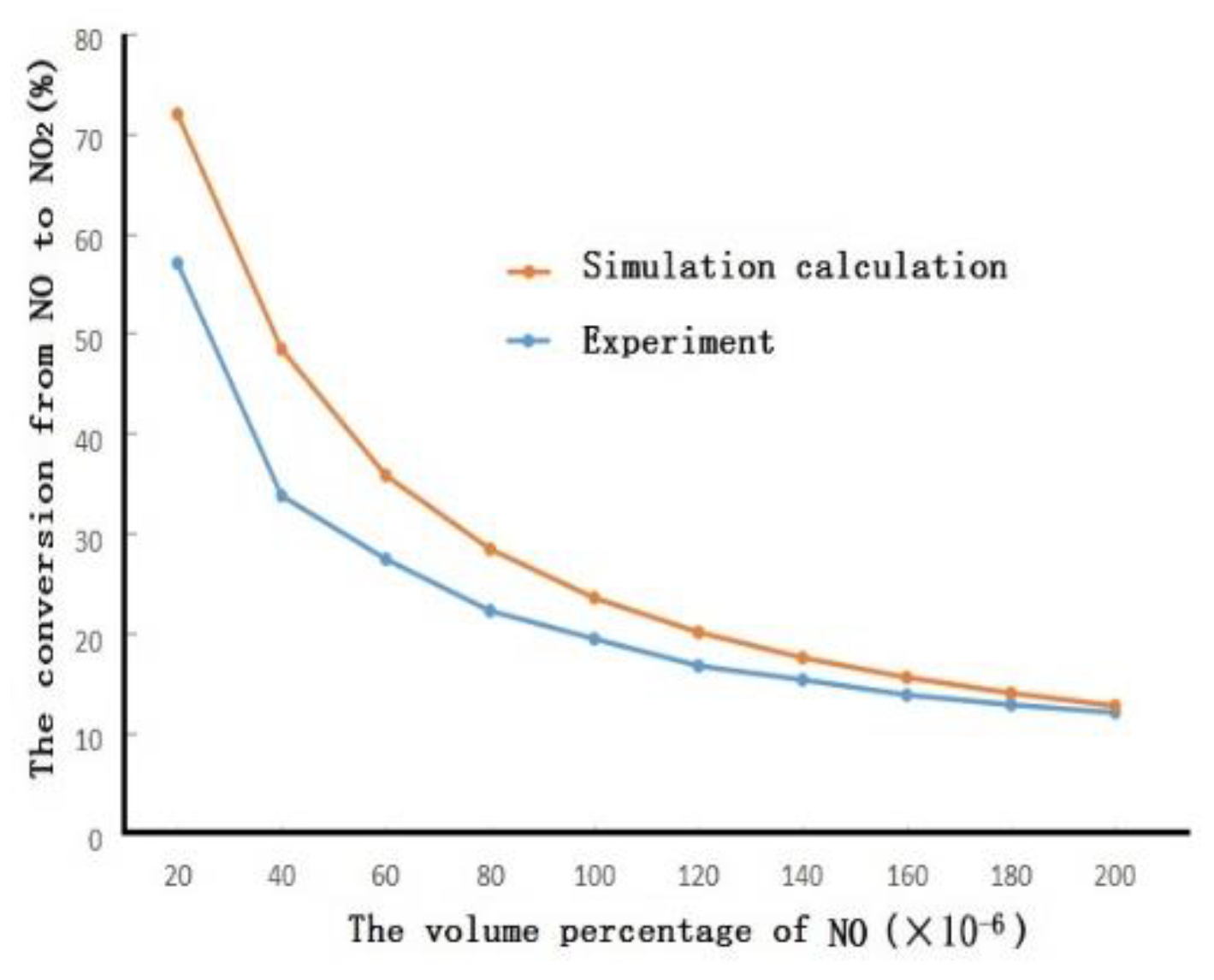
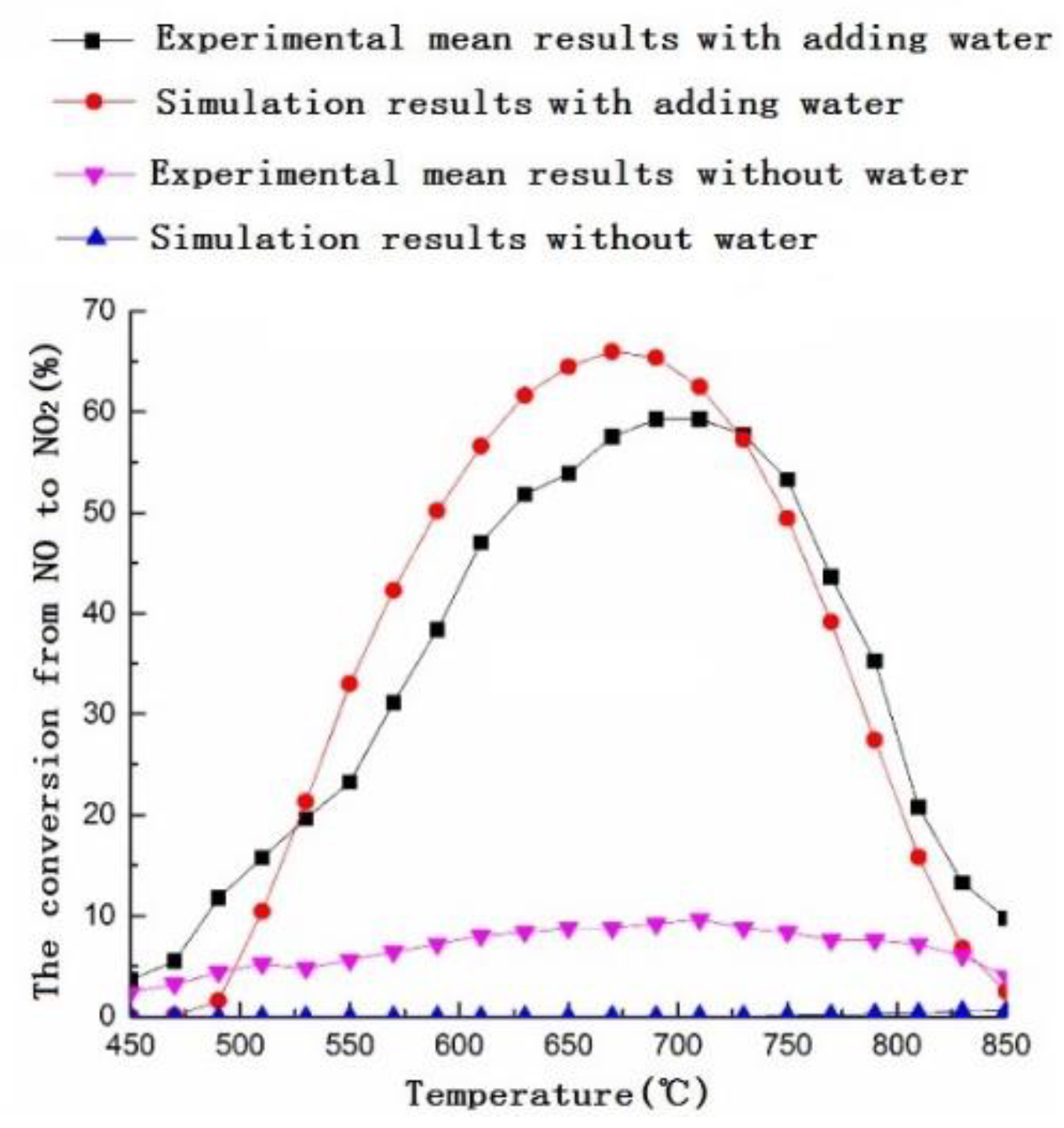
3.1. Numerical Simulation
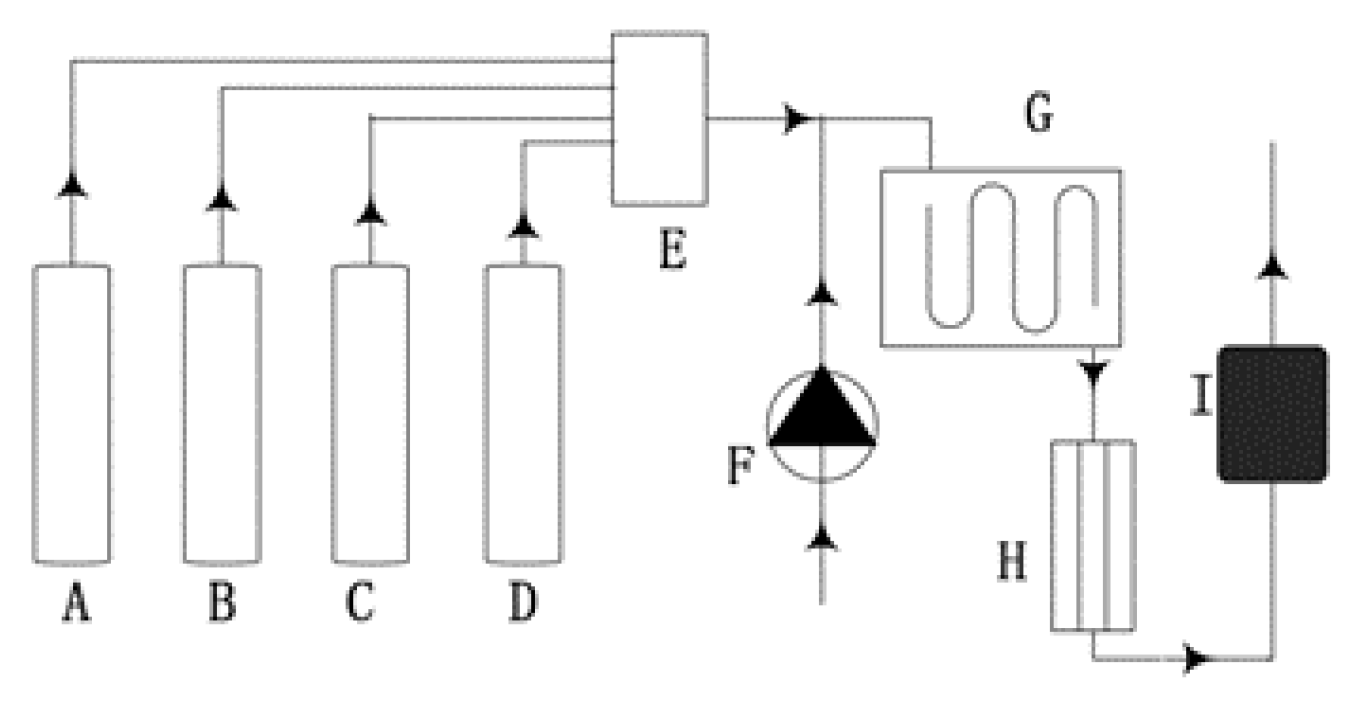
3.2. Experimental Research
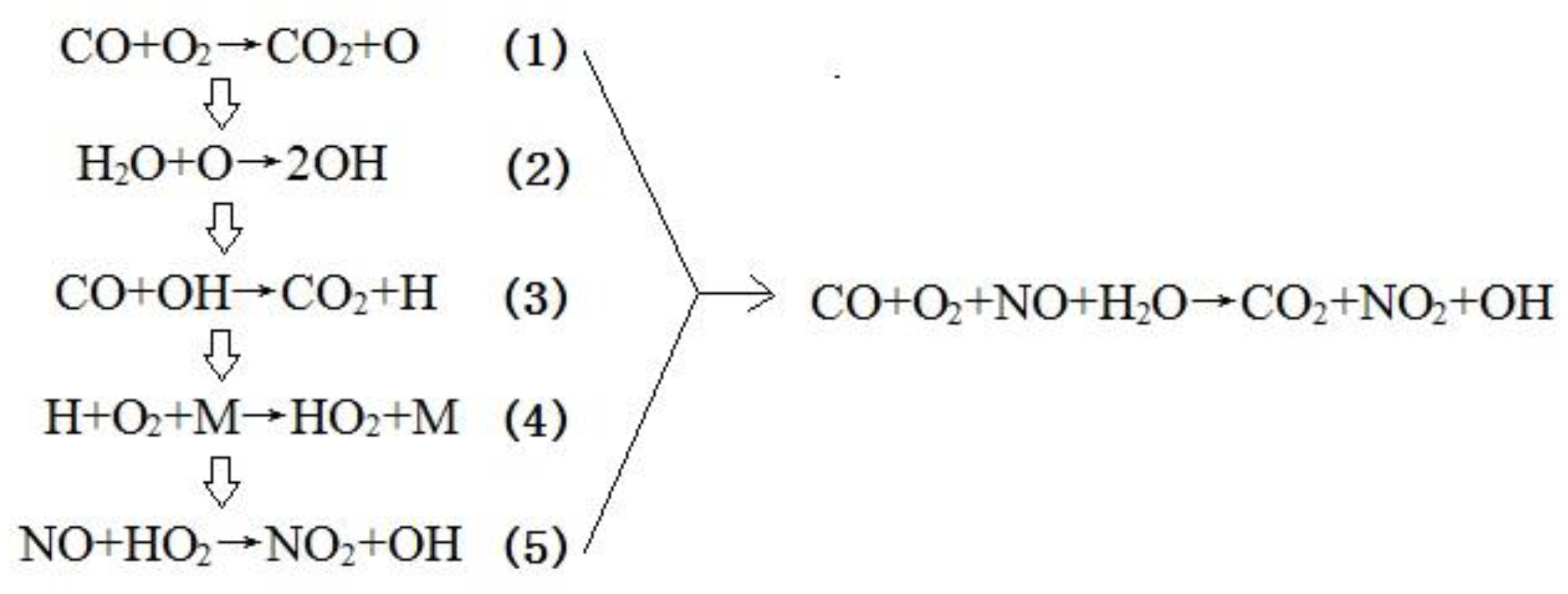
3.3. Mechanism Analysis
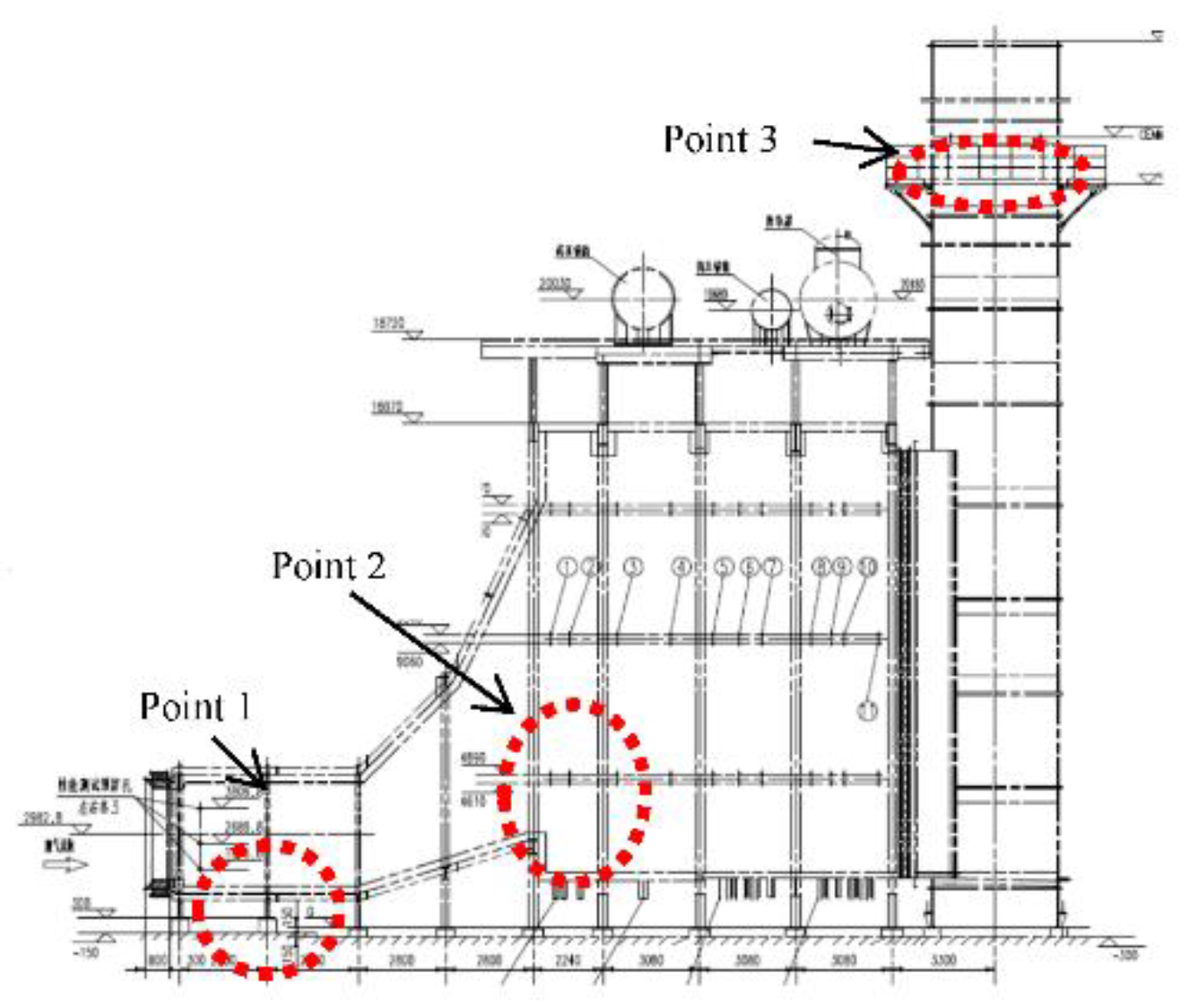
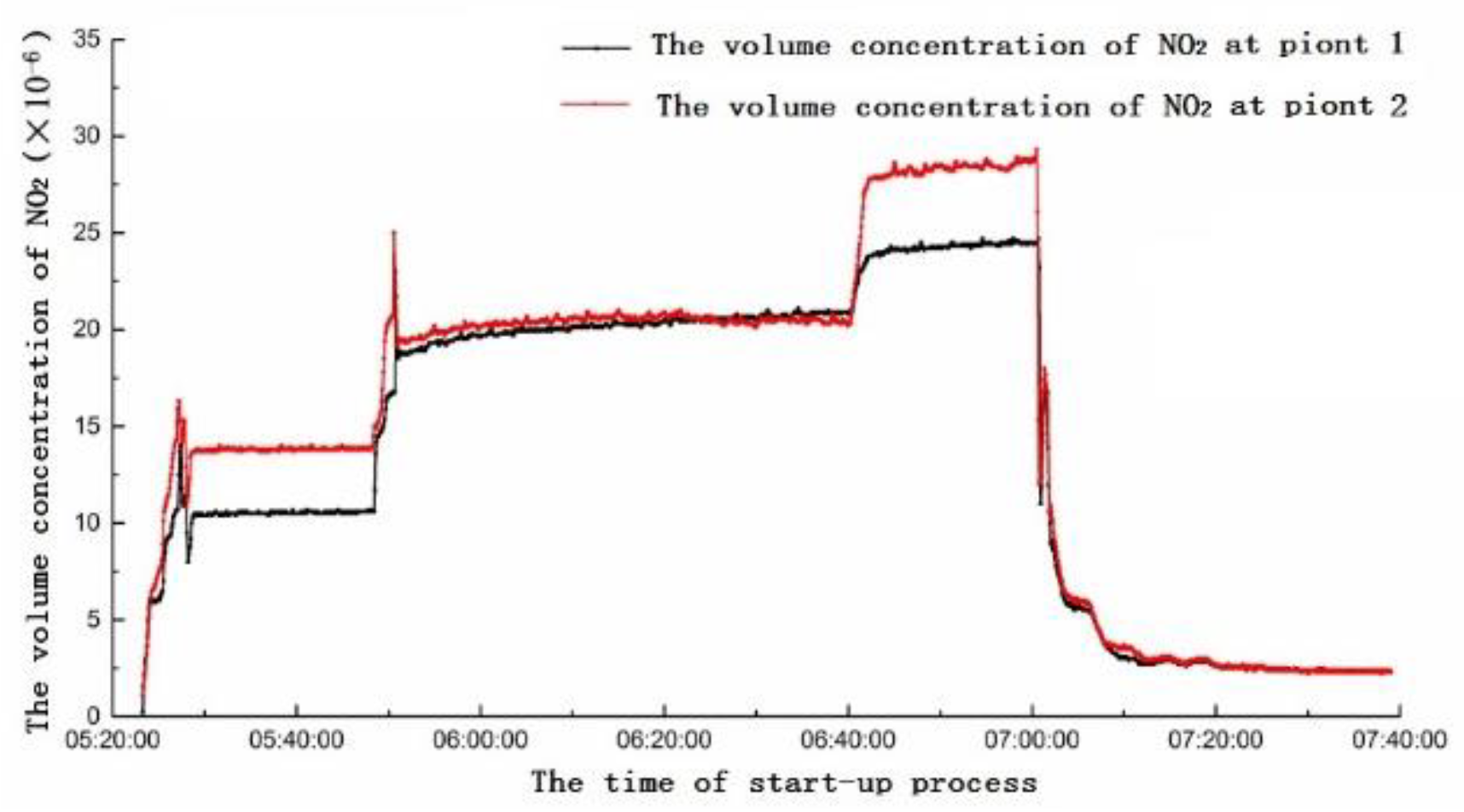
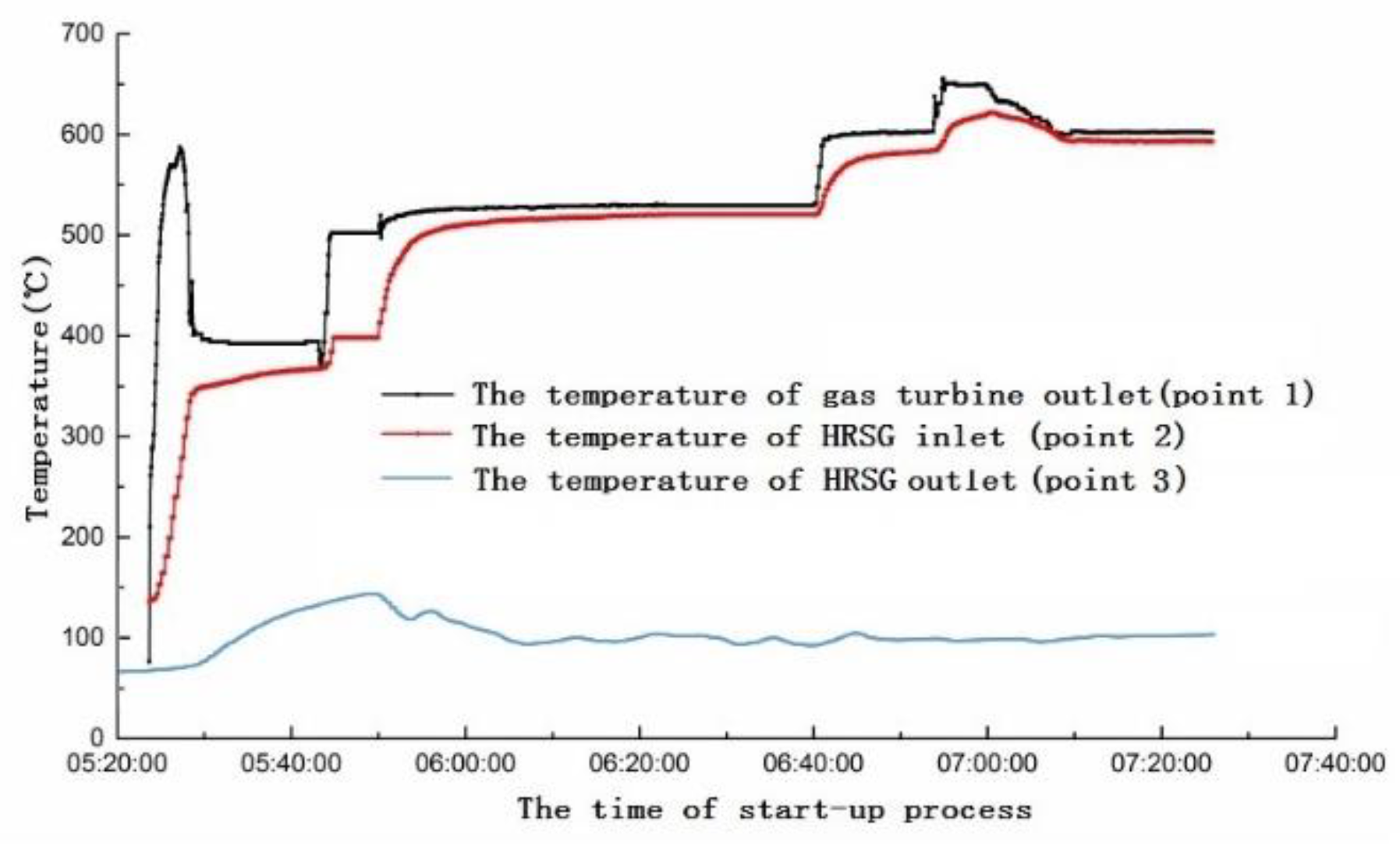
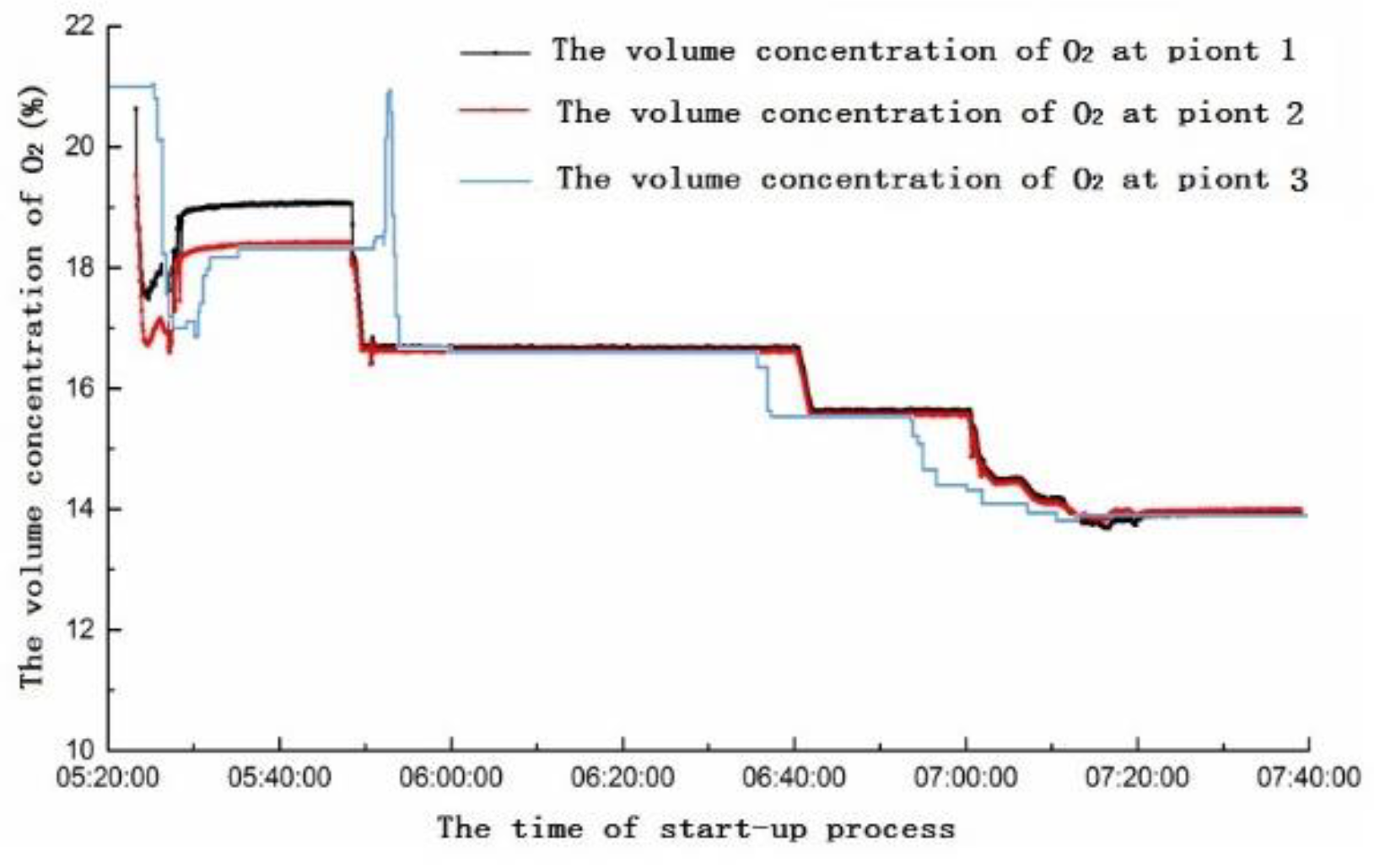
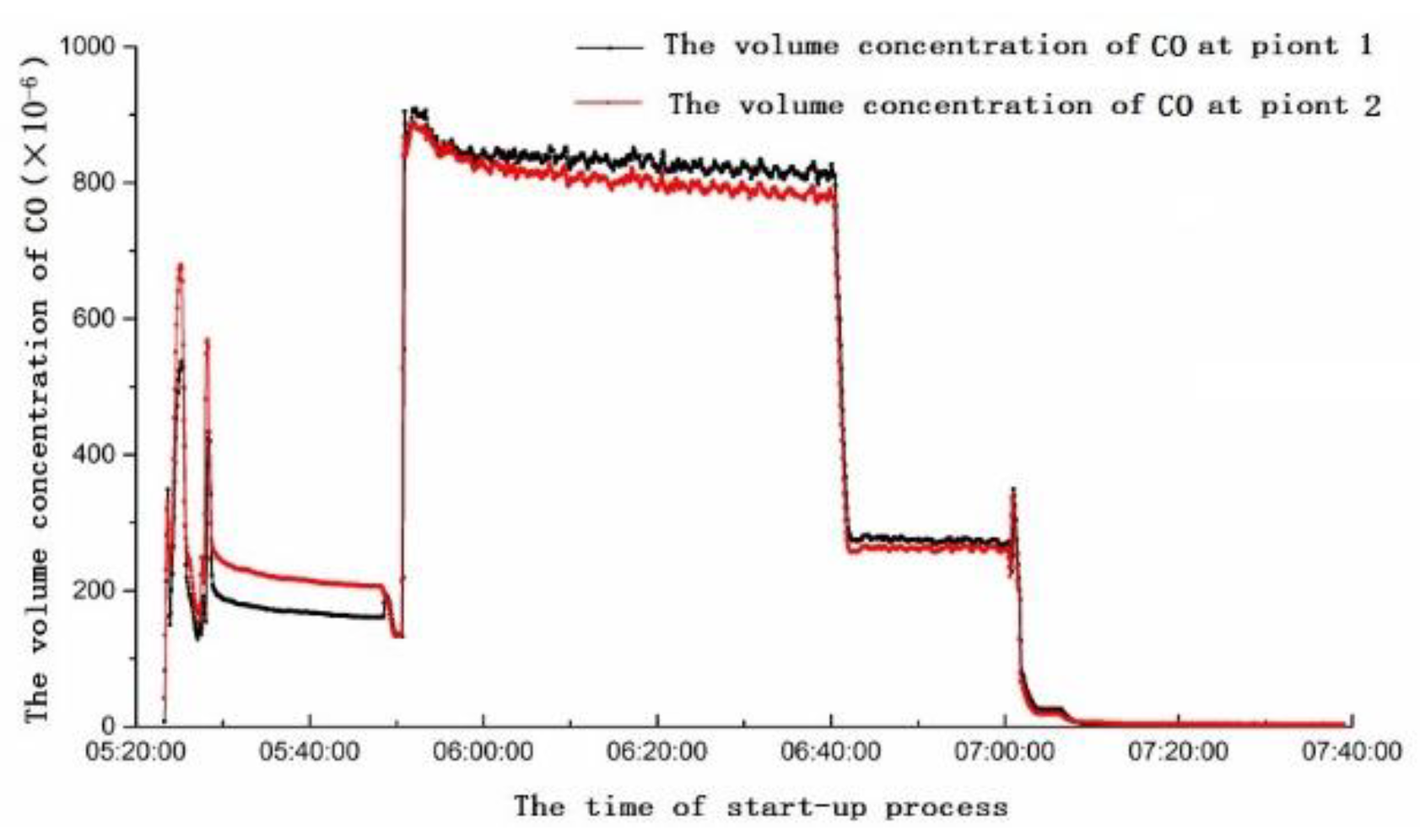
4. On-Site Measurement of the 6F Unit
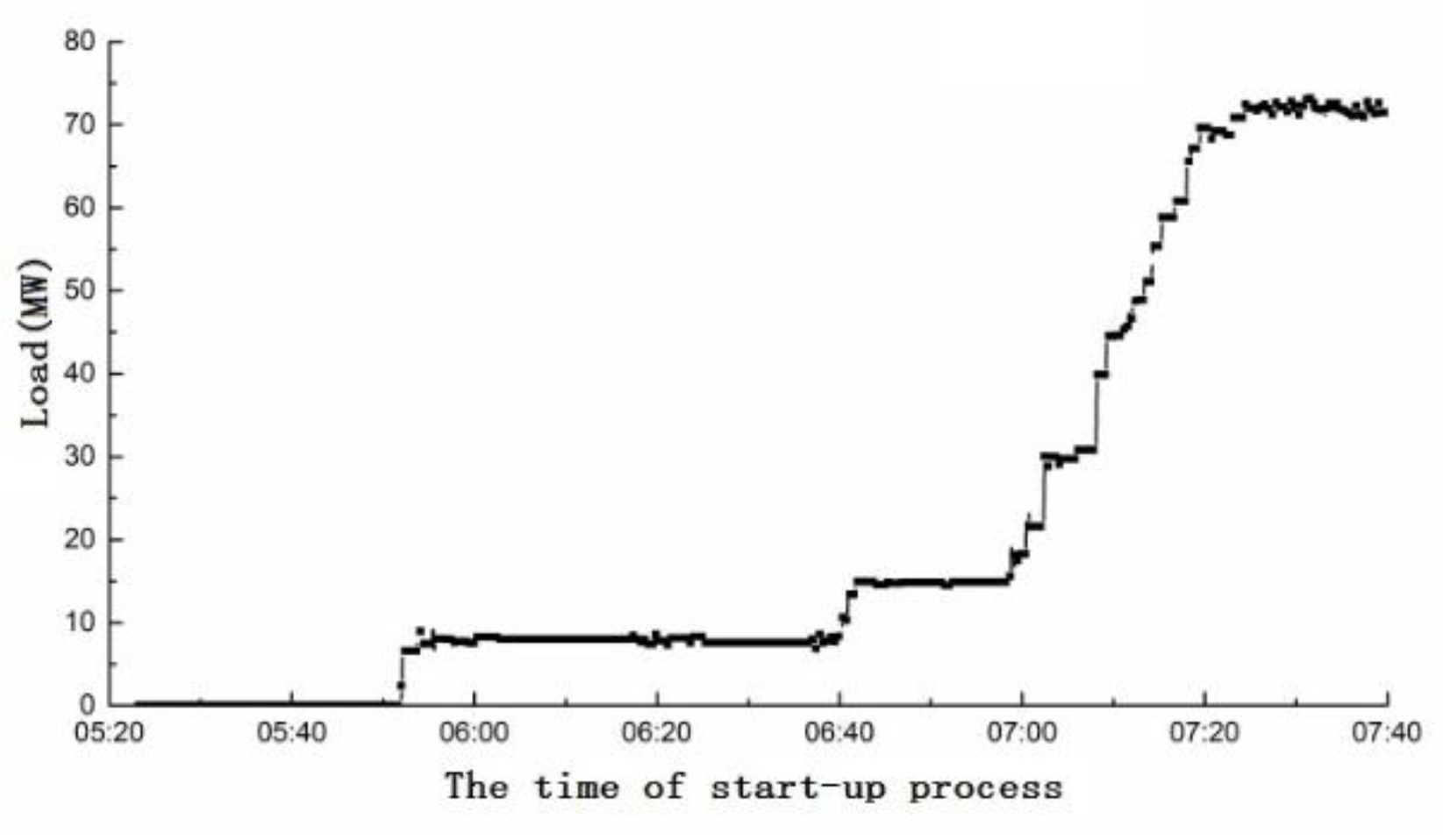
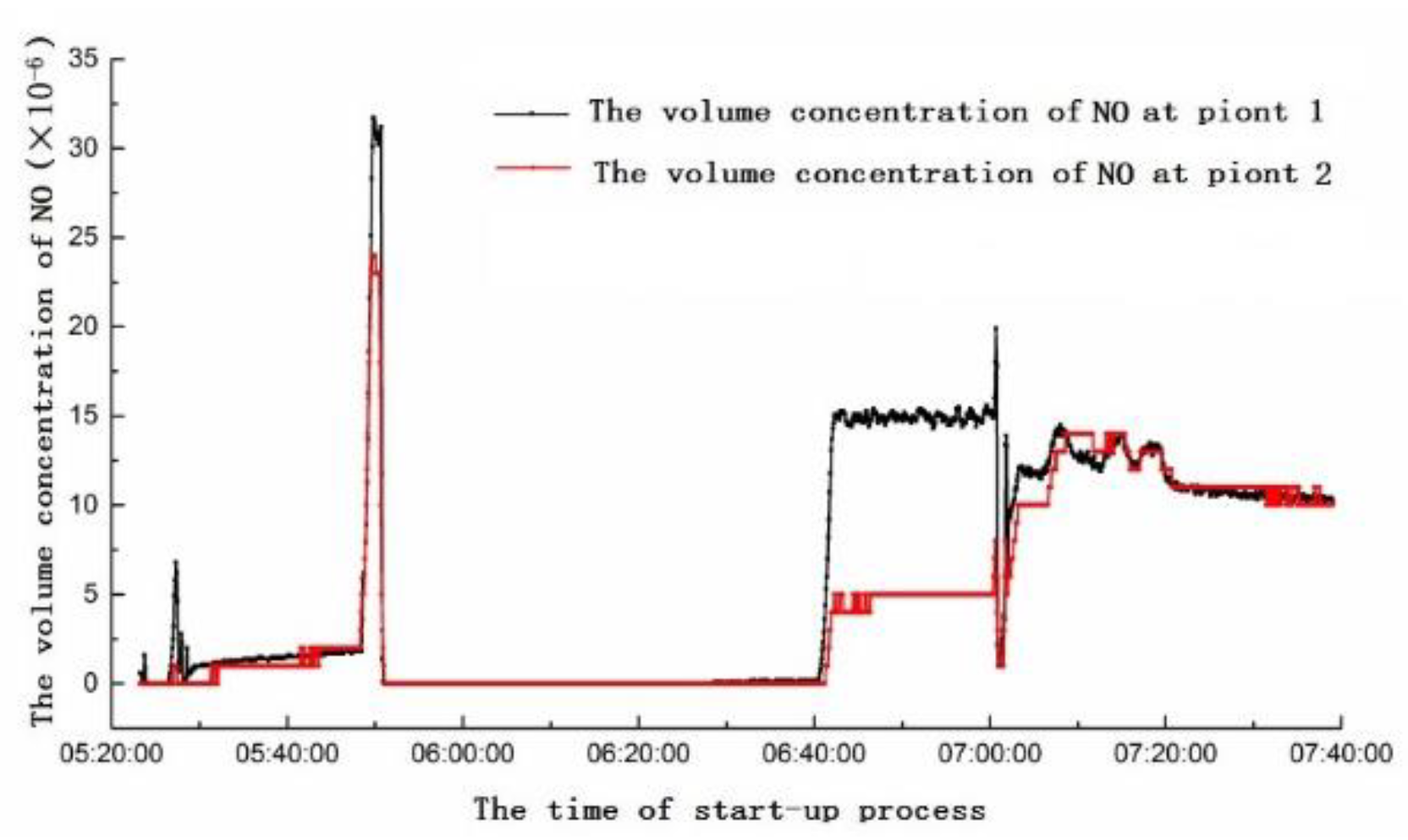
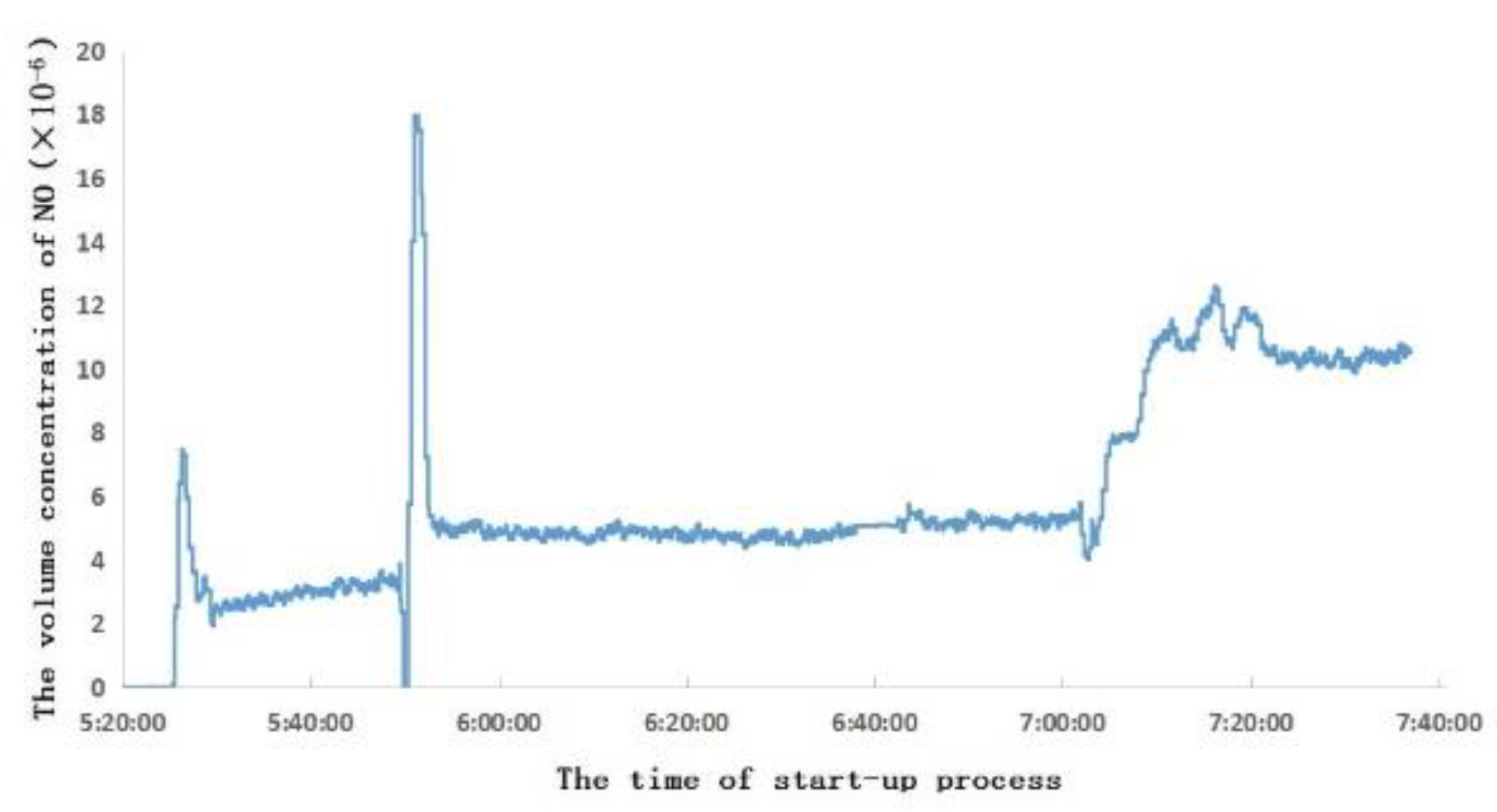
4.1. Analysis of Yellow Plume Formation at Different Stages of the Start-Up Period
4.2. Analysis of the Position of Yellow Plume Formation in HRSG
5. Analysis of Countermeasures for Yellow Plume
5.1. Discussion on Control Measures from Gas Turbine Side
- (1)
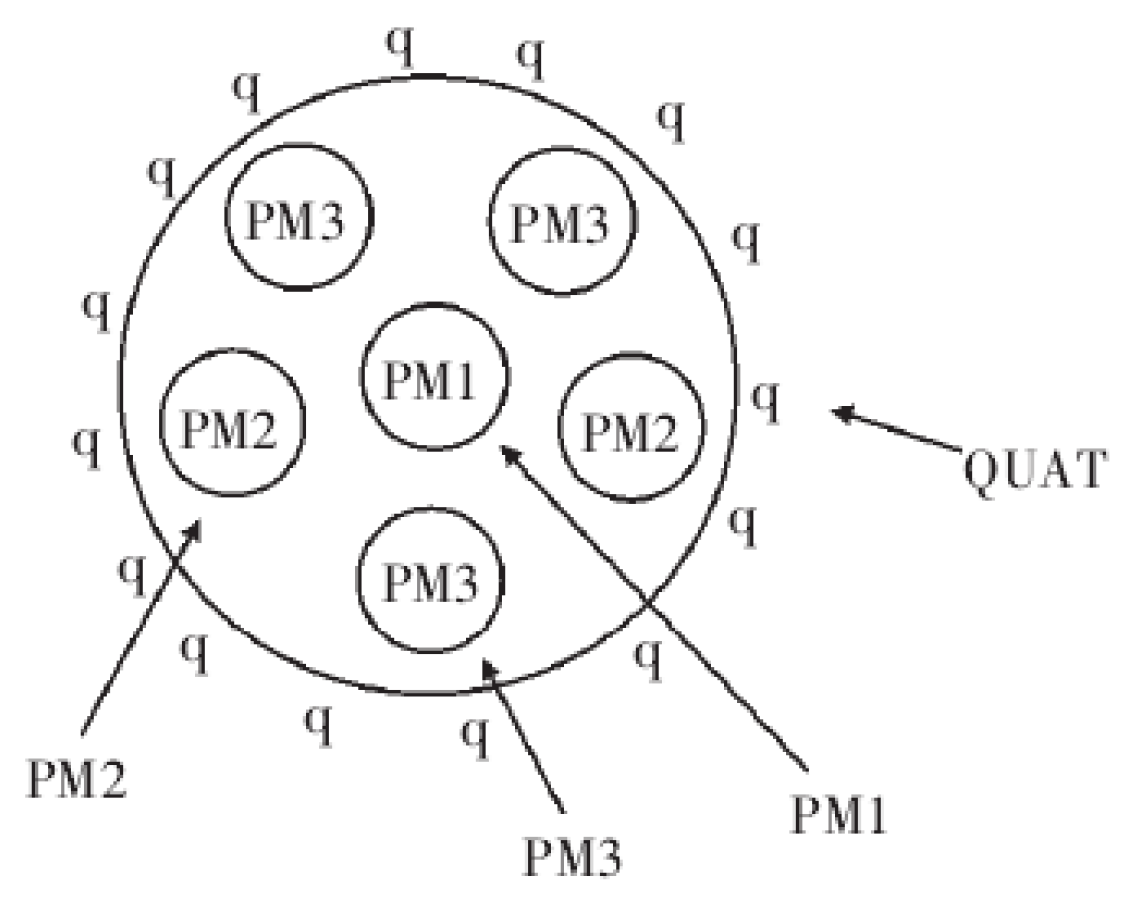
- The gas turbine starts to ignite, PM1 and PM2 open simultaneously. With the success of ignition, gas flow of PM2 gradually increases, while gas flow of PM1 gradually decreases. When only the PM2 nozzle is opened, the NO is relatively low. When the open degree of PM2 reaches 80%, there is a sudden increase of NO concentration.
- (2)
- When the rated speed of gas turbine reaches 95%, PM1 nozzle begins to open, while PM2 gradually reduces the amount of fuel until the fuel valve is fully closed. When only PM1 nozzle is open, the unit and the NOx concentrations change little. Then, the unit starts to connect to the grid.
- (3)
- When the gas turbine is connected to the grid successfully and begins to load up, the combustion mode is switched over and PM2 is put into operation. In the switching process, the combustion temperature is relatively high, and the NO reaches the highest value. With the open degrees of PM2 and PM1 entering a stable state, the NO rapidly drops back to normal, and the NO2 enters a stable and high concentration period. After that, the open degree of PM1 increases, and the gas turbine load increases. With the increase of combustion temperature, NO concentration increases slightly from the previous stage, and NO2 enters the highest concentration period.
- (4)
- When the gas turbine needs to increase to the base load, the combustion mode changes again. The open degrees of PM2 and PM1 decrease, PM3 and QUAT nozzles gradually open, and the switching moment has the characteristics of sudden increase of NO concentration. After switching successfully, PM1, PM2, PM3 and QUAT nozzles all open. Then, the nozzles gradually increase open degree, and the gas engine load increase. At this time, the combustion temperature of gas turbine increases gradually, the concentration of NO increases gradually, and the concentration of NO2 has a gradual downward trend. Because the open degree of each nozzle is changing, the concentration of NO has small fluctuations. Although PM1 is in premixed mode, its fuel coefficient (fuel equivalent ratio) is relatively high, which plays a role in stabilizing the flame and adjusting combustion dynamic stability. PM2 and PM3 nozzles are in the lean combustion limit region or even slightly lower. Subsequently, the gas turbine reaches the stable base load; at this time, the fuel flow rate of each nozzle reaches the optimal ratio, and the concentrations of NO and NO2 reach a steady low state.
- (1)
- The time from the unit being connected to the grid successfully to full load should be reduced, and the rate of load rise needs to increase. For the 6F unit, the running time of PM1 + PM2 premixed mode should be reduced to shorten the yellow plume time.
- (2)
- The start-up strategy of the gas turbine should be adjusted. The exhaust temperature of gas turbine during the start-up period should not exceed 500 °C to reduce the NO conversion to NO2 in the HRSG, which can alleviate the phenomenon of yellow plume.
- (3)
- The combustion efficiency during the start-up period should be increased, then the content of CO is reduced, thus the yellow plume could be alleviated. For example, upgrading the combustion system of the gas turbine could make CO in the combustion chamber stay a sufficient time to complete combustion, thus the content of CO is reduced.
5.2. Discussion on Control Measures from the HRSG Side
- (1)
- CO catalytic oxidation device [20] should be installed in the diffusion section, which connects the gas turbine with the HRSG, which can reduce the content of CO and NO2. Simultaneously, the flue gas temperature should rise to improve the efficiency of the HRSG.
- (2)
- Catalytic reduction method [21] should be adopted in the flue. The NO2 would be reduced to NO, thus yellow plume would be eliminated.
- (3)
- The flue gas should be sprayed and NO2 absorbed in the flue or chimney of the HRSG [22], thus the sewage would be neutralized by alkaline substances, or the alkaline solution could be directly sprayed inside the chimney.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Li, Y.; Wang, K. The Environmental Protection Status Quo of China’s gas Power Plants and the Trending in Policies. Electr. Power 2018, 51, 147–153. [Google Scholar]
- Liu, Z.; Wang, K.; Li, Y. Study on denitration scheme of gas power plant based on environmental protection standard. Electr. Power Technol. Environ. Prot. 2017, 33, 18–21. [Google Scholar]
- Tuttle, J.H.; Shisler, R.A.; Mellor, A.M. Nitrogen Dioxide Formation in gas Turbine Engines: Measurements and Measurement Methods. Combust. Sci. Technol. 1974, 9, 261–271. [Google Scholar] [CrossRef]
- Laurendeau, N.M. Fast Nitrogen Dioxide Reactions: Significance during NO Decomposition and NO2 Formation. Combust. Sci. Technol. 1975, 11, 89–96. [Google Scholar] [CrossRef]
- Chen, R.H. NOx and NO2 Emission of Swirl-Stabilized Nonpremixed Flames of a H2/CH4 Mixture. Combust. Sci. Technol. 2012, 120, 321–333. [Google Scholar] [CrossRef]
- Schäfer, K.; Brockmann, K.; Heland, J.; Wiesen, P.; Jahn, C.; Legras, O. Multipass open-path Fourier-transform infrared measurements for nonintrusive monitoring of gas turbine exhaust composition. Appl. Opt. 2005, 44, 2189. [Google Scholar] [CrossRef] [PubMed]
- Feitelberg, A.S.; Correa, S.M. The Role of Carbon Monoxide in NO2 Plume Formation. In ASME 1999 International Gas Turbine and Aeroengine Congress and Exhibition; American Society of Mechanical Engineers: New York, NY, USA, 2000; p. V002T02A005. [Google Scholar]
- Sorrentino, G.; Sabia, P.; Bozza, P.; Ragucci, R.; de Joannon, M. Impact of external operating parameters on the performance of a cyclonic burner with high level of internal recirculation under MILD combustion conditions. Energy 2017, 137, 1167–1174. [Google Scholar] [CrossRef]
- Galbiati, M.A.; Cavigiolo, A.; Effuggi, A.; Gelosa, D.; Rota, R. Mild combustion for fuel-NOx reduction. Combust. Sci. Technol. 2004, 176, 1035–1054. [Google Scholar] [CrossRef]
- Xu, H.; Hu, D.; Zhou, X.; Liu, H. Study on NOx emission from gas turbine and denitrification technology. Energy Environ. 2017, 5, 29–31. [Google Scholar]
- Wang, W.; Hua, W.; Sun, H. The causes and solutions of gas turbine chimney emission yellow smoke. Electr. Power Technol. Environ. Prot. 2016, 32, 33–35. [Google Scholar]
- Institute of Power Machinery and Engineering, Department of Thermal Engineering, Tsinghua University. Gas Turbine and Gas-Steam Combined Cycle Device; China Electric Power Press: Beijing, China, 2007. [Google Scholar]
- Zhu, B. Chemical Reaction Engineering; Chemical Industry Press: Beijing, China, 2012. [Google Scholar]
- Wei, Y.; Zhang, D.; Ran, B.; Mao, Y. SCR Process Simulation and Test Study Based on Catalytic Reaction Kinetics. Dongfang Electr. Rev. 2015, 29, 14–19. [Google Scholar]
- Yao, Q.; Li, S.; Wang, Y. Introduction to Combustion: Concept and Application; Tsinghua University Press: Beijing, China, 2008. [Google Scholar]
- Turns, S.R. An Introduction to Combustion Conceps and Applications; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Glassman, I. Combustion; Academic Press: Orlando, FL, USA, 2008. [Google Scholar]
- Cai, G. Application of fully premixed DLN2.6+ combustor in China. Power Energy 2017, 38, 315–319. [Google Scholar]
- Lou, H.; Chen, X.; Mao, Z. Brief Analysis of DLN-2.6 Combustion System of GE gas Turbine. Zhejiang Electr. Power 2011, 30, 70–73. [Google Scholar]
- McDeed, D.; Pyros, G.; Bravato, A. Operating A Gas Turbine Power Plant at Low Load Conditions. U.S. Patent 2015/011563, 23 July 2015. [Google Scholar]
- Yang, J.H.; Yang, J.; Chun, D.H.; Kim, H.; Lee, H.; Jung, H. Catalytic process for decolorizing yellow plume. Korean J. Chem. Eng. 2011, 28, 418–423. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Cheng, S.; Li, P.; Xue, Z.; Shao, J. A Device for Eliminating Yellow Smoke from Chimneys in the Early Stages of Gas Turbine Startup. Patent CN107261842A, 20 October 2017. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, Y.; Wang, K.; Shao, W.; Wang, B.; Sun, C. Mechanism Research and Countermeasure Analysis of Yellow Plume during the Gas Turbine Start-Up Period. Appl. Sci. 2019, 9, 251. https://doi.org/10.3390/app9020251
Liu Z, Li Y, Wang K, Shao W, Wang B, Sun C. Mechanism Research and Countermeasure Analysis of Yellow Plume during the Gas Turbine Start-Up Period. Applied Sciences. 2019; 9(2):251. https://doi.org/10.3390/app9020251
Chicago/Turabian StyleLiu, Zhitan, Yugang Li, Kai Wang, Weiwei Shao, Bo Wang, and Chen Sun. 2019. "Mechanism Research and Countermeasure Analysis of Yellow Plume during the Gas Turbine Start-Up Period" Applied Sciences 9, no. 2: 251. https://doi.org/10.3390/app9020251
APA StyleLiu, Z., Li, Y., Wang, K., Shao, W., Wang, B., & Sun, C. (2019). Mechanism Research and Countermeasure Analysis of Yellow Plume during the Gas Turbine Start-Up Period. Applied Sciences, 9(2), 251. https://doi.org/10.3390/app9020251



