Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: A Preliminary Multiscale Numerical Investigation
Abstract
1. Introduction
2. Methods
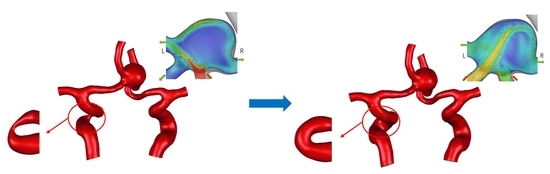
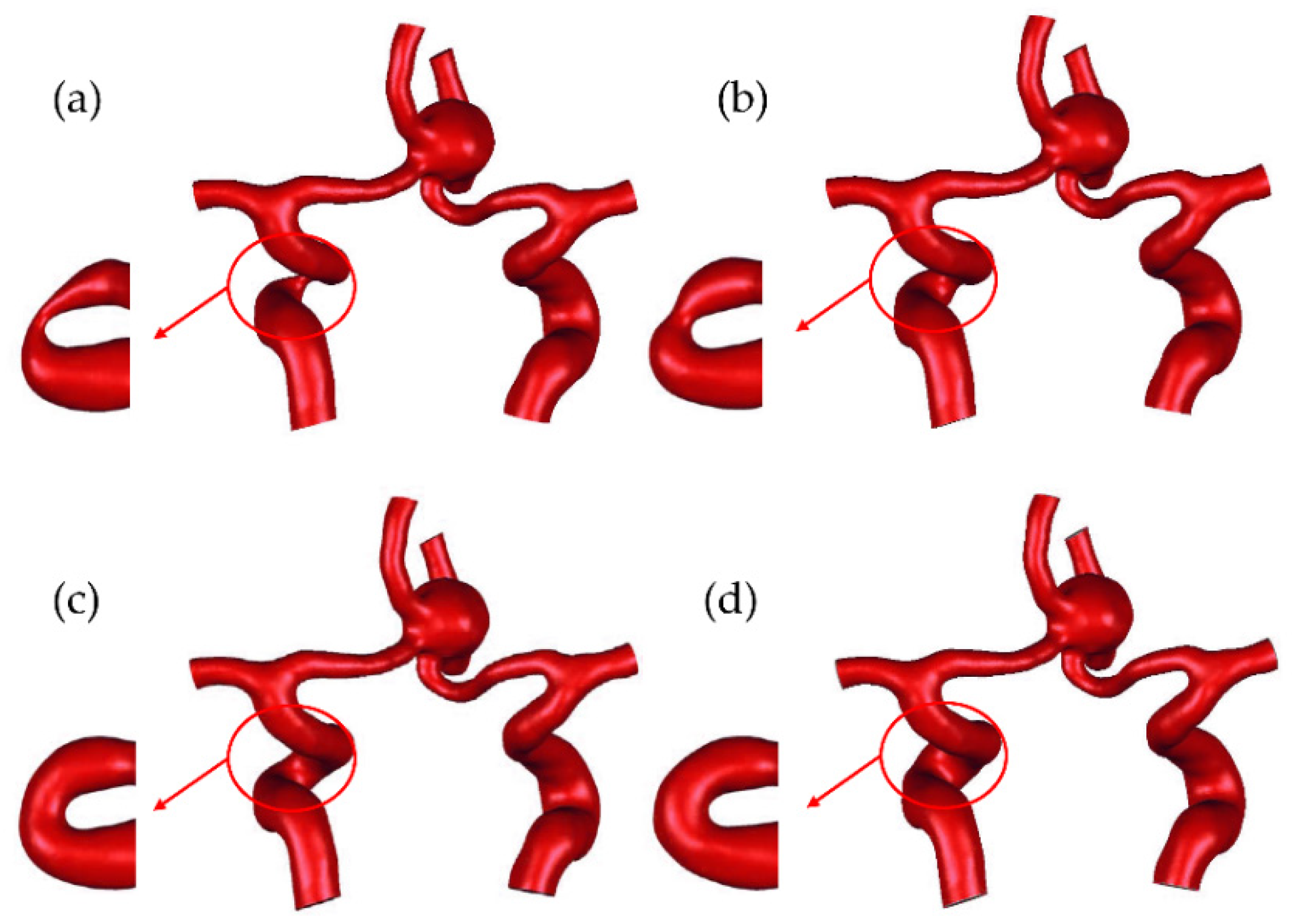
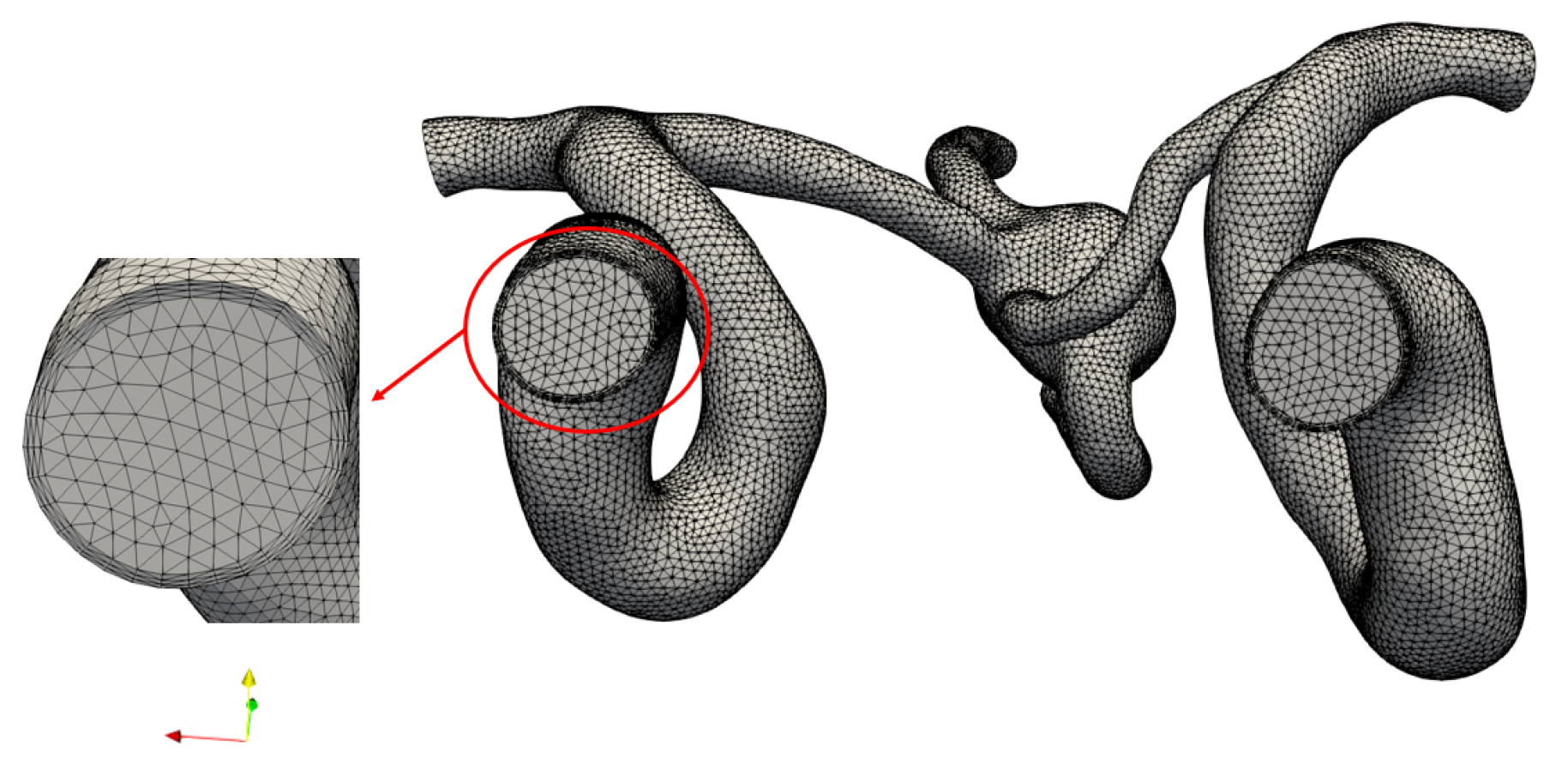
2.1. Multiscale Modeling Approach
2.2. Data Analysis
2.3. Validation of Numerical Methods
3. Results
3.1. The Impact of Revascularization on the CBF
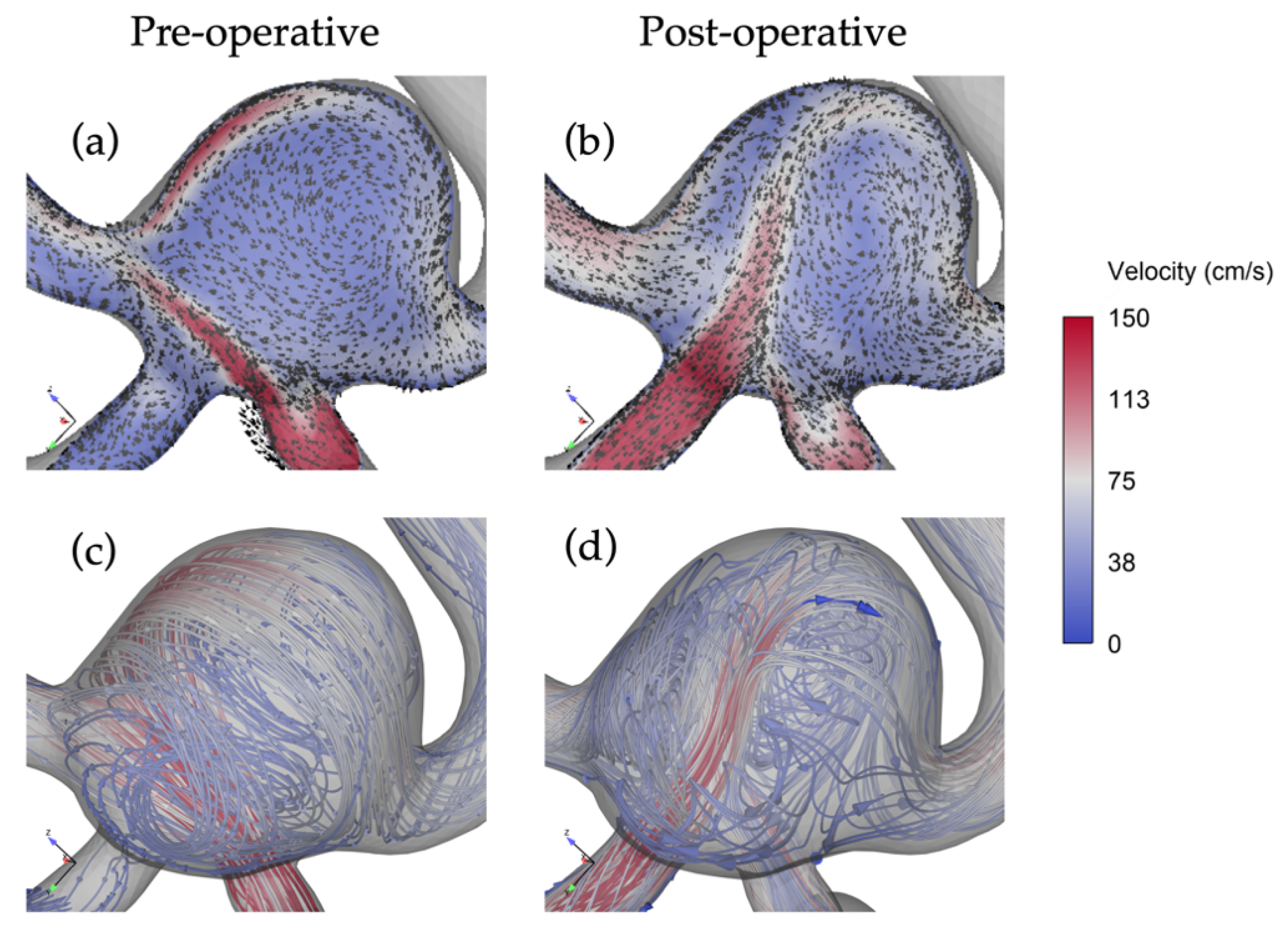
3.2. The Impact of Revascularization on Flow Patterns
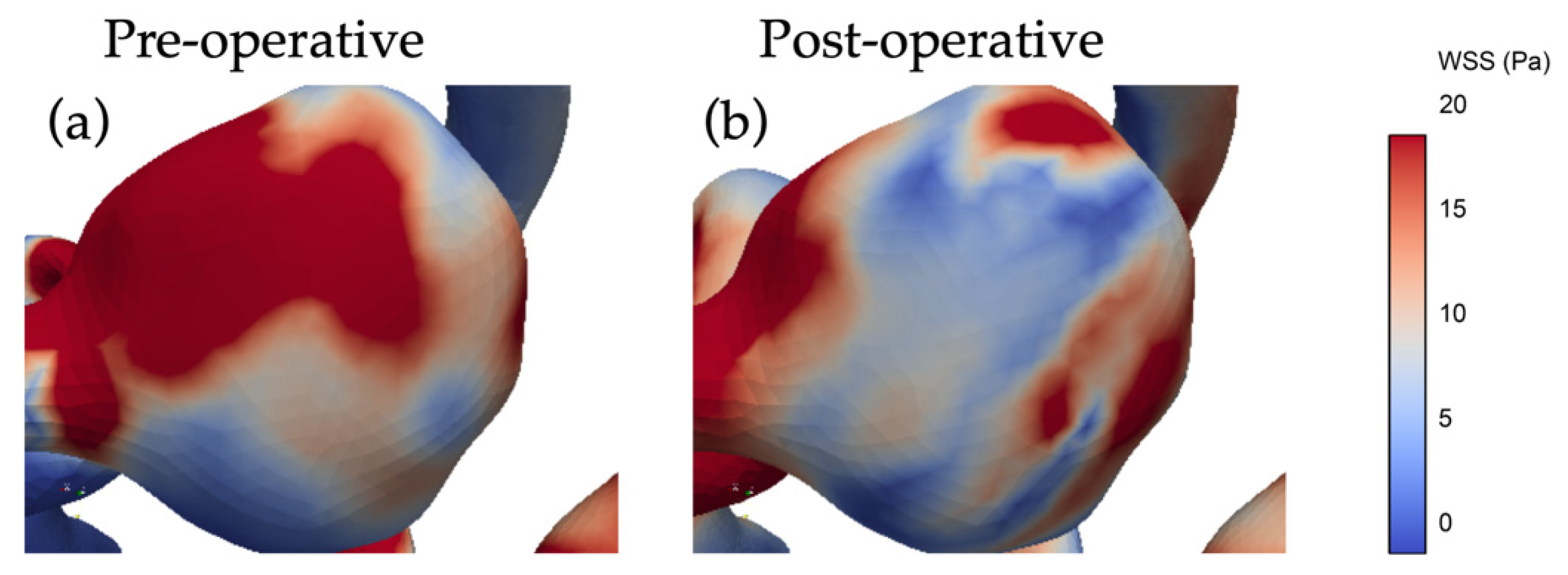
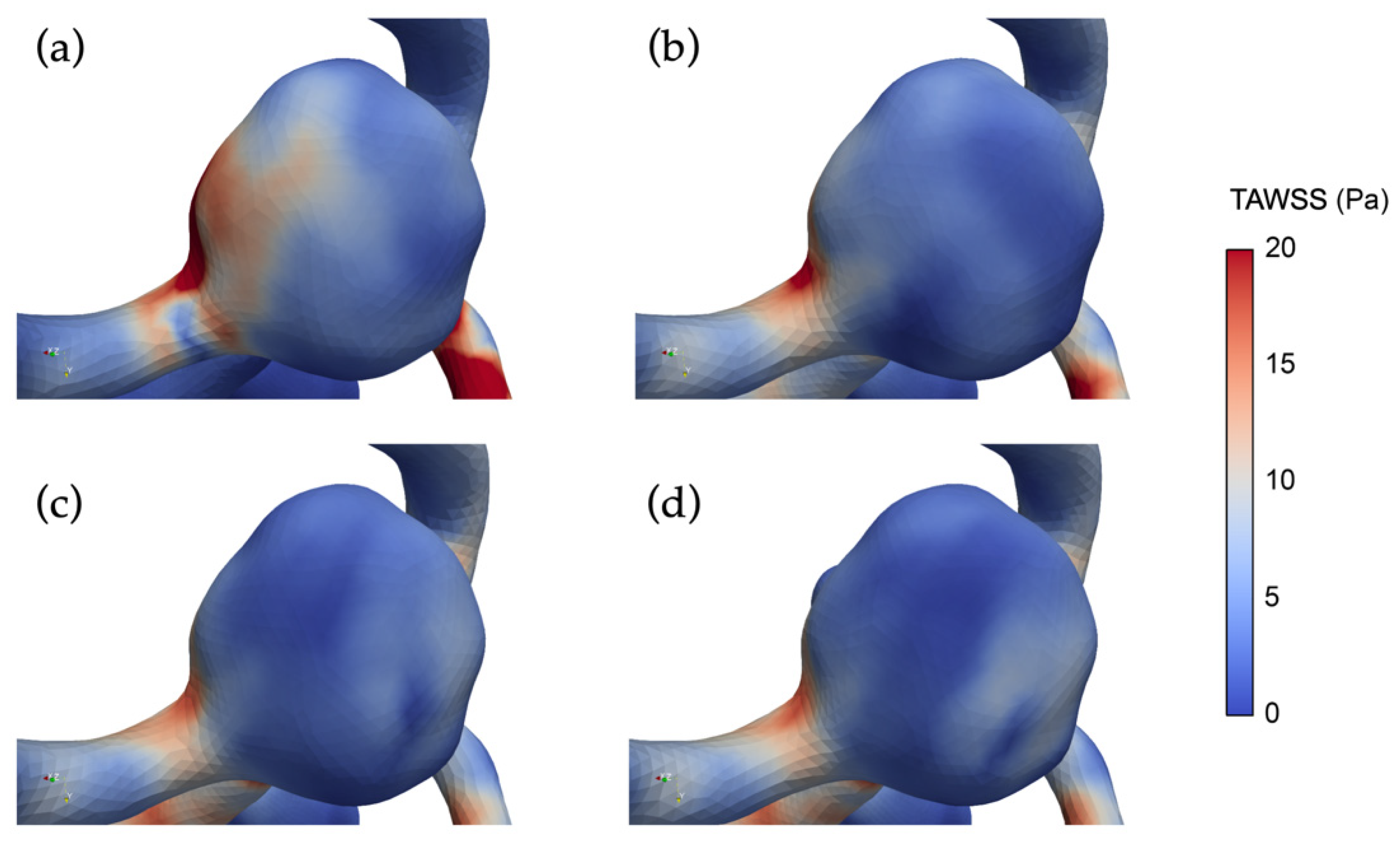
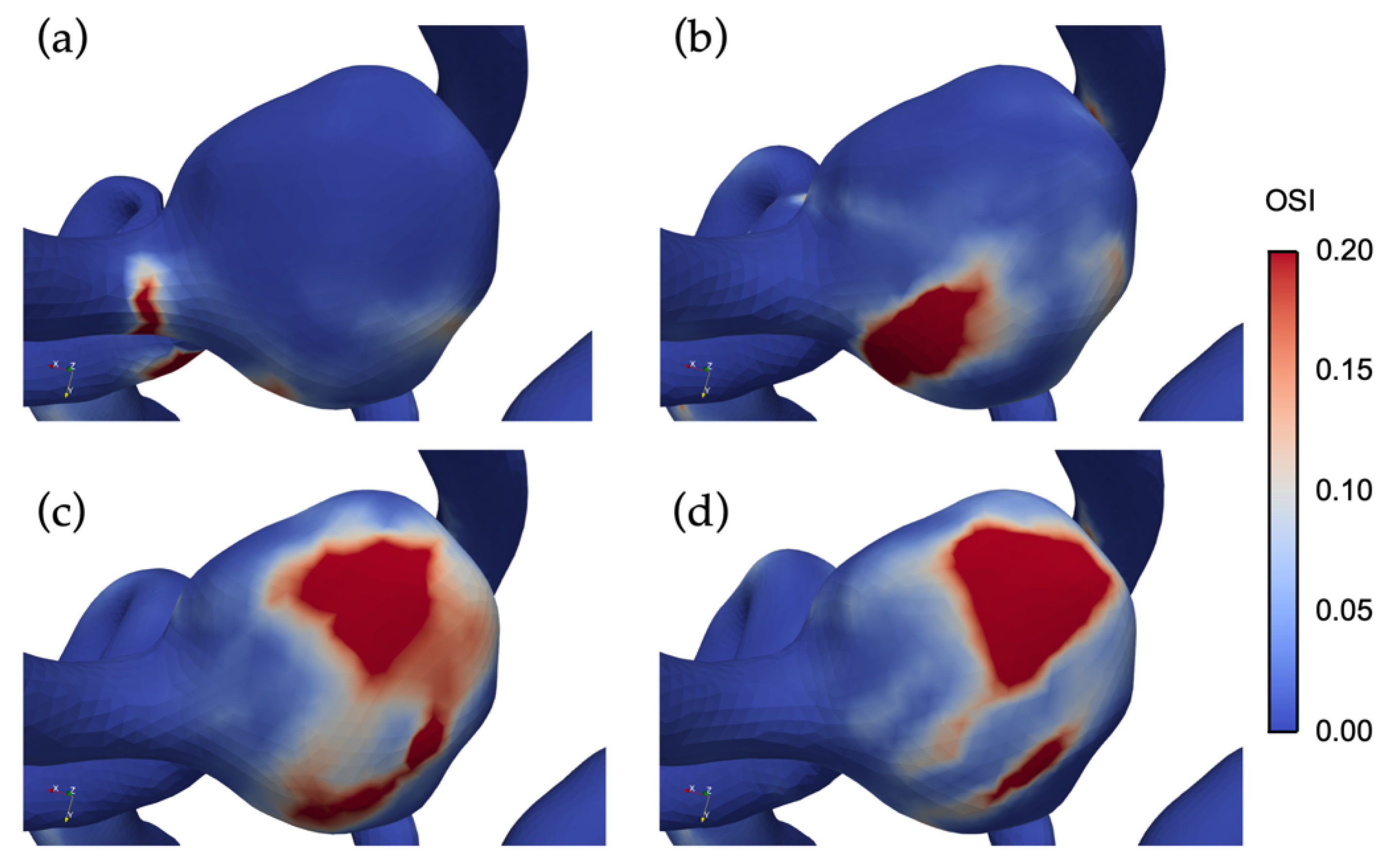
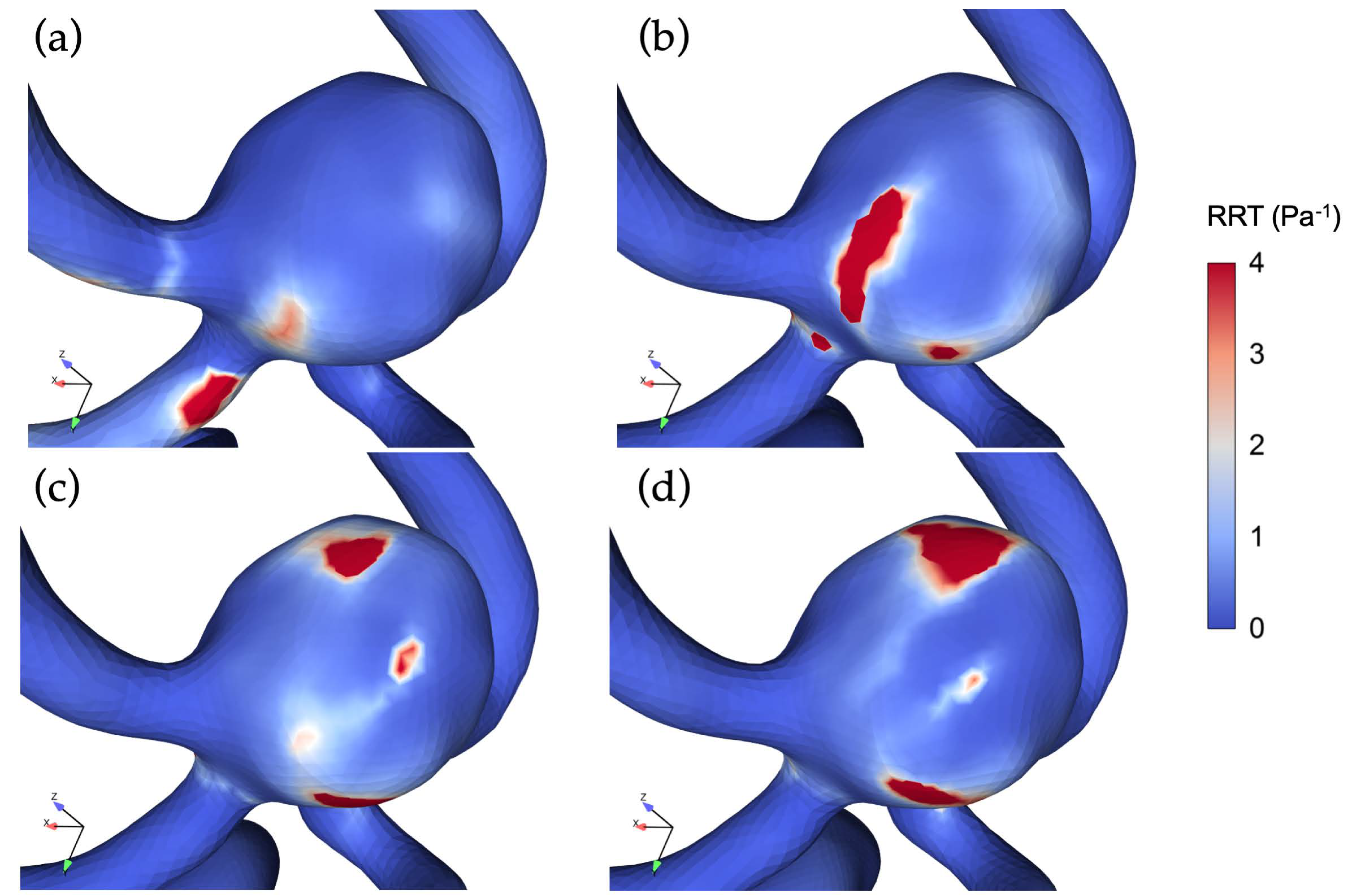
3.3. The Impact of Revascularization on the Hemodynamics Parameters
4. Discussion
4.1. Modeling Considerations
4.2. Impacts of Unilateral ICA Revascularization on ACoAA Flow Patterns
4.3. Impacts of Unilateral ICA Revascularization on ACoAA Hemodynamics Characteristics
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, U.A.; Thapar, A.; Shalhoub, J.; Davies, A.H. Risk of intracerebral aneurysm rupture during carotid revascularization. J. Vasc. Surg. 2012, 56, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, J.; Wang, J.; Wang, L.; Qi, P.; Hu, S.; Chen, K.; Wang, D. A clinical study and meta-analysis of carotid stenosis with coexistent intracranial aneurysms. J. Clin. Neurosci. 2018, 52, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Cvetic, V.; Dragas, M.; Colic, M.; Vukasinovic, I.; Radmili, O.; Ilic, N.; Koncar, I.; Bascarevic, V.; Ristanovic, N.; Davidovic, L. Simultaneous Endovascular Treatment of Tandem Internal Carotid Lesions. Vasc. Endovasc. Surg. 2016, 50, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Villoria, F.; Fortea, F.; Carrera, J.; Mateo, O.; Sanchez-Alarcos, S.; Reparaz, L. Simultaneous Cerebral Aneurysms and Carotid Disease Should the Symptomatic Lesion always be the first to be Treated? Interv. Neuroradiol. 2003, 9, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Awad, I.A.; Wiebers, D.O.; Piepgras, D.; Haley, E.C.; Brott, T.; Hademenos, G.; Chyatte, D.; Rosenwasser, R.; Caroselli, C. Recommendations for the management of patients with unruptured intracranial aneurysms: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation 2000, 102, 2300–2308. [Google Scholar] [CrossRef]
- Hartmann, M.; Weber, R.; Zoubaa, S.; Schranz, C.; Knauth, M. Fatal subarachnoid hemorrhage after carotid stenting. J. Neuroradiol. 2004, 31, 63–66. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J.; Meissner, I.; Brown, R.D.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet (Lond. Engl.) 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Kappelle, L.J.; Eliasziw, M.; Fox, A.J.; Barnett, H.J.M. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis. Neurology 2000, 55, 307–309. [Google Scholar] [CrossRef]
- Tallarita, T.; Sorenson, T.J.; Rinaldo, L.; Oderich, G.S.; Bower, T.C.; Meyer, F.B.; Lanzino, G. Management of carotid artery stenosis in patients with coexistent unruptured intracranial aneurysms. J. Neurosurg. 2019, 1–4. [Google Scholar] [CrossRef]
- Suh, B.-Y.; Yun, W.-S.; Kwun, W.-H. Carotid artery revascularization in patients with concomitant carotid artery stenosis and asymptomatic unruptured intracranial artery aneurysm. Ann. Vasc. Surg. 2011, 25, 651–655. [Google Scholar] [CrossRef]
- Uchida, H.; Endo, H.; Fujimura, M.; Endo, T.; Niizuma, K.; Tominaga, T. Intra-operative hemorrhage due to hyperperfusion during direct revascularization surgery in an adult patient with moyamoya disease: A case report. Neurosurg. Rev. 2017, 40, 679–684. [Google Scholar] [CrossRef]
- Riphagen, J.H.; Bernsen, H.J.J.A. Rupture of an intracerebral aneurysm after carotid endarterectomy: A case report. Acta Neurol. Belg. 2009, 109, 314–316. [Google Scholar]
- Batjer, H.; Mickey, B.; Samson, D. Enlargement and rupture of distal basilar artery aneurysm after iatrogenic carotid occlusion. Neurosurgery 1987, 20, 624–628. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or Low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef]
- Cebral, J.R.; Detmer, F.; Chung, B.J.; Choque-Velasquez, J.; Rezai, B.; Lehto, H.; Tulamo, R.; Hernesniemi, J.; Niemela, M.; Yu, A.; et al. Local hemodynamic conditions associated with focal changes in the intracranial aneurysm wall. Am. J. Neuroradiol. 2019, 40, 510–516. [Google Scholar]
- Liang, L.; Steinman, D.A.; Brina, O.; Chnafa, C.; Cancelliere, N.M.; Pereira, V.M. Towards the Clinical utility of CFD for assessment of intracranial aneurysm rupture—A systematic review and novel parameter-ranking tool. J. Neurointerv. Surg. 2019, 11, 153–158. [Google Scholar] [CrossRef]
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef]
- Bacigaluppi, S.; Piccinelli, M.; Antiga, L.; Veneziani, A.; Passerini, T.; Rampini, P.; Zavanone, M.; Severi, P.; Tredici, G.; Zona, G.; et al. Factors affecting formation and rupture of intracranial saccular aneurysms. Neurosurg. Rev. 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Brinjikji, W.; Chung, B.J.; Jimenez, C.; Putman, C.; Kallmes, D.F.; Cebral, J.R. Hemodynamic differences between unstable and stable unruptured aneurysms independent of size and location: A pilot study. J. Neurointerv. Surg. 2017, 9, 376–380. [Google Scholar] [CrossRef]
- Dhar, S.; Tremmel, M.; Mocco, J.; Kim, M.; Yamamoto, J.; Siddiqui, A.H.; Hopkins, L.N.; Meng, H. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008, 63, 185–196; discussion 196–197. [Google Scholar] [CrossRef]
- Alfano, J.M.; Kolega, J.; Natarajan, S.K.; Xiang, J.; Paluch, R.A.; Levy, E.I.; Siddiqui, A.H.; Meng, H. Intracranial Aneurysms Occur More Frequently at Bifurcation Sites That Typically Experience Higher Hemodynamic Stresses. Neurosurgery 2013, 73, 497–505. [Google Scholar] [CrossRef]
- Rinkel, G.J.E.; Djibuti, M.; Algra, A.; van Gijn, J. Prevalence and Risk of Rupture of Intracranial Aneurysms. Stroke 1998, 29, 251–256. [Google Scholar] [CrossRef]
- Castro, M.A.; Putman, C.M.; Cebral, J.R. Patient-Specific Computational Modeling of Cerebral Aneurysms with Multiple Avenues of Flow from 3D Rotational Angiography Images. Acad. Radiol. 2006, 13, 811–821. [Google Scholar] [CrossRef]
- Zhu, G.; Yuan, Q.; Yang, J.; Yeo, J.H. The role of the circle of Willis in internal carotid artery stenosis and anatomical variations: A computational study based on a patient-specific three-dimensional model. Biomed. Eng. Online 2015, 14, 107. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Yuan, Q.; Yang, J.; Yeo, J.H. Experimental study of hemodynamics in the Circle of Willis. Biomed. Eng. Online 2015, 14, S10. [Google Scholar] [CrossRef]
- NASCET. North american symptomatic sarotid endarterectomy trial. Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar] [CrossRef]
- Miura, Y.; Ishida, F.; Umeda, Y.; Tanemura, H.; Suzuki, H.; Matsushima, S.; Shimosaka, S.; Taki, W. Low Wall Shear Stress Is Independently Associated With the Rupture Status of Middle Cerebral Artery Aneurysms. Stroke 2013, 44, 519–521. [Google Scholar] [CrossRef]
- Catalán-Echeverría, B.; Kelly, M.; Peeling, L.; Bergstrom, D.; Chen, X.; Malvè, M. CFD-Based Comparison Study of a New Flow Diverting Stent and Commercially-Available Ones for the Treatment of Cerebral Aneurysms. Appl. Sci. 2019, 9, 1341. [Google Scholar] [CrossRef]
- Updegrove, A.; Wilson, N.M.; Merkow, J.; Lan, H.; Marsden, A.L.; Shadden, S.C. SimVascular: An Open Source Pipeline for Cardiovascular Simulation. Ann. Biomed. Eng. 2017, 45, 525–541. [Google Scholar] [CrossRef]
- Enzmann, D.R.; Ross, M.R.; Marks, M.P.; Pelc, N.J. Blood flow in major cerebral arteries measured by phase-contrast cine MR. Am. J. Neuroradiol. 1994, 15, 123–129. [Google Scholar]
- Zhao, M.; Amin-Hanjani, S.; Ruland, S.; Curcio, A.P.; Ostergren, L.; Charbel, F.T. Regional cerebral blood flow using quantitative MR angiography. Am. J. Neuroradiol. 2007, 28, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Cieslicki, K.; Ciesla, D. Investigations of flow and pressure distributions in physical model of the circle of Willis. J. Biomech. 2005, 38, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Zarrinkoob, L.; Ambarki, K.; Wåhlin, A.; Birgander, R.; Eklund, A.; Malm, J. Blood flow distribution in cerebral arteries. J. Cereb. Blood Flow Metab. 2015, 35, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Skodvin, T.Ø.; Evju, Ø.; Helland, C.A.; Isaksen, J.G. Rupture prediction of intracranial aneurysms: A nationwide matched case-control study of hemodynamics at the time of diagnosis. J. Neurosurg. 2018, 129, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Kadasi, L.M.; Dent, W.C.; Malek, A.M. Colocalization of thin-walled dome regions with low hemodynamic wall shear stress in unruptured cerebral aneurysms. J. Neurosurg. 2013, 119, 172–179. [Google Scholar] [CrossRef]
- Shojima, M. Magnitude and Role of Wall Shear Stress on Cerebral Aneurysm. Computational Fluid Dynamic Study of 20 Middle Cerebral Artery Aneurysms. Stroke 2004, 35, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, Z.-Q.; Karuna, T.; He, X.-Y.; Wang, X.-M.; Li, X.-F.; Liu, W.-C.; Li, R.; Guo, S.-Q.; Chen, Y.-C.; et al. The role of wall shear stress in the parent artery as an independent variable in the formation status of anterior communicating artery aneurysms. Eur. Radiol. 2019, 29, 689–698. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of hemodynamic characteristics and cerebral aneurysm rupture. Am. J. Neuroradiol. 2011, 32, 264–270. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C. Quantitative Characterization of the Hemodynamic Environment in Ruptured and Unruptured Brain Aneurysms. Am. J. Neuroradiol. 2011, 32, 145–151. [Google Scholar] [CrossRef]
- Castro, M.A.; Putman, C.M.; Sheridan, M.J.; Cebral, J.R. Hemodynamic Patterns of Anterior Communicating Artery Aneurysms: A Possible Association with Rupture. Am. J. Neuroradiol. 2009, 30, 297–302. [Google Scholar] [CrossRef]
- Cebral, J.R.; Duan, X.; Gade, P.S.; Chung, B.J.; Mut, F.; Aziz, K.; Robertson, A.M. Regional Mapping of Flow and Wall Characteristics of Intracranial Aneurysms. Ann. Biomed. Eng. 2016, 44, 3553–3567. [Google Scholar] [CrossRef] [PubMed]
- Jou, L.-D.; Lee, D.H.; Mawad, M.E. Cross-flow at the anterior communicating artery and its implication in cerebral aneurysm formation. J. Biomech. 2010, 43, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Oshima, M.; Huang, H.; Liu, H.; Takagi, S. Numerical Study of Cerebroarterial Hemodynamic Changes Following Carotid Artery Operation: A Comparison Between Multiscale Modeling and Stand-Alone Three-Dimensional Modeling. J. Biomech. Eng. 2015, 137, 101011. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Torres, F. Effects of Hypertension and Pressure Gradient in A Human Cerebral Aneurysm Using Fluid Structure Interaction simulations. J. Mech. Med. Biol. 2017, 17, 1750018. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, L.; Zhang, Y.; Liu, J.; Yang, X. Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: Case report and literature review. BMC Neurol. 2016, 16, 231. [Google Scholar] [CrossRef]
- Russin, J.; Babiker, H.; Ryan, J.; Rangel-Castilla, L.; Frakes, D.; Nakaji, P. Computational Fluid Dynamics to Evaluate the Management of a Giant Internal Carotid Artery Aneurysm. World Neurosurg. 2015, 83, 1057–1065. [Google Scholar] [CrossRef]
- Karmonik, C.; Diaz, O.; Klucznik, R.; Grossman, R.G.; Zhang, Y.J.; Britz, G.; Lv, N.; Huang, Q. Quantitative comparison of hemodynamic parameters from steady and transient CFD simulations in cerebral aneurysms with focus on the aneurysm ostium. J. Neurointerv. Surg. 2015, 7, 367–372. [Google Scholar] [CrossRef]
- Pewowaruk, R.; Roldán-Alzate, A. 4D Flow MRI Estimation of Boundary Conditions for Patient Specific Cardiovascular Simulation. Ann. Biomed. Eng. 2019, 47, 1786–1798. [Google Scholar] [CrossRef]
- Vignon, I.E.; Taylor, C.A. Outflow boundary conditions for one-dimensional finite element modeling of blood flow and pressure waves in arteries. Wave Motion 2004, 39, 361–374. [Google Scholar] [CrossRef]
- Xiang, J.; Natarajan, S.K.; Tremmel, M.; Ma, D.; Mocco, J.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I.; Meng, H. Hemodynamic–Morphologic Discriminants for Intracranial Aneurysm Rupture. Stroke 2011, 42, 144–152. [Google Scholar] [CrossRef]
- Lee, U.Y.; Jung, J.; Kwak, H.S.; Lee, D.H.; Chung, G.H.; Park, J.S.; Koh, E.J. Wall shear stress and flow patterns in unruptured and ruptured anterior communicating artery aneurysms using computational fluid dynamics. J. Korean Neurosurg. Soc. 2018, 61, 689–699. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Ujiie, H.; Haida, S.; Nakazawa, N.; Hori, T. Velocity profile and wall shear stress of saccular aneurysms at the anterior communicating artery. Heart Vessels 2008, 23, 60–66. [Google Scholar] [CrossRef]
- Fukazawa, K.; Ishida, F.; Umeda, Y.; Miura, Y.; Shimosaka, S.; Matsushima, S.; Taki, W.; Suzuki, H. Using computational fluid dynamics analysis to characterize local hemodynamic features of middle cerebral artery aneurysm rupture points. World Neurosurg. 2015, 83, 80–86. [Google Scholar] [CrossRef]
- Xiang, J.; Yu, J.; Snyder, K.V.; Levy, E.I.; Siddiqui, A.H.; Meng, H. Hemodynamic-morphological discriminant models for intracranial aneurysm rupture remain stable with increasing sample size. J. Neurointerv. Surg. 2016, 8, 104–110. [Google Scholar] [CrossRef]
- Can, A.; Du, R. Association of hemodynamic factors with intracranial aneurysm formation and rupture: Systematic review and meta-analysis. Neurosurgery 2016, 78, 510–519. [Google Scholar] [CrossRef]
- Le, W.-J.; Zhu, Y.-Q.; Li, M.-H.; Yan, L.; Tan, H.-Q.; Xiao, S.-M.; Cheng, Y.-S. New method for retrospective study of hemodynamic changes before and after aneurysm formation in patients with ruptured or unruptured aneurysms. BMC Neurol. 2013, 13, 166. [Google Scholar] [CrossRef]
- Sadasivan, C.; Fiorella, D.J.; Woo, H.H.; Lieber, B.B. Physical Factors Effecting Cerebral Aneurysm Pathophysiology. Ann. Biomed. Eng. 2013, 41, 1347–1365. [Google Scholar] [CrossRef]
- Varble, N.; Tutino, V.M.; Yu, J.; Sonig, A.; Siddiqui, A.H.; Davies, J.M.; Meng, H. Shared and Distinct Rupture Discriminants of Small and Large Intracranial Aneurysms. Stroke 2018, 49, 856–864. [Google Scholar] [CrossRef]
- Murayama, Y.; Fujimura, S.; Suzuki, T.; Takao, H. Computational fluid dynamics as a risk assessment tool for aneurysm rupture. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Wu, J.; Chen, X.; Li, M.; Li, Z.; Yang, S.; Guo, R.; Gao, B.; Cao, Y.; et al. A Novel Scoring System for Rupture Risk Stratification of Intracranial Aneurysms: A Hemodynamic and Morphological Study. Front. Neurosci. 2018, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Selimovic, A.; Thompson, H.; Chiarini, A.; Penrose, J.; Ventikos, Y.; Watton, P.N. Investigating the Influence of Haemodynamic Stimuli on Intracranial Aneurysm Inception. Ann. Biomed. Eng. 2013, 41, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Torii, R.; Oshima, M.; Kobayashi, T.; Takagi, K.; Tezduyar, T.E. Influencing factors in image-based fluid-structure interaction computation of cerebral aneurysms. Int. J. Numer. Methods Fluids 2011, 65, 324–340. [Google Scholar] [CrossRef]
- Bazilevs, Y.; Hsu, M.-C.; Zhang, Y.; Wang, W.; Kvamsdal, T.; Hentschel, S.; Isaksen, J.G. Computational vascular fluid-structure interaction: Methodology and application to cerebral aneurysms. Biomech. Model. Mechanobiol. 2010, 9, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Cebral, J.R. CFD for Evaluation and Treatment Planning of Aneurysms: Review of Proposed Clinical Uses and Their Challenges. Ann. Biomed. Eng. 2014, 43, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liang, F.; Gu, L.; Liu, H. Flow instability detected in ruptured versus unruptured cerebral aneurysms at the internal carotid artery. J. Biomech. 2018, 72, 187–199. [Google Scholar] [CrossRef]
- Hippelheuser, J.E.; Lauric, A.; Cohen, A.D.; Malek, A.M. Realistic non-Newtonian viscosity modelling highlights hemodynamic differences between intracranial aneurysms with and without surface blebs. J. Biomech. 2014, 47, 3695–3703. [Google Scholar] [CrossRef]
- Fisher, C.; Rossmann, J.S. Effect of non-newtonian behavior on hemodynamics of cerebral aneurysms. J. Biomech. Eng. 2009, 131, 091004. [Google Scholar] [CrossRef]
- Valencia, A.; Zarate, A.; Galvez, M.; Badilla, L. Non-Newtonian blood flow dynamics in a right internal carotid artery with a saccular aneurysm. Int. J. Numer. Methods Fluids 2006, 50, 751–764. [Google Scholar] [CrossRef]
- Khan, M.O.; Steinman, D.A.; Valen-Sendstad, K. Non-Newtonian versus numerical rheology: Practical impact of shear-thinning on the prediction of stable and unstable flows in intracranial aneurysms. Int. J. Numer. Methods Biomed. Eng. 2017, 33, 1–10. [Google Scholar] [CrossRef]
- Evju, Ø.; Valen-Sendstad, K.; Mardal, K.A. A study of wall shear stress in 12 aneurysms with respect to different viscosity models and flow conditions. J. Biomech. 2013, 46, 2802–2808. [Google Scholar] [CrossRef] [PubMed]


| Rp (dyne·s/cm5) | C (cm5/dyne) | Rd (dyne·s/cm5) | |
|---|---|---|---|
| Left Middle Cerebral Artery (LMCA) | 4168.21 | 6.50368 × 10−6 | 41,682.1 |
| Right Middle Cerebral Artery (RMCA) | 3989.86 | 6.7944 × 10−6 | 39,898.6 |
| Left Anterior Cerebral Artery (LACA) | 6304.05 | 4.30021 × 10−6 | 63,040.5 |
| Right Anterior Cerebral Artery (RACA) | 5891.02 | 4.6017 × 10−6 | 58,910.2 |
| Study | Volumetric Flow Rate (mL/s) | |||
|---|---|---|---|---|
| LACA | RACA | LMCA | RMCA | |
| Current Study | 1.81 | 1.93 | 2.85 | 2.79 |
| Zhu et al. [24] | 1.39 | 1.39 | 2.78 | 2.78 |
| Zhu et al. [25] | 1.34 | 1.34 | 3.26 | 3.26 |
| Enzmann et al. [30] | 1.25 ± 0.17 | 1.47 ± 0.18 | 1.80 ± 0.12 | 2.12 ± 0.12 |
| Zhao et al. [31] | 1.42 ± 0.43 | 1.33 ± 0.47 | 2.5 ± 0.52 | 2.42 ± 0.45 |
| Cieslicki et al. [32] | 1.54 ± 0.03 | 1.56 ± 0.03 | 2.44 ± 0.05 | 2.41 ± 0.05 |
| Zarrinkoob et al. [33] | 1.54 ± 0.56 | 1.68 ± 0.56 | 2.94 ± 0.42 | 2.94 ± 0.28 |
| Cases | Area (mm2) | |
|---|---|---|
| Low TAWSS | High TAWSS | |
| Severe stenosis | 50 | 65 |
| Moderate stenosis | 72 | 28 |
| Mild stenosis | 69 | 18 |
| Non-stenotic | 66 | 21 |
| TAWSS Magnitude/Pa | ||||
|---|---|---|---|---|
| Severe Stenosis | Moderate Stenosis | Mild Stenosis | Non-Stenotic | |
| Space-averaged | 7.02 | 4.03 | 3.57 | 3.63 |
| Maximum | 77.77 | 40.91 | 24.27 | 25.34 |
| Severe Stenosis | Moderate Stenosis | Mild Stenosis | Non-Stenotic |
|---|---|---|---|
| 0.020 | 0.035 | 0.059 | 0.057 |
| RRT/Pa−1 | ||||
|---|---|---|---|---|
| Severe Stenosis | Moderate Stenosis | Mild Stenosis | Non-Stenotic | |
| Maximum | 28.63 | 58.94 | 70.52 | 184.82 |
| Space-averaged | 0.39 | 0.67 | 0.83 | 1.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.-Y.; Wei, Y.; Su, Y.-L.; Yuan, Q.; Yang, C.-F. Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: A Preliminary Multiscale Numerical Investigation. Appl. Sci. 2019, 9, 4143. https://doi.org/10.3390/app9194143
Zhu G-Y, Wei Y, Su Y-L, Yuan Q, Yang C-F. Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: A Preliminary Multiscale Numerical Investigation. Applied Sciences. 2019; 9(19):4143. https://doi.org/10.3390/app9194143
Chicago/Turabian StyleZhu, Guang-Yu, Yuan Wei, Ya-Li Su, Qi Yuan, and Cheng-Fu Yang. 2019. "Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: A Preliminary Multiscale Numerical Investigation" Applied Sciences 9, no. 19: 4143. https://doi.org/10.3390/app9194143
APA StyleZhu, G.-Y., Wei, Y., Su, Y.-L., Yuan, Q., & Yang, C.-F. (2019). Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: A Preliminary Multiscale Numerical Investigation. Applied Sciences, 9(19), 4143. https://doi.org/10.3390/app9194143