Stomatal Response of Maize (Zea mays L.) to Crude Oil Contamination in Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Seed of Maize and Soil
2.2. Experimental Design and Management
2.3. Analytical Methods
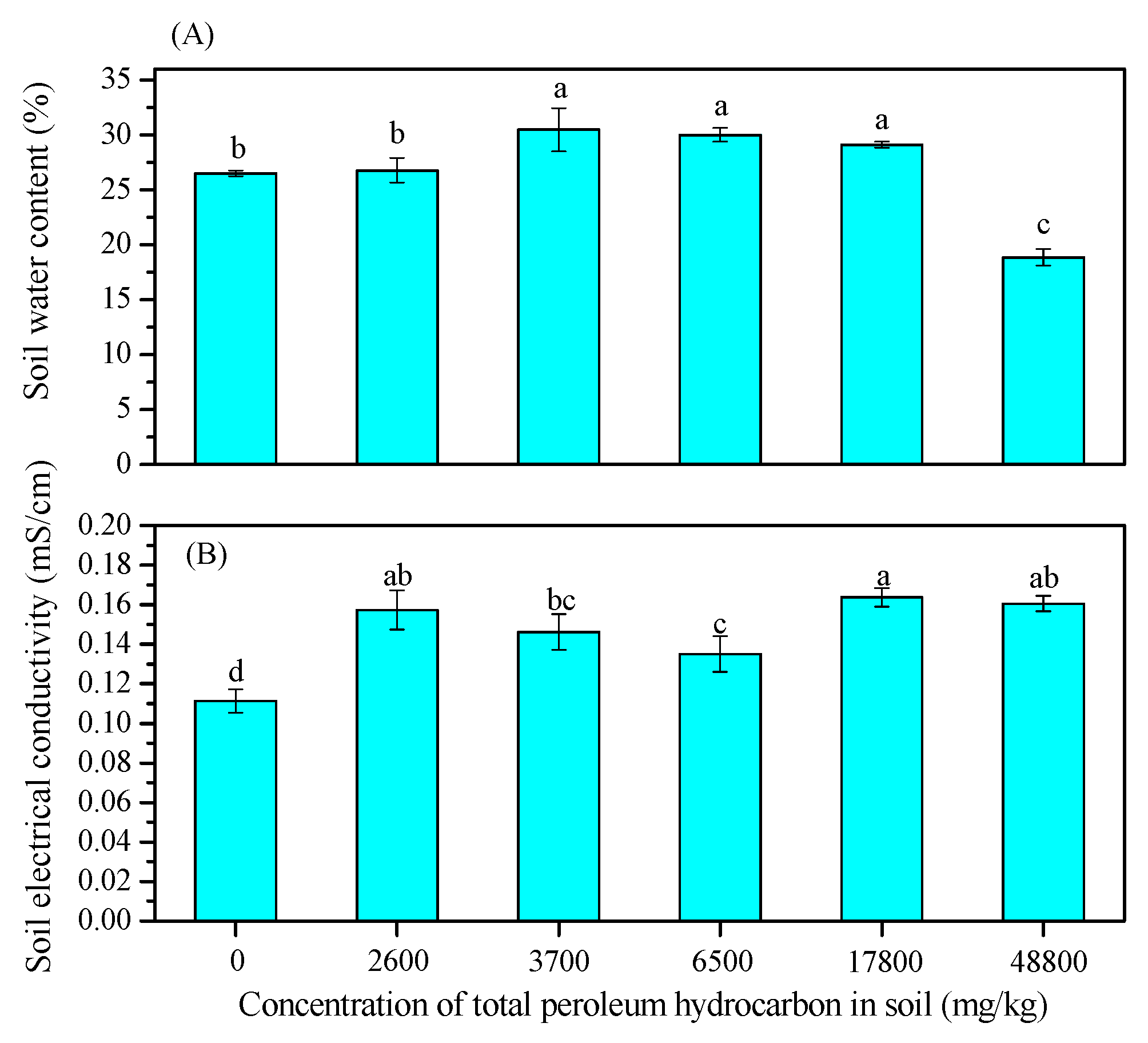
2.3.1. Soil Water Content and Soil Electrical Conductivity
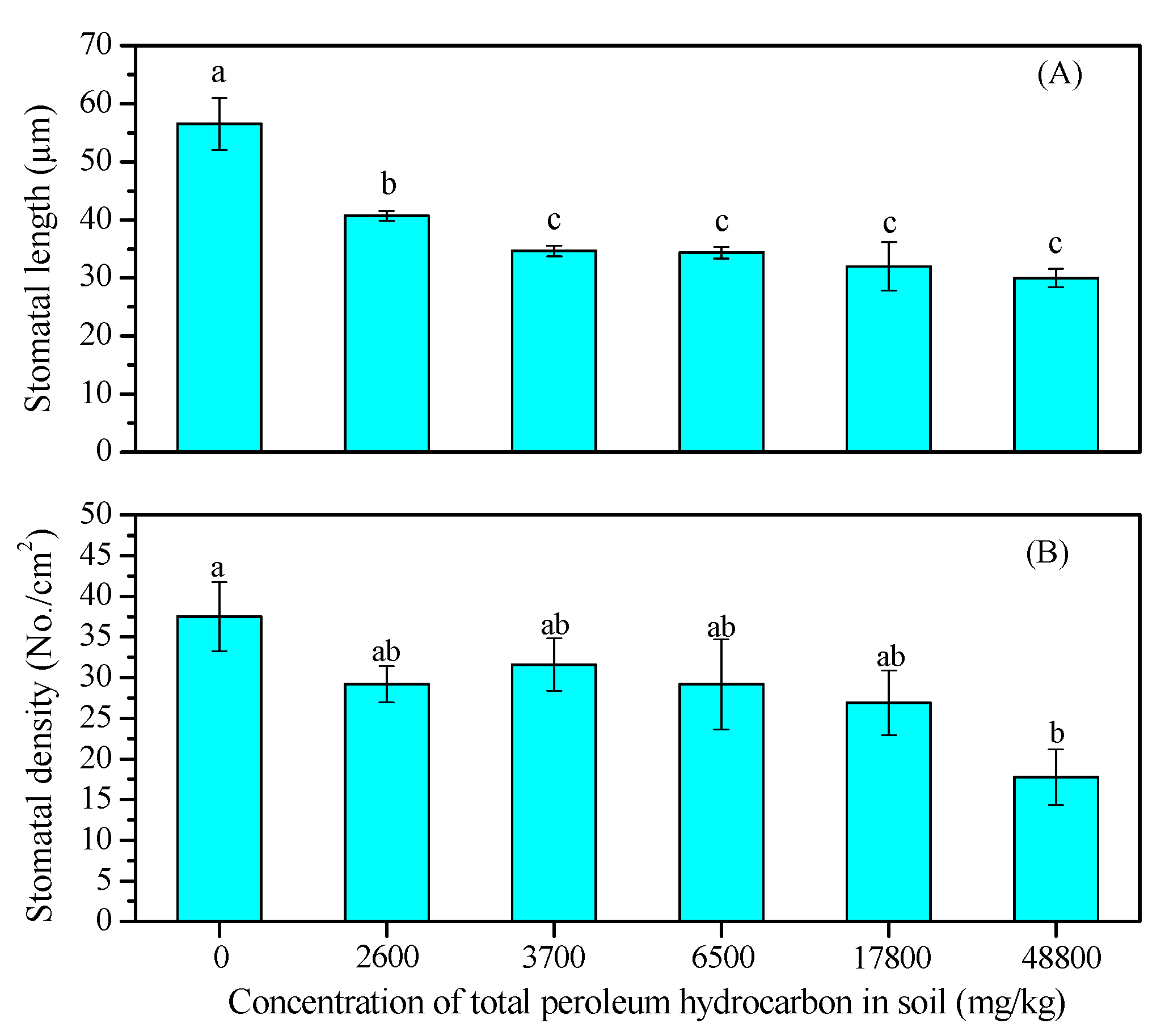
2.3.2. Determination of Stomatal Traits
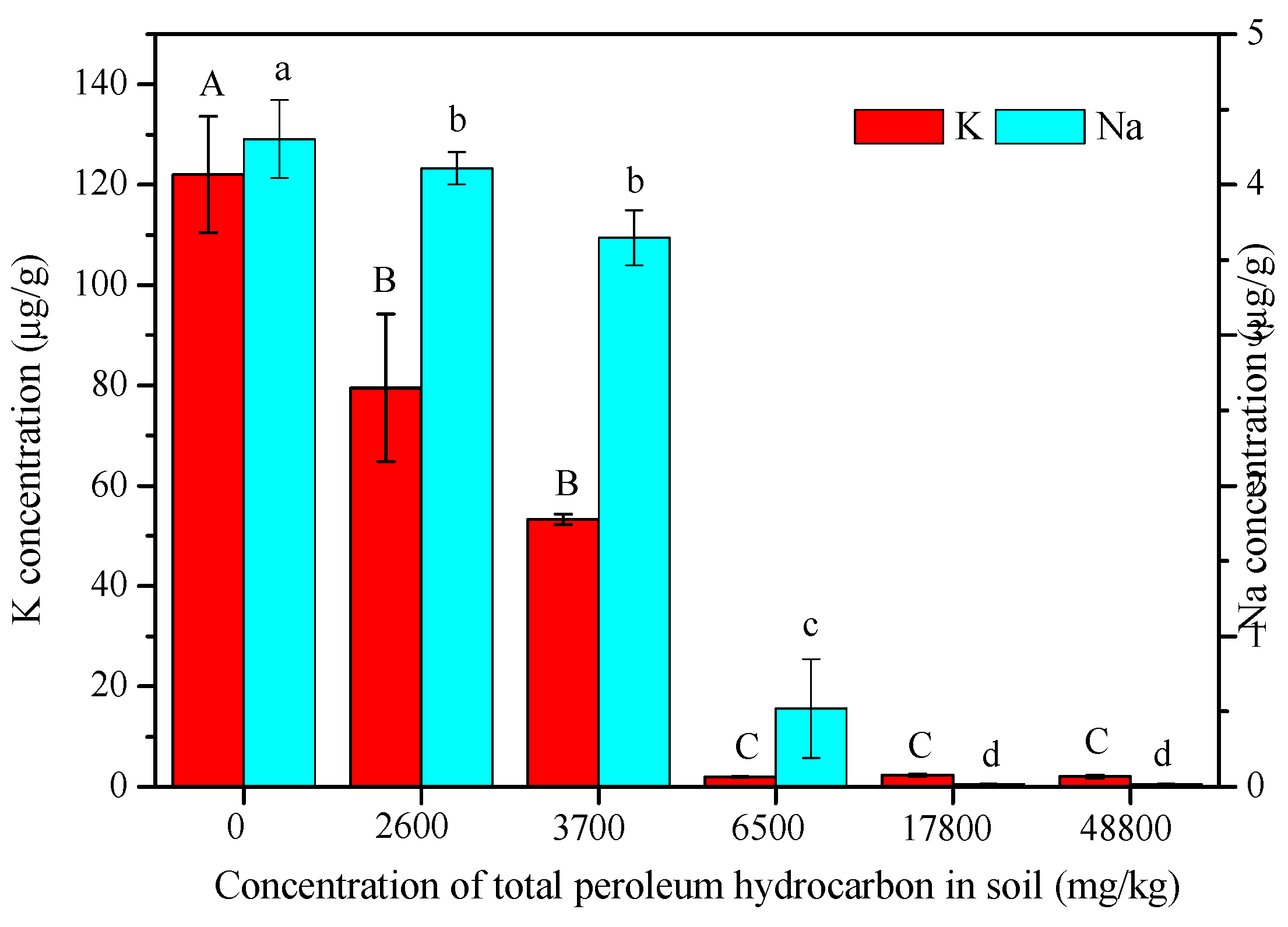
2.3.3. Determination of K and Na Concentration in Maize Leaves
2.3.4. Determination of PHE in Maize Leaves
2.4. Statistical Analysis
3. Results
3.1. Changes in Soil Properties
3.2. Leaf Growth and Stomatal Density and Length
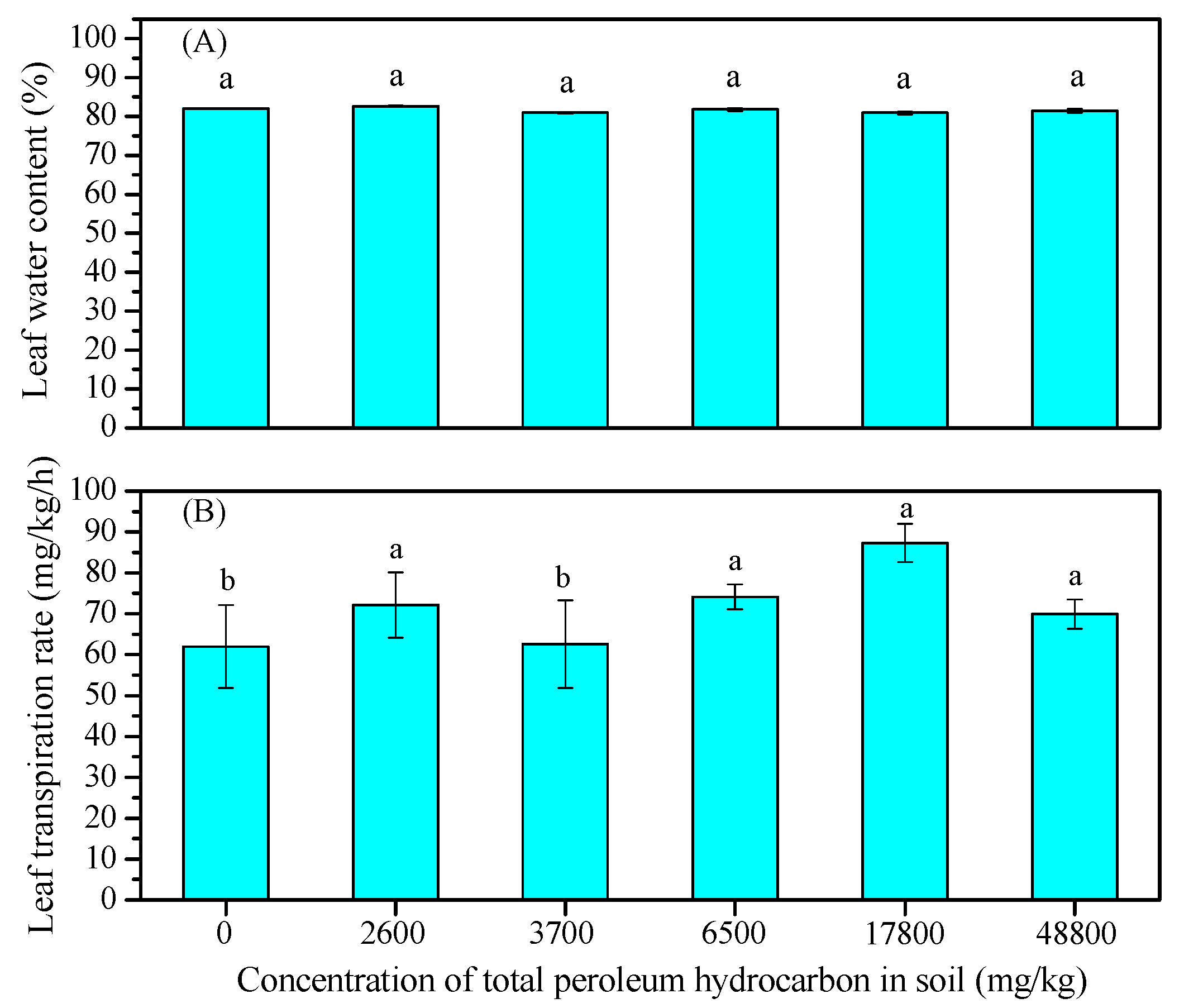
3.3. Water Content and Transpiration Rate
3.4. Concentrations of K and Na in Maize Leaf
3.5. Phenanthrene Concentration in Maize Leaf
3.6. Relationship
4. Discussion
4.1. Soil Properties in Crude Oil Contaminated Soil
4.2. Stomata and Other Leaf Parameters
4.3. PHE Uptake and Stomata
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klamerus-Iwan, A.; Błońska, E.; Lasota, J.; Kalandyk, A.; Waligórski, P. Influence of oil contamination on physical and biological properties of forest soil after chainsaw use. Water Air Soil Poll. 2015, 226, 389. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Moore, P.S.; Ennas, M.G.; Tocco, M.G.; Ibba, A.; Mattuzzi, S.; Meloni, M.; Monne, M.; Piras, G.; Collu, S. Effect of urban traffic, individual habits, and genetic polymorphisms on background urinary 1-hydroxypyrene excretion. Ann. Epidemiol. 2007, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Desalme, D.; Binet, P.; Chiapusio, G. Challenges in Tracing the Fate and Effects of Atmospheric Polycyclic Aromatic Hydrocarbon Deposition in Vascular Plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, J.; Zhao, J.M. Effects of crude oil residuals on soil chemical properties in oil sites, Momoge Wetland, China. Environ. Monit. Assess. 2010, 161, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kyunghwa, B.; Heesik, K.; Heemock, O.; Byungdae, Y.; Jaisoo, K.; Insook, L. Effects of crude oil, oil components, and bioremediation on plant growth. J. Environ. Sci. Health Part A 2004, 39, 2465–2472. [Google Scholar]
- Kisic, I.; Mesic, S.; Basic, F.; Brkic, V.; Mesic, M.; Durn, G.; Zgorelec, Z.; Bertovic, L. The effect of drilling fluids and crude oil on some chemical characteristics of soil and crops. Geoderma 2009, 149, 209–216. [Google Scholar] [CrossRef]
- Merkl, N.; Schultzekraft, R.; Infante, C. Assessment of tropical grasses and legumes for phytoremediation of petroleum-contaminated soils. Water Air Soil Pollut. 2005, 165, 195–209. [Google Scholar] [CrossRef]
- Frei, E.R.; Ghazoul, J.; Matter, P.; Heggli, M.; Pluess, A.R. Plant population differentiation and climate change: Responses of grassland species along an elevational gradient. Glob. Chang. Biol. 2014, 20, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Baruah, P.; Saikia, R.R.; Baruah, P.P.; Deka, S. Effect of crude oil contamination on the chlorophyll content and morpho-anatomy of Cyperus brevifolius (Rottb.) Hassk. Environ. Sci. Pollut. Res. 2014, 21, 12530. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniyam, A.; Harvey, P.J. Scanning electron microscopic investigations of root structural modifications arising from growth in crude oil-contaminated sand. Environ. Sci. Pollut. Res. 2014, 21, 12651–12661. [Google Scholar] [CrossRef] [PubMed]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.Q.; Zhang, S.Z.; Zhu, Y.G.; Christie, P. Uptake and acropetal translocation of polycyclic aromatic hydrocarbons by wheat (Triticum aestivum L.) grown in field-contaminated soil. Environ. Sci. Technol. 2009, 43, 3556–3560. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Fryer, M.; Grosso, A. Plant uptake of non ionic organic chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Buckley, T.N.; Mott, K.A. Modelling stomatal conductance in response to environmental factors. Plant Cell Environ. 2013, 36, 1691. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Hardie, W.J.; Smith, J.P. Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Aust. J. Grape Wine Res. 2011, 17, 147–152. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Laclau, J.-P.; Beri, C.; Mietton, L.; Almeida Muniz, M.R.; Arenque, B.C.; Piccolo, M.d.C.; Jordan-Meille, L.; Bouillet, J.-P.; Nouvellon, Y. Photosynthetic and anatomical responses of Eucalyptus grandis leaves to potassium and sodium supply in a field experiment. Plant Cell Environ. 2014, 37, 70–81. [Google Scholar] [CrossRef]
- Ahmad, S.H.; Reshi, Z.; Ahmad, J.; Iqbal, M. Morpho-anatomical responses of Trigonella foenum graecum Linn. to induced cadmium and lead stress. J. Plant Biol. 2005, 48, 64–84. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Shahsavari, E.; Adetutu, E.M.; Anderson, P.A.; Ball, A.S. Tolerance of selected plant species to petrogenic hydrocarbons and effect of plant rhizosphere on the microbial removal of hydrocarbons in contaminated soil. Water Air Soil Poll. 2013, 224, 1–14. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Ziółkowska, A. Content of polycyclic aromatic hydrocarbons in soils polluted with petrol and diesel oil after remediationwith plants and various substances. Plant Soil Environ. 2013, 59, 287–294. [Google Scholar] [CrossRef]
- Liao, C.; Xu, W.; Lu, G.; Deng, F.; Liang, X.; Guo, C.; Dang, Z. Biosurfactant-enhanced phytoremediation of soils contaminated by crude oil using maize (Zea mays. L). Ecol. Eng. 2016, 92, 10–17. [Google Scholar] [CrossRef]
- Liao, C.; Xu, W.; Lu, G.; Liang, X.; Guo, C.; Yang, C.; Dang, Z. Accumulation of hydrocarbons by maize (Zea mays L.) in remediation of soils contaminated with crude oil. Int. J. Phytoremediat. 2015, 17, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, B.; Zheng, X.; Liu, G. Plant biomass, soil water content and soil N:P ratio regulating soil microbial functional diversity in a temperate steppe: A regional scale study. Soil Biol. Biochem. 2010, 42, 445–450. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, M.; Hou, R.; Shen, R.; Qiu, S.; Ouyang, Z. Effects of experimental warming on stomatal traits in leaves of maize (Zea may L.). Ecol. Evol. 2013, 3, 3095–3111. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, N.; Çakirlar, H. The effect of salinity on some physiological parameters in two maize cultivars. Bulg. J. Plant Physiol. 2002, 28, 66–74. [Google Scholar]
- Tao, S.; Jiao, X.C.; Chen, S.H.; Liu, W.X.; Jr, C.R.; Zhu, L.Z.; Luo, Y.M. Accumulation and distribution of polycyclic aromatic hydrocarbons in rice (Oryza sativa). Environ. Pollut. 2006, 140, 406–415. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, M.; Liu, H.Y. Micro-Column convenient chromatography for separation of aromatic hydrocarbon compound and Gc/Irms analysis. Pet. Geol. Exp. 2013, 35, 347–350. (In Chinese) [Google Scholar]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Hudson, B. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Aal, G.Z.A.; Slater, L.D.; Atekwana, E.A. Induced-polarization measurements on unconsolidated sediments from a site of active hydrocarbon biodegradation. Geophysics 2006, 71, H13–H24. [Google Scholar] [CrossRef]
- Pal, A.; Kulshreshtha, K.; Ahmad, K.J.; Behl, H.M. Do leaf surface characters play a role in plant resistance to auto-exhaust pollution? Flora-Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 47–55. [Google Scholar] [CrossRef]
- Rai, V.; Khatoon, S.; Bisht, S.S.; Mehrotra, S. Effect of cadmium on growth, ultramorphology of leaf and secondary metabolites of Phyllanthus amarus Schum. and Thonn. Chemosphere 2005, 61, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.; Oliver, J.; Casson, S.; Gray, J.E. Putting the brakes on: Abscisic acid as a central environmental regulator of stomatal development. New Phytol. 2014, 202, 376–391. [Google Scholar] [CrossRef]
- Rai, M.K.; Shekhawat, N.S.; Gupta, A.K.; Phulwaria, M.; Ram, K.; Jaiswal, U. The role of abscisic acid in plant tissue culture: A review of recent progress. Plant Cell Tissue Organ Cult. 2011, 106, 179–190. [Google Scholar] [CrossRef]
- Serna, L. The role of brassinosteroids and abscisic acid in stomatal development. Plant Sci. 2014, 225, 95–101. [Google Scholar] [CrossRef]
- Váňová, L.; Kummerová, M.; Klemš, M.; Zezulka, Š. Fluoranthene influences endogenous abscisic acid level and primary photosynthetic processes in pea (Pisum sativum L.) plants in vitro. Plant Growth Regul. 2009, 57, 39–47. [Google Scholar] [CrossRef]
- Outlaw, W.H., Jr. Current concepts on the role of potassium in stomatal movements. Physiol. Plant. 2010, 59, 302–311. [Google Scholar] [CrossRef]
- Verónica, B.; Leidi, E.O.; Zaida, A.; Lourdes, R.; Anna, D.L.; Fernández, J.A.; Beatriz, C.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Lin, H.; Tao, S.; Zuo, Q.; Coveney, R.M. Uptake of polycyclic aromatic hydrocarbons by maize plants. Environ. Pollut. 2007, 148, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Vegetation-atmosphere partitioning of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 1994, 28, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Adachi, Y.; Ye, W.; Hayashi, M.; Nakamura, Y.; Kinoshita, T.; Mori, I.C.; Murata, Y. Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiol. 2013, 163, 600–610. [Google Scholar] [CrossRef] [PubMed]
- She, X.P.; Huang, A.X.; Li, J.; Han, X.Z. Inhibition of dark-induced stomatal closure by fusicoccin involves a removal of hydrogen peroxide in guard cells of Vicia faba. Physiol. Plant. 2010, 140, 258–268. [Google Scholar] [CrossRef] [PubMed]

| TPH | SW | EC | LW | TR | SL | SD | K | Na | PHE | |
|---|---|---|---|---|---|---|---|---|---|---|
| TPH | 1.00 | |||||||||
| SW | −0.82 * | 1.00 | ||||||||
| EC | 0.55 | −0.22 | 1.00 | |||||||
| LW | -0.10 | −0.25 | 0.23 | 1.00 | ||||||
| TR | 0.27 | 0.10 | 0.65 | −0.28 | 1.00 | |||||
| SL | −0.59 | 0.10 | −0.83 * | 0.05 | −0.55 | 1.00 | ||||
| SD | −0.91 * | 0.65 | −0.78 | −0.10 | −0.46 | 0.81 | 1.00 | |||
| K | −0.62 | 0.11 | −0.66 | 0.30 | −0.67 | 0.92 ** | 0.79 | 1.00 | ||
| Na | −0.71 | 0.27 | −0.54 | 0.44 | −0.72 | 0.75 | 0.78 | 0.94 ** | 1.00 | |
| PHE | −0.59 | 0.09 | −0.71 | 0.11 | −0.40 | 0.98 ** | 0.76 | 0.89 * | 0.72 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Huang, H.; Zhou, Y.; Lin, H.; Xie, T.; Liao, C. Stomatal Response of Maize (Zea mays L.) to Crude Oil Contamination in Soils. Appl. Sci. 2019, 9, 4074. https://doi.org/10.3390/app9194074
Zhang C, Huang H, Zhou Y, Lin H, Xie T, Liao C. Stomatal Response of Maize (Zea mays L.) to Crude Oil Contamination in Soils. Applied Sciences. 2019; 9(19):4074. https://doi.org/10.3390/app9194074
Chicago/Turabian StyleZhang, Chaolan, He Huang, Yongxin Zhou, Haiying Lin, Tian Xie, and Changjun Liao. 2019. "Stomatal Response of Maize (Zea mays L.) to Crude Oil Contamination in Soils" Applied Sciences 9, no. 19: 4074. https://doi.org/10.3390/app9194074
APA StyleZhang, C., Huang, H., Zhou, Y., Lin, H., Xie, T., & Liao, C. (2019). Stomatal Response of Maize (Zea mays L.) to Crude Oil Contamination in Soils. Applied Sciences, 9(19), 4074. https://doi.org/10.3390/app9194074




