Physiological Driver Monitoring Using Capacitively Coupled and Radar Sensors
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
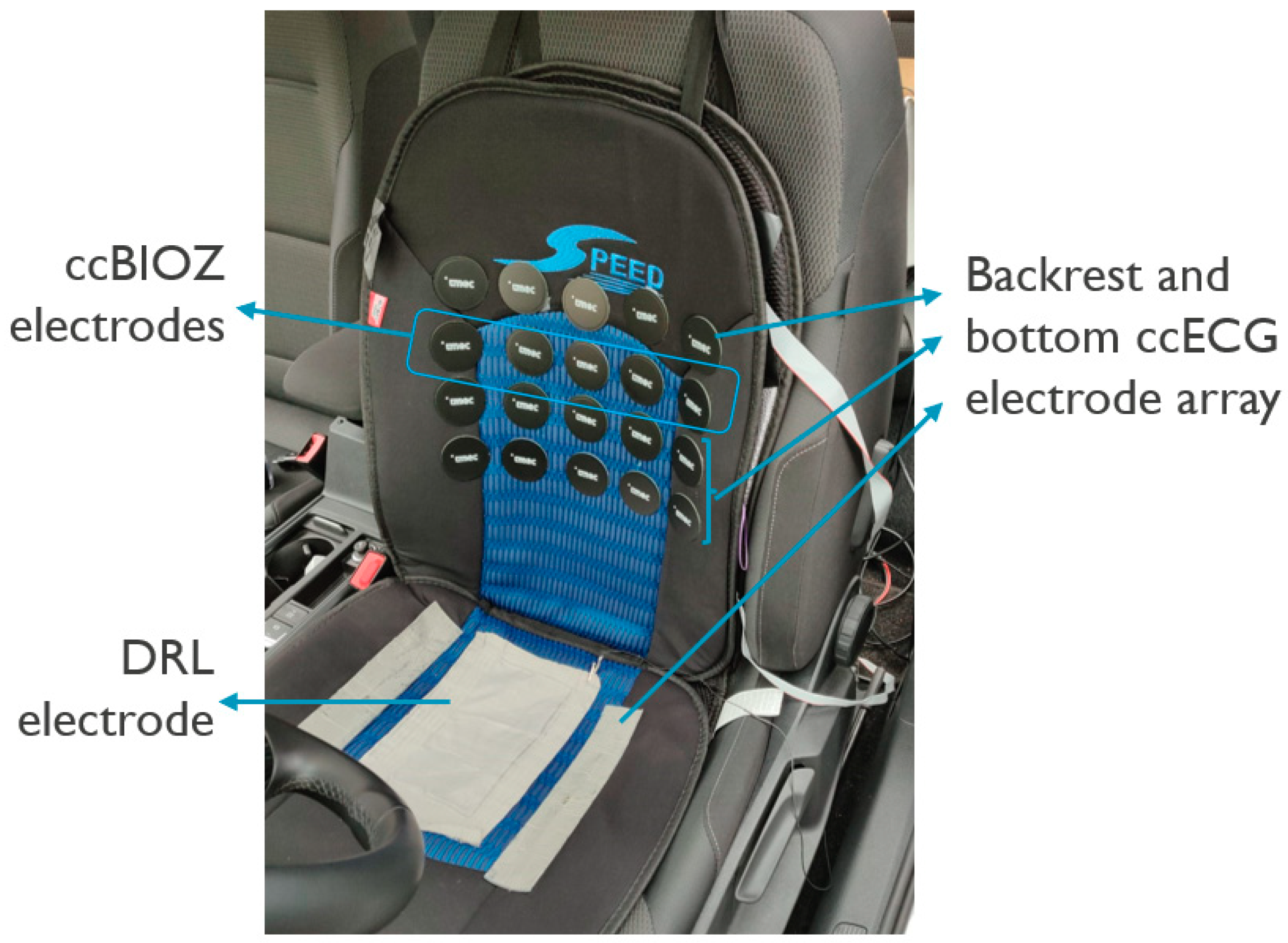
2.1. Capacitively-Coupled ECG and Bio-Impedance System and Positioning in the Car Seat
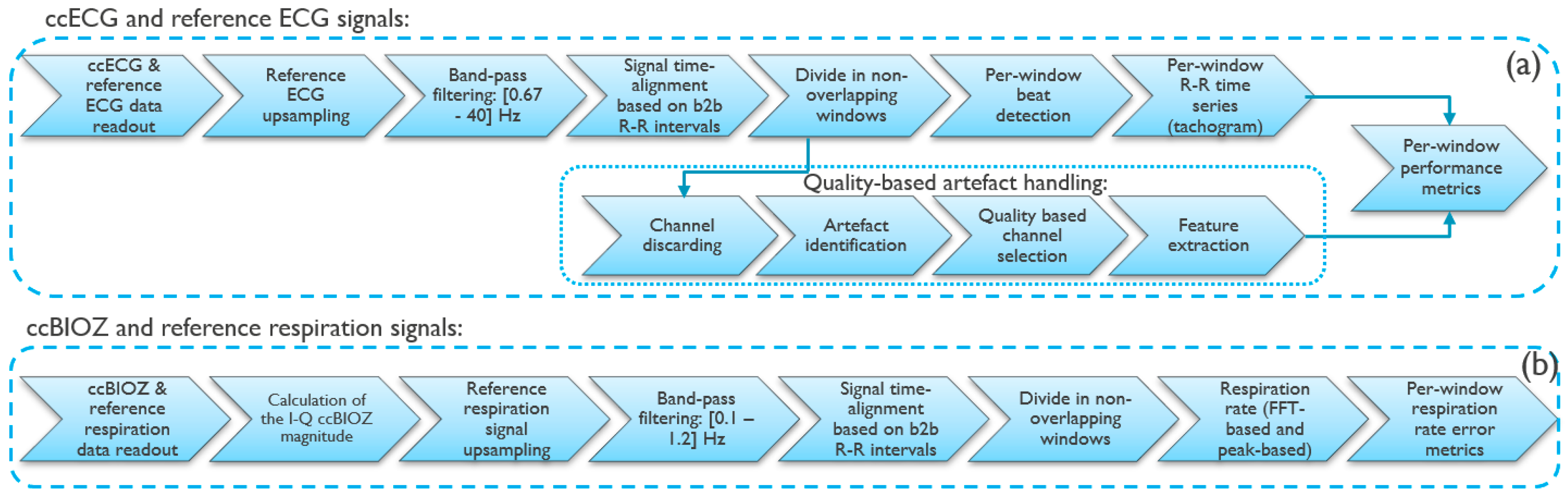
2.2. Signal Processing on Capacitively-Coupled Signals
- A method to discard from the set of 4 ECG channels the channels with no potential valuable information. This uses a classifier described in more detail in [28];
- The identification of artefacts using a template-based signal quality metric previously evaluated by the authors [29], in combination with an amplitude-based metric calculated as the percentage of 0.5-s sub-windows that have data within [0.05–3] mV range;
- The selection of the channel. This selection uses the same signal quality metric as in step 2;
- The extraction of features from the signal parts with useful information. The parts of the signal that have been identified as containing artefacts in step 2 are omitted from the feature calculation.
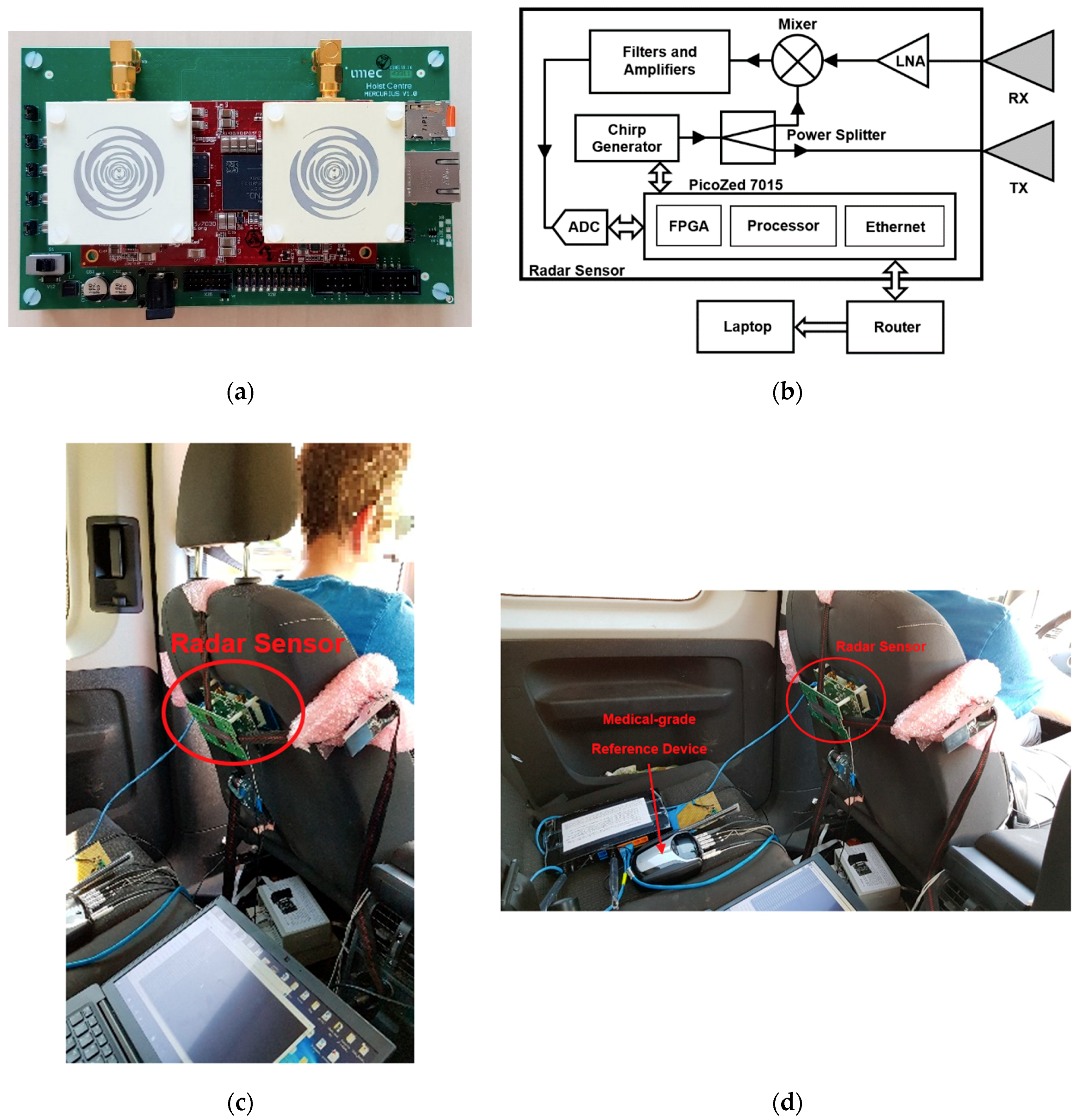
2.3. Radar Sensor System and Positioning in the Car Seat
2.4. Signal Processing on Radar Signals
3. Results
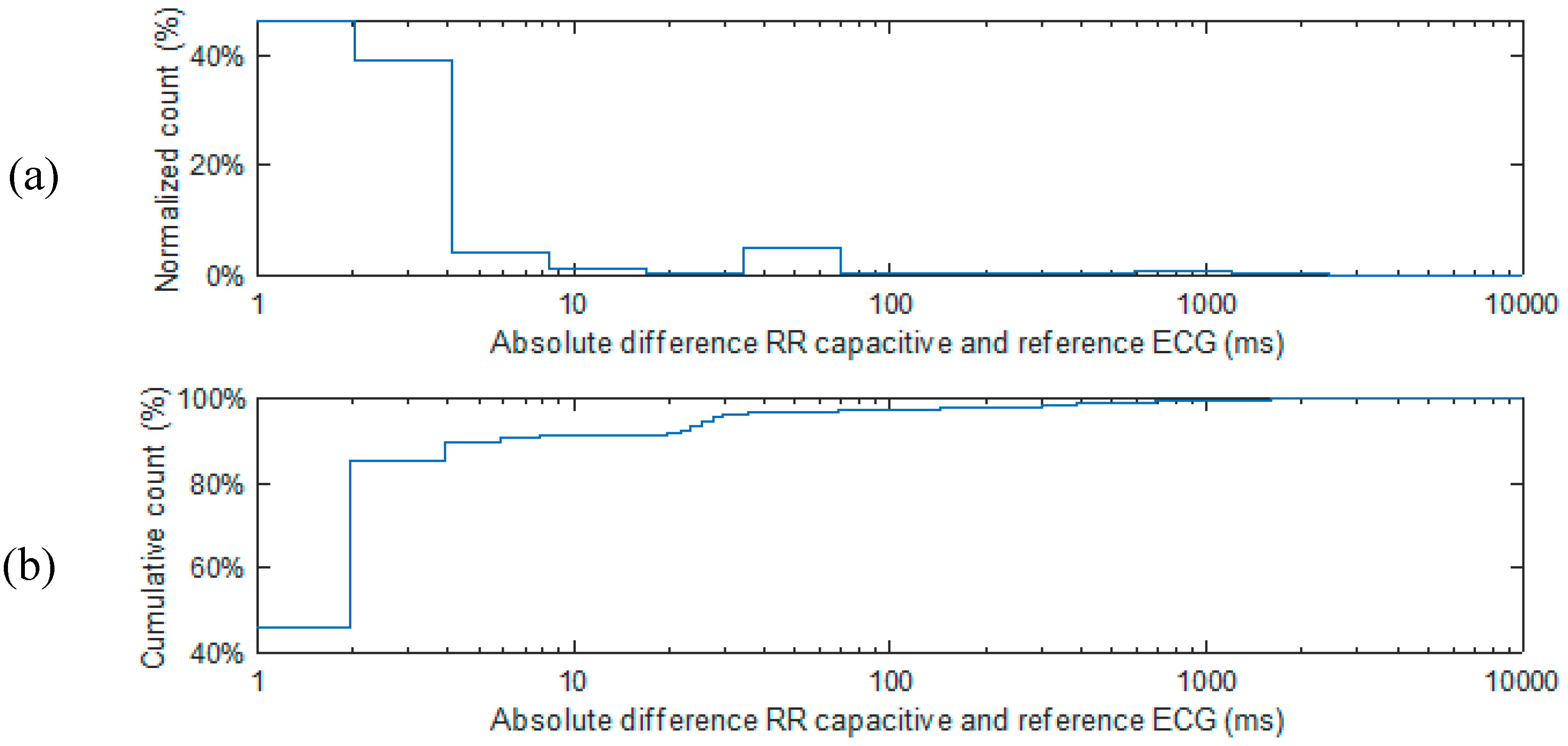
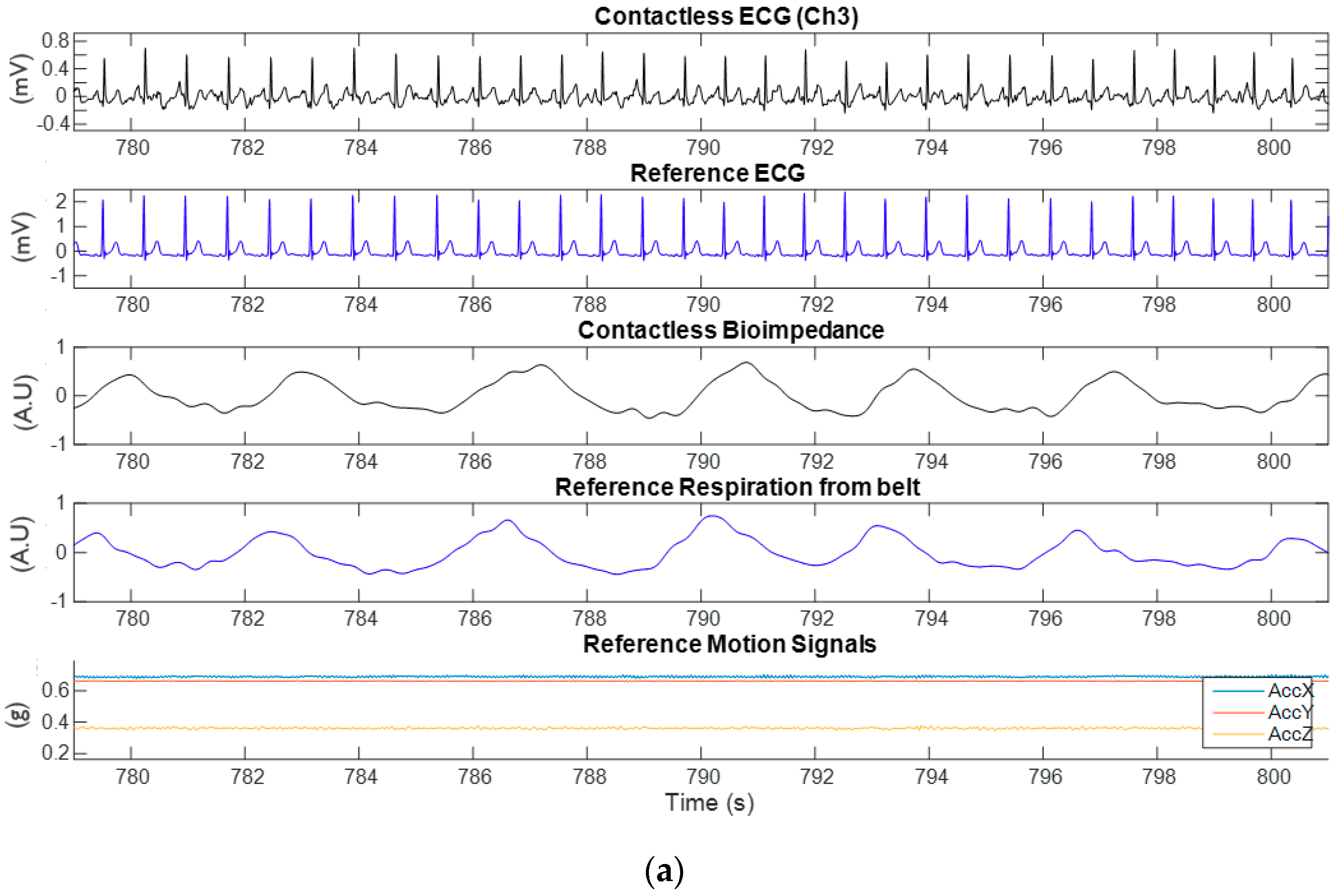
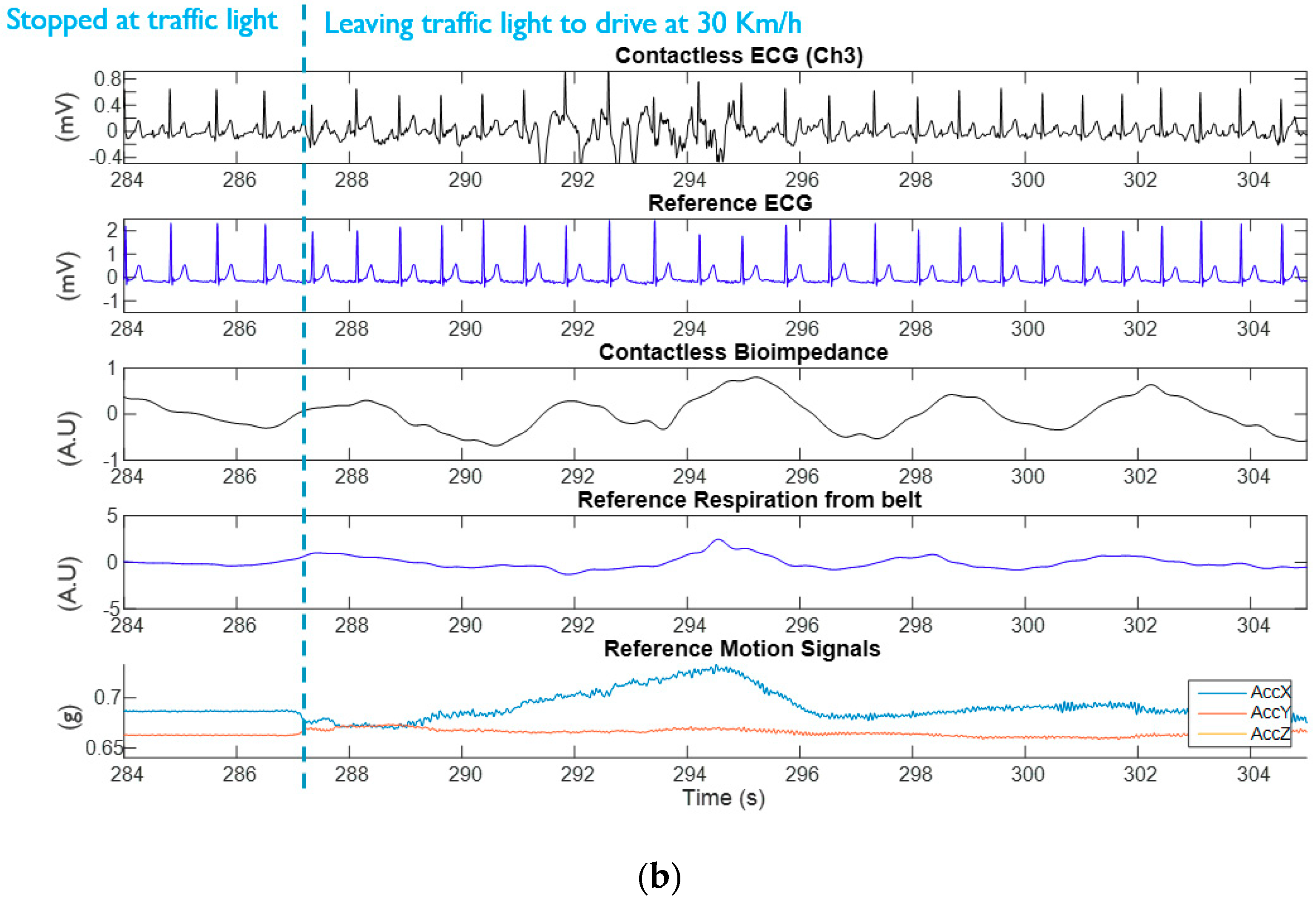
3.1. Experimental Results from Capacitively-Coupled ECG and Bio-Impedance
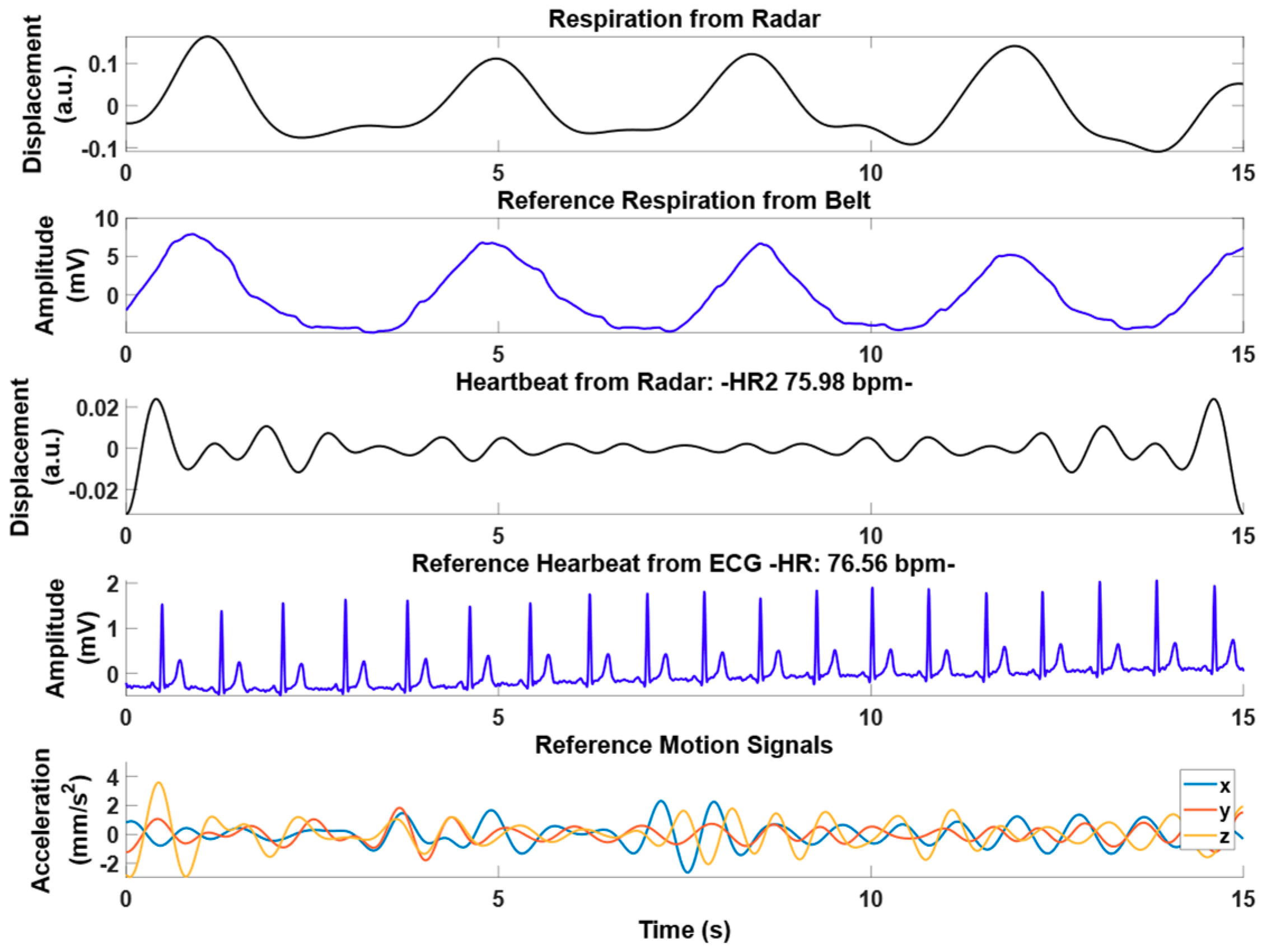
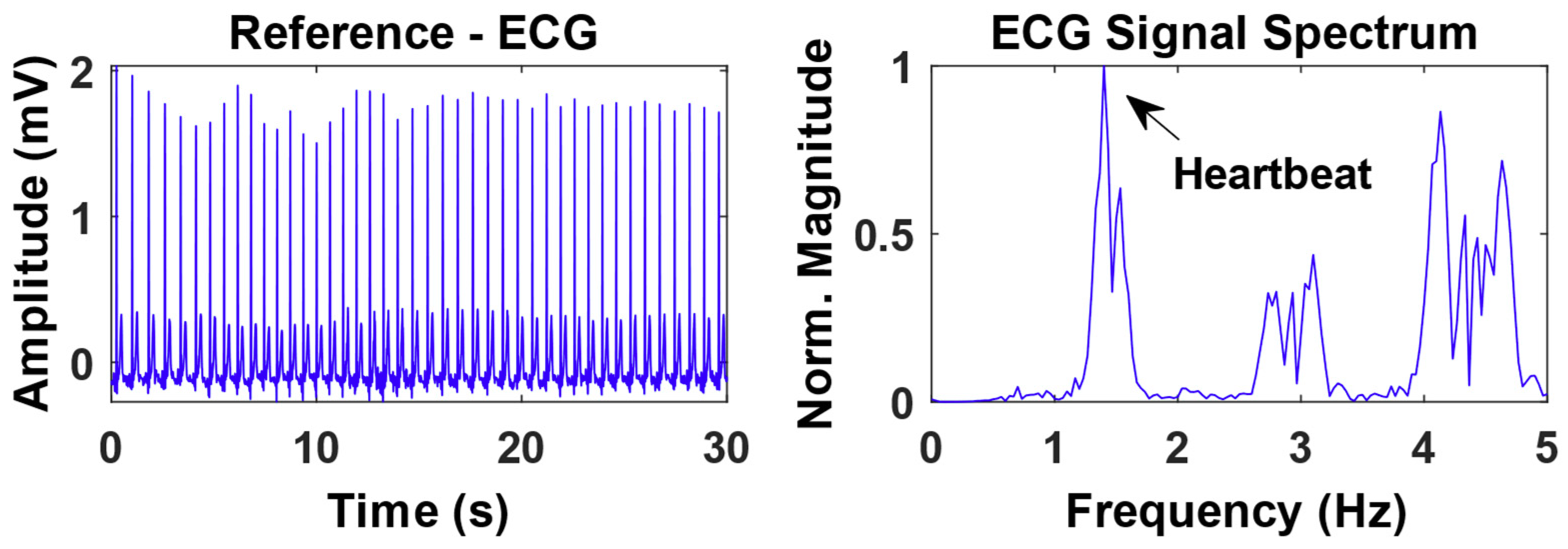
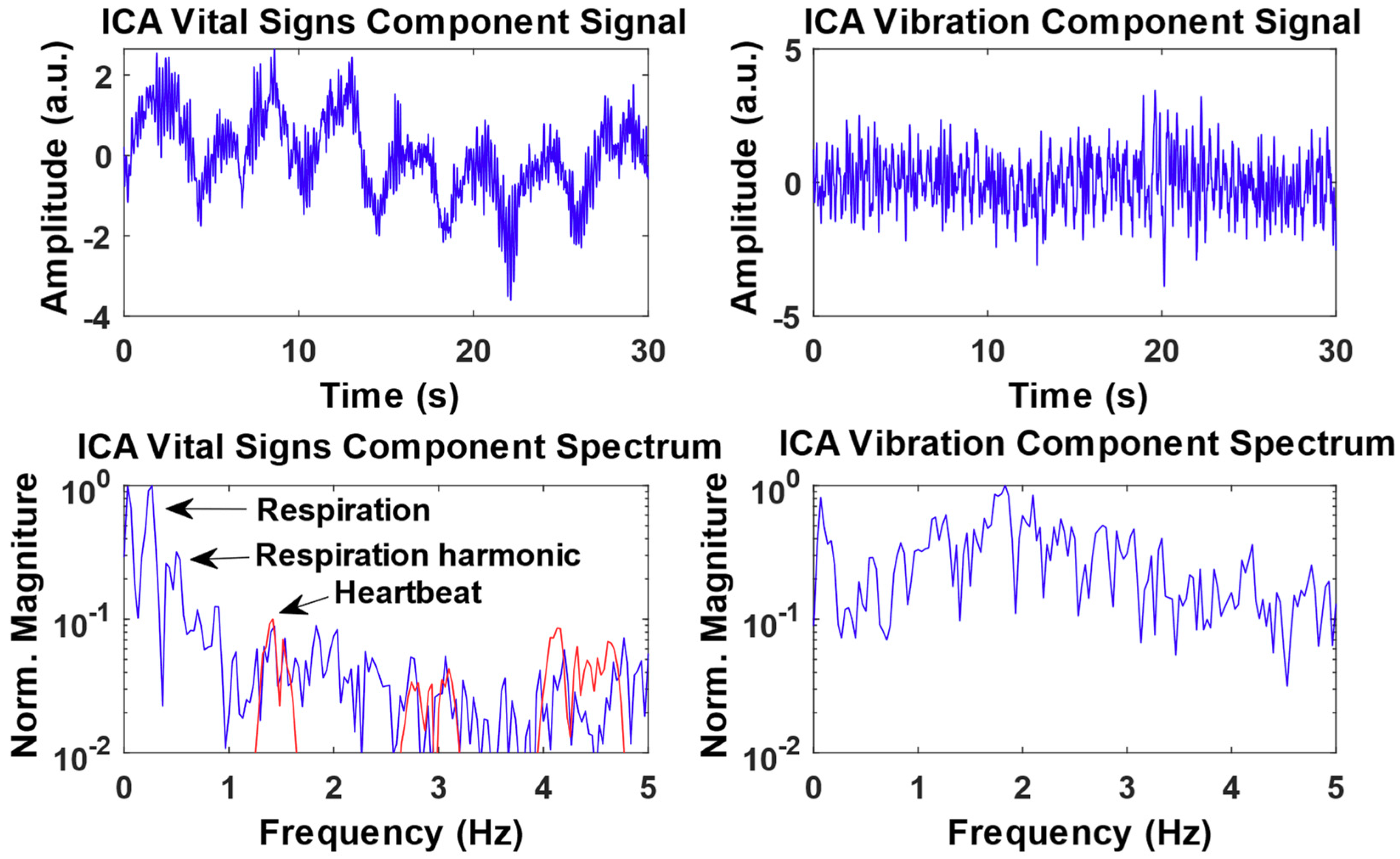
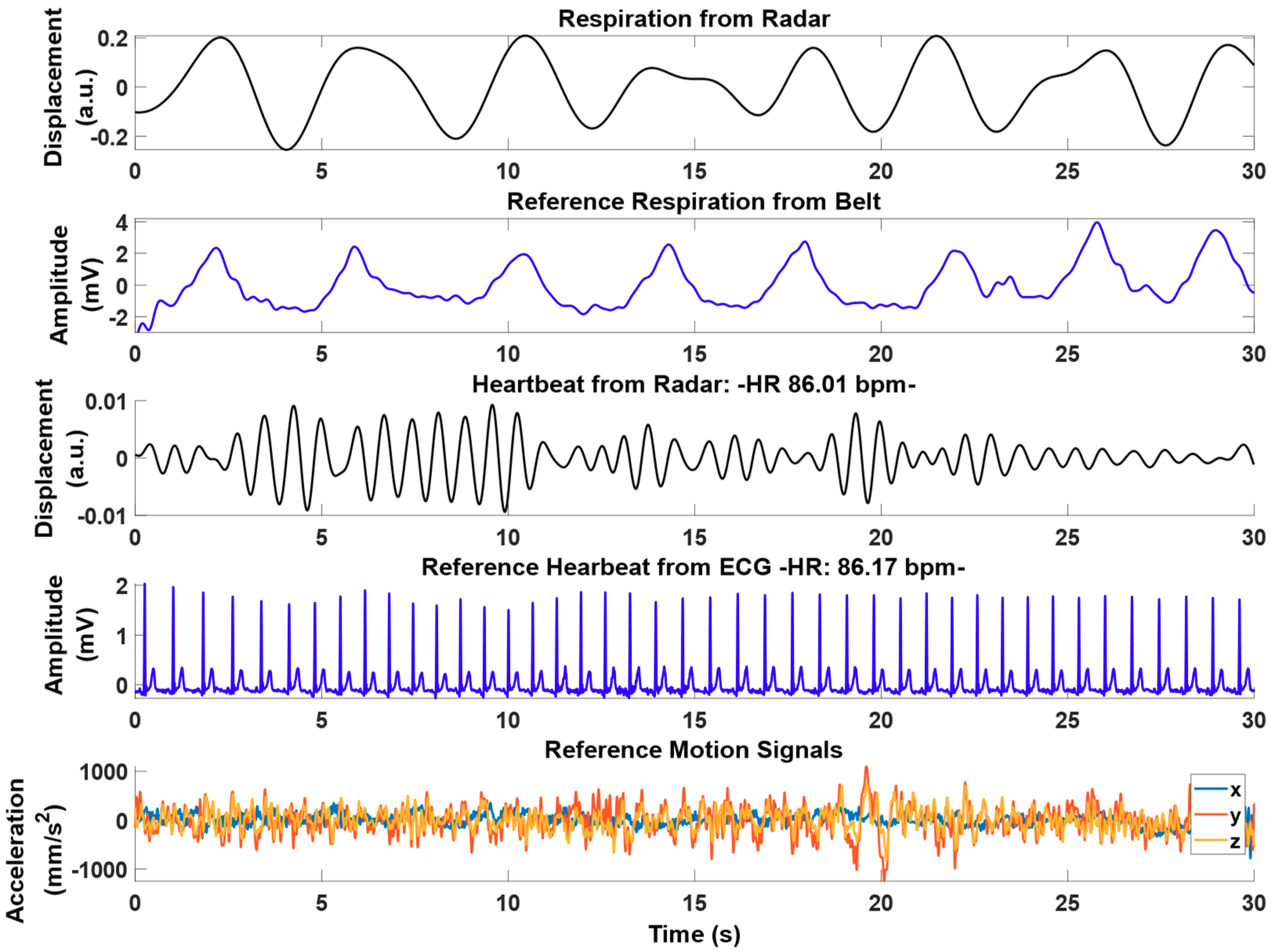
3.2. Experimental Results from Radar
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vicente, J.; Laguna, P.; Bartra, A.; Bailón, R. Drowsiness detection using heart rate variability. Med. Biol. Eng. Comput. 2016, 54, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Taelman, J.; Vandeput, S.; Spaepen, A.; van Huffel, S. Influence of Mental Stress on Heart Rate and Heart Rate Variability. IFMBE Proc. 2009, 22, 1366–1369. [Google Scholar]
- Valderas, M.T.; Bolea, J.; Laguna, P.; Vallverdú, M.; Bailón, R. Human emotion recognition using heart rate variability analysis with spectral bands based on respiration. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6134–6137. [Google Scholar]
- Vandecasteele, K.; De Cooman, T.; Gu, Y.; Cleeren, E.; Claes, K.; Paesschen, W.V.; Huffel, S.V.; Hunyadi, B. Automated epileptic seizure detection based on wearable ECG and PPG in a hospital environment. Sensors 2017, 17, 2338. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, G.; Fanucchi, S.; Strata, G.; Pataleo, L.; Landucci Pellegrini, L.; Prontera, C.; Martini, A.; Murri, L. Transient autonomic nervous system dysfunction during hyperacute stroke. Acta Neurol. Scand. 2000, 102, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Glob. Heart 2012, 7, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Pacela, A.F. Impedance pneumography—A survey of instrumentation techniques. Med. Biol. Eng. 1966, 4, 1–15. [Google Scholar] [CrossRef]
- Castro, I.D.; Morariu, R.; Torfs, T.; van Hoof, C.; Puers, R. Robust wireless capacitive ECG system with adaptive signal quality and motion artifact reduction. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar]
- Obeid, D.; Zaharia, G.; Sadek, S.; Zein, G. Microwave doppler radar for heartbeat detection vs electrocardiogram. Microw. Opt. Technol. Lett. 2012, 54, 2610–2617. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Peng, Z.; Huang, T.; Fan, T.; Wang, F.; Horng, T.; Muñoz-Ferreras, J.; Gómez-García, R.; Ran, L.; Lin, J. A Review on recent progress of portable short range noncontact microwave radar systems. IEEE Trans. Microw. Theory Tech. 2017, 65, 1692–1706. [Google Scholar] [CrossRef]
- Mercuri, M.; Soh, P.J.; Pandey, G.; Karsmakers, P.; Vandenbosch, G.A.E.; Leroux, P.; Schreurs, D. Analysis of an indoor biomedical radar based system for health monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2061–2068. [Google Scholar] [CrossRef]
- Wang, F.K.; Chou, Y.R.; Chiu, Y.C.; Horng, T.S. Chest-worn health monitor based on a bistatic self injection-locked radar. IEEE Trans. Biomed. Eng. 2015, 62, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.R.; Zierdt, M.G. Novel signal processing techniques for Doppler radar cardiopulmonary sensing. Signal Process. 2009, 89, 45–66. [Google Scholar] [CrossRef]
- Mikhelson, I.V.; Bakhtiari, S.; Elmer, T.W., II; Sahakian, A.V. Email author Remote sensing of patterns of cardiac activity on an ambulatory subject using millimeter-wave interferometry and statistical methods. Med. Biol. Eng. Comput. 2013, 51, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jenshan, L. Random Body Movement Cancellation in Doppler Radar Vital Sign Detection. IEEE Trans. Microw. Theory Tech. 2008, 56, 3143–3152. [Google Scholar]
- Peng, Z.; Muñoz-Ferreras, J.M.; Tang, Y.; Liu, C.; Gómez-García, R.; Ran, L.; Li, C. A portable FMCW interferometry radar with programmable low-IF architecture for localization, ISAR imaging, and vital sign tracking. IEEE Trans. Microw. Theory Tech. 2017, 65, 1334–1344. [Google Scholar] [CrossRef]
- Hui, X.; Kan, E.C. Monitoring vital signs over multiplexed radio by near-field coherent sensing. Nat. Electron. 2018, 1, 74–78. [Google Scholar] [CrossRef]
- Mercuri, M.; Liu, Y.-H.; Lorato, I.; Torfs, T.; Bourdoux, A.; Van Hoof, C. Frequency-Tracking CW Doppler Radar Solving Small-Angle Approximation and Null Point Issues in Non-Contact Vital Signs Monitoring. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 671–680. [Google Scholar] [CrossRef]
- Mercuri, M.; Liu, Y.-H.; Young, A.; Torfs, T.; Bourdoux, A.; Van Hoof, C.C. Digital Phase-Tracking Doppler Radar for Accurate Displacement Measurements and Vital Signs Monitoring. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium (IMS), Honolulu, HI, USA, 4–9 June 2017; pp. 1–4. [Google Scholar]
- Mercuri, M.; Liu, Y.-H.; Lorato, I.; Torfs, T.; Wieringa, F.; Bourdoux, A.; Van Hoof, C. A Direct Phase-Tracking Doppler Radar Using Wavelet Independent Component Analysis for Non-Contact Respiratory and Heart Rate Monitoring. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 632–643. [Google Scholar] [CrossRef]
- Mercuri, M.; Liu, Y.-H.; Sheelavant, S.; Polito, S.; Torfs, T.; Van Hoof, C. Digital Linear Discrete FMCW Radar for Healthcare Application. In Proceedings of the 2019 IEEE MTT-S International Microwave Symposium (IMS), Boston, MA, USA, 2–7 June 2019; pp. 1–3. [Google Scholar]
- Mercuri, M.; Lorato, I.; Liu, Y.-H.; Wieringa, F.; Van Hoof, C.; Torfs, T. Vital-sign monitoring and spatial tracking of multiple people using a contactless radar-based sensor. Nat. Electron. 2019, 2, 252–262. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Sheelavant, S.; Mercuri, M.; Mateman, P.; Dijkhuis, J.; Zomagboguelou, W.; Breeschoten, A.; Traferro, S.; Zhan, Y.; Torfs, T. 9.3 A680 μW Burst-Chirp UWB Radar Transceiver for Vital Signs and Occupancy Sensing up to 15m Distance. In Proceedings of the 2019 IEEE International Solid- State Circuits Conference—(ISSCC), San Francisco, CA, USA, 17–21 February 2019; pp. 166–167. [Google Scholar]
- Schires, E.; Georgiou, P.; Lande, T.S. Vital Sign Monitoring Through the Back Using an UWB Impulse Radar With Body Coupled Antennas. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 292–302. [Google Scholar] [CrossRef]
- Castro, I.D.; Mercuri, M.; Torfs, T.; Lorato, I.; Puers, R.; van Hoof, C. Sensor Fusion of Capacitively Coupled ECG and Continuous-Wave Doppler Radar for Improved Unobtrusive Heart Rate Measurements. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 316–328. [Google Scholar] [CrossRef]
- Castro, I.D.; Patel, A.; Torfs, T.; Puers, R.; van Hoof, C. Capacitive multi-electrode array with real-time electrode selection for unobtrusive ECG & BIOZ monitoring. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Castro, I.D.; Varon, C.; Moeyersons, J.; Gomez, A.V.; Morales, J.; Deviaene, M.; Torfs, T.; van Huffel, S.; Puers, R.; van Hoof, C. Data Quality Assessment of Capacitively-coupled ECG signals. In Proceedings of the 2019 Computing in Cardiology Conference (CinC), Singapore, 8–11 September 2019. [Google Scholar]
- Castro, I.D.; Varon, C.; Torfs, T.; van Huffel, S.; Puers, R.; van Hoof, C. Evaluation of a multichannel non-contact ECG system and signal quality algorithms for sleep apnea detection and monitoring. Sensors 2018, 18, 577. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Jeong, J.J.; Kim, S.H.; Kim, S.W. Reduction of motion artifacts and improvement of R peak detecting accuracy using adjacent non-intrusive ECG sensors. Sensors 2016, 16, 715. [Google Scholar] [CrossRef] [PubMed]
- Antink, C.H.; Breuer, E.; Uguz, D.U.; Leonhardt, S. Signal-Level Fusion With Convolutional Neural Networks for Capacitively Coupled ECG in the Car. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018. [Google Scholar]

| Subject | Recording Length | Number of Clothing Layers Worn | |
|---|---|---|---|
| City | Highway | ||
| 1 | 44 min | 35 min | 1 |
| 2 | 16 min | 15 min | 2 |
| 3 | n/a | 32 min | 1 |
| 4 | 38 min | 46 min | 1 |
| 5 | 39 min | 36 min | 2 |
| Subj. | Traffic | Before Motion Artefact Algorithms | After Motion Artefact Algorithms | |||||
|---|---|---|---|---|---|---|---|---|
| R Peak Detection Sensitivity | R Peak Detection Positive Predictivity | HR Mean Absolute Error (bpm) | R Peak Detection Sensitivity | R Peak Detection Positive Predictivity | Hr Mean Absolute Error (bpm) | % of Signal Removed | ||
| 1 | highway | 94.67% | 95.15% | 2.28 | 97.58% | 98.26% | 0.86 | 10.3% |
| city | 89.46% | 92.34% | 2.18 | 97.11% | 97.23% | 1.28 | 29.7% | |
| 2 | highway | 76.71% | 84.65% | 3.06 | 98.28% | 99.03% | 1.57 | 47.3% |
| city | 59.27% | 66.53% | 12.68 | 99.36% | 100.00% | 0.93 | 82.5% | |
| 3 | highway | 98.05% | 98.04% | 1.10 | 99.58% | 99.49% | 0.36 | 9.8% |
| city | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| 4 | highway | 99.63% | 99.89% | 0.05 | 99.82% | 99.92% | 0.01 | 2.8% |
| city | 99.28% | 98.97% | 0.35 | 99.31% | 99.05% | 0.22 | 0.3% | |
| 5 | highway | 89.92% | 86.57% | 3.37 | 96.65% | 91.51% | 2.30 | 20.6% |
| city | 92.74% | 87.45% | 6.56 | 93.99% | 88.64% | 5.80 | 11.2% | |
| Median | highway | 94.67% | 95.15% | 2.28 | 98.28% | 99.03% | 0.86 | 10.29% |
| city | 91.10% | 89.90% | 4.37 | 98.21% | 98.14% | 1.11 | 20.41% | |
| overall | 92.74% | 92.34% | 2.28 | 98.28% | 99.03% | 0.93 | 11.15% | |
| Mean | highway | * | * | 1.97 | * | * | 1.02 | * |
| city | * | * | 5.44 | * | * | 2.06 | * | |
| overall | * | * | 3.51 | * | * | 1.48 | * | |
| Subj. | Traffic | ECG after Motion Artefact Algorithms | Respiration | ||||
|---|---|---|---|---|---|---|---|
| R-R Interval Mean Absolute Error (ms) | R-R Interval Mean Absolute Percentual Error (%) | 95th Percentile Absolute R-R Interval Error (ms) | SDNN Mean Absolute Error (ms) | Coverage R-R Interval within 5 ms | Coverage Respiration Rate within 3 bpm | ||
| 1 | highway | 18.67 | 1.39% | 25.39 | 52.43 | 91.16% | 68.57% |
| city | 25.44 | 1.98% | 52.73 | 64.77 | 90.15% | 61.36% | |
| 2 | highway | 28.64 | 2.54% | 23.24 | 119.12 | 93.85% | 26.67% |
| city | 25.00 | 0.84% | 50.78 | 58.11 | 94.61% | 25.00% | |
| 3 | highway | 5.21 | 0.39% | 1.95 | 10.09 | 95.93% | 68.75% |
| city | n/a | n/a | n/a | n/a | n/a | n/a | |
| 4 | highway | 1.48 | 0.11% | 1.95 | 0.13 | 97.50% | 26.09% |
| city | 5.43 | 0.41% | 5.86 | 8.17 | 94.34% | 47.37% | |
| 5 | highway | 67.72 | 4.05% | 391.21 | 88.34 | 59.37% | 66.67% |
| city | 130.40 | 6.38% | 369.48 | 132.58 | 58.94% | 64.10% | |
| Median | highway | 18.67 | 1.39% | 23.24 | 52.43 | 93.85% | 66.67% |
| city | 25.22 | 1.41% | 51.76 | 61.44 | 92.25% | 54.37% | |
| overall | 25.00 | 1.39% | 25.39 | 58.11 | 93.85% | 61.36% | |
| Mean | highway | 24.34 | * | 88.75 | 54.02 | * | * |
| city | 46.57 | * | 119.71 | 65.91 | * | * | |
| overall | 34.22 | * | 102.51 | 59.30 | * | * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, I.D.; Mercuri, M.; Patel, A.; Puers, R.; Van Hoof, C.; Torfs, T. Physiological Driver Monitoring Using Capacitively Coupled and Radar Sensors. Appl. Sci. 2019, 9, 3994. https://doi.org/10.3390/app9193994
Castro ID, Mercuri M, Patel A, Puers R, Van Hoof C, Torfs T. Physiological Driver Monitoring Using Capacitively Coupled and Radar Sensors. Applied Sciences. 2019; 9(19):3994. https://doi.org/10.3390/app9193994
Chicago/Turabian StyleCastro, Ivan D., Marco Mercuri, Aakash Patel, Robert Puers, Chris Van Hoof, and Tom Torfs. 2019. "Physiological Driver Monitoring Using Capacitively Coupled and Radar Sensors" Applied Sciences 9, no. 19: 3994. https://doi.org/10.3390/app9193994
APA StyleCastro, I. D., Mercuri, M., Patel, A., Puers, R., Van Hoof, C., & Torfs, T. (2019). Physiological Driver Monitoring Using Capacitively Coupled and Radar Sensors. Applied Sciences, 9(19), 3994. https://doi.org/10.3390/app9193994





