The Application of Alginate Coated Iron Hydroxide for the Removal of Cu(II) and Phosphate
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
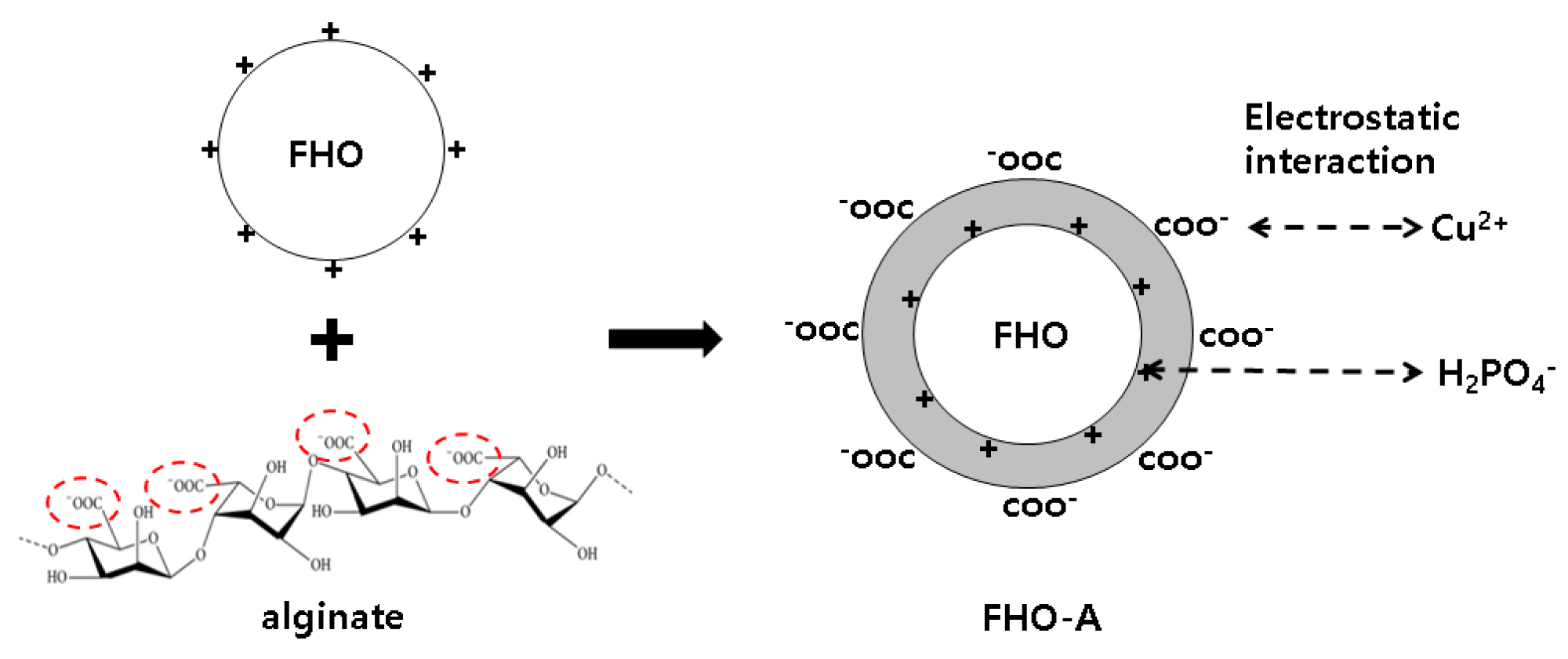
2.2. Synthesis of Alginate Coated Iron Hydroxide
2.3. FTIR Analysis
2.4. Bath Equilibrium Test
2.5. Kinetic Tests
2.6. Instrumental Techniques
3. Results and Discussion
3.1. FHO-A
3.2. FTIR
3.3. Cu(II) and Phosphate Removal Efficiency
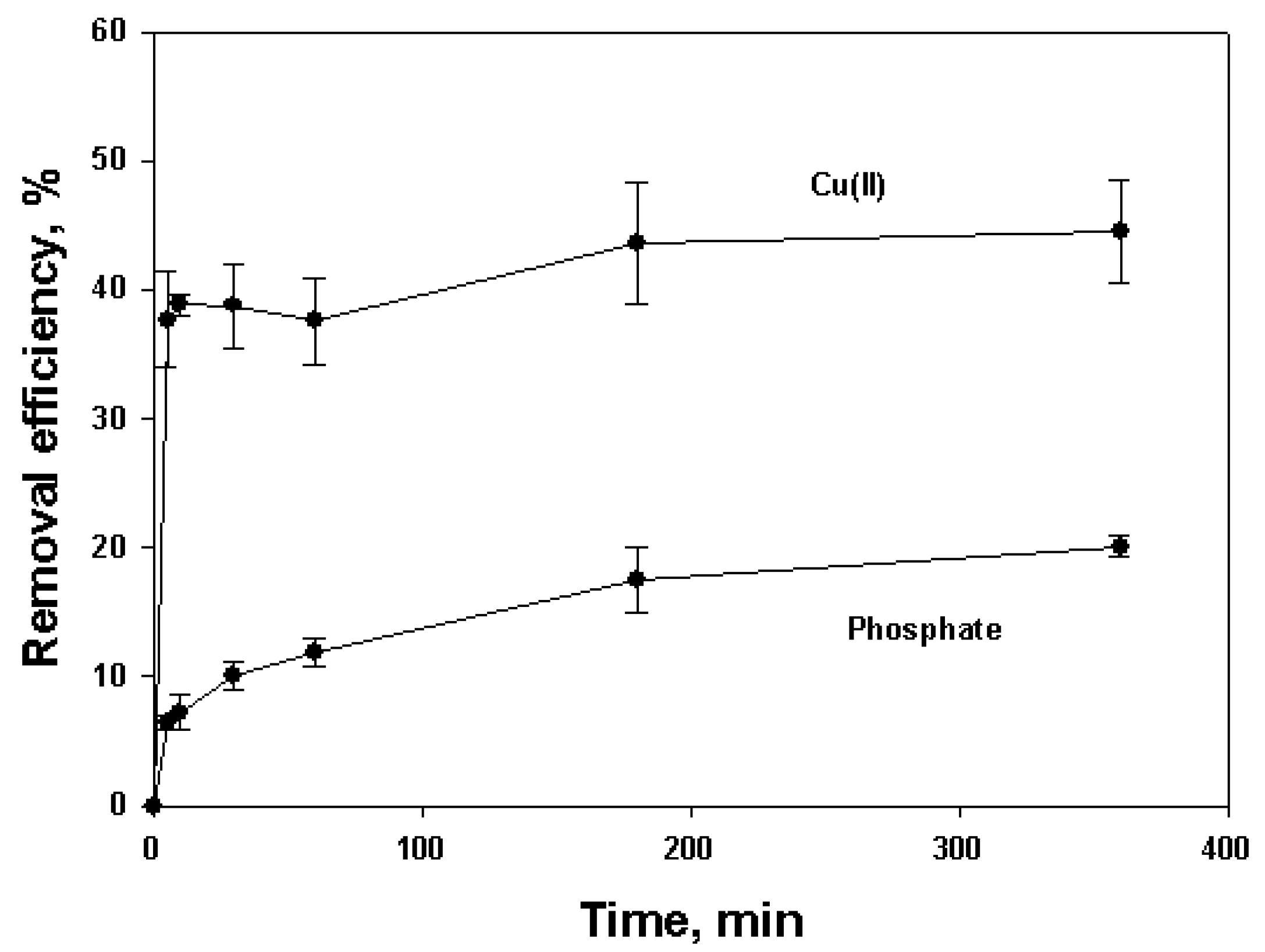
3.4. Kinetic Test
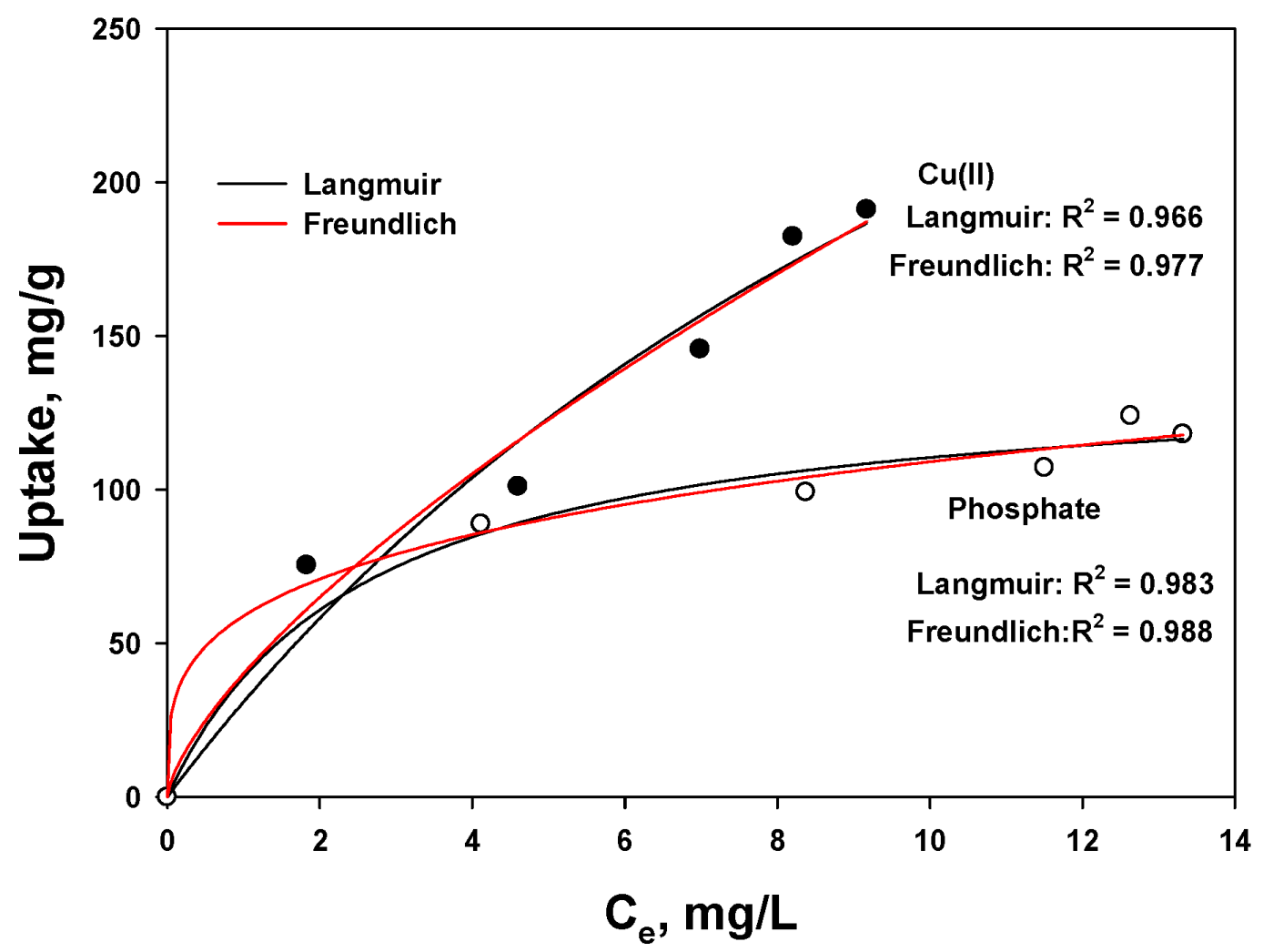
3.5. Equilibrium Isotherm
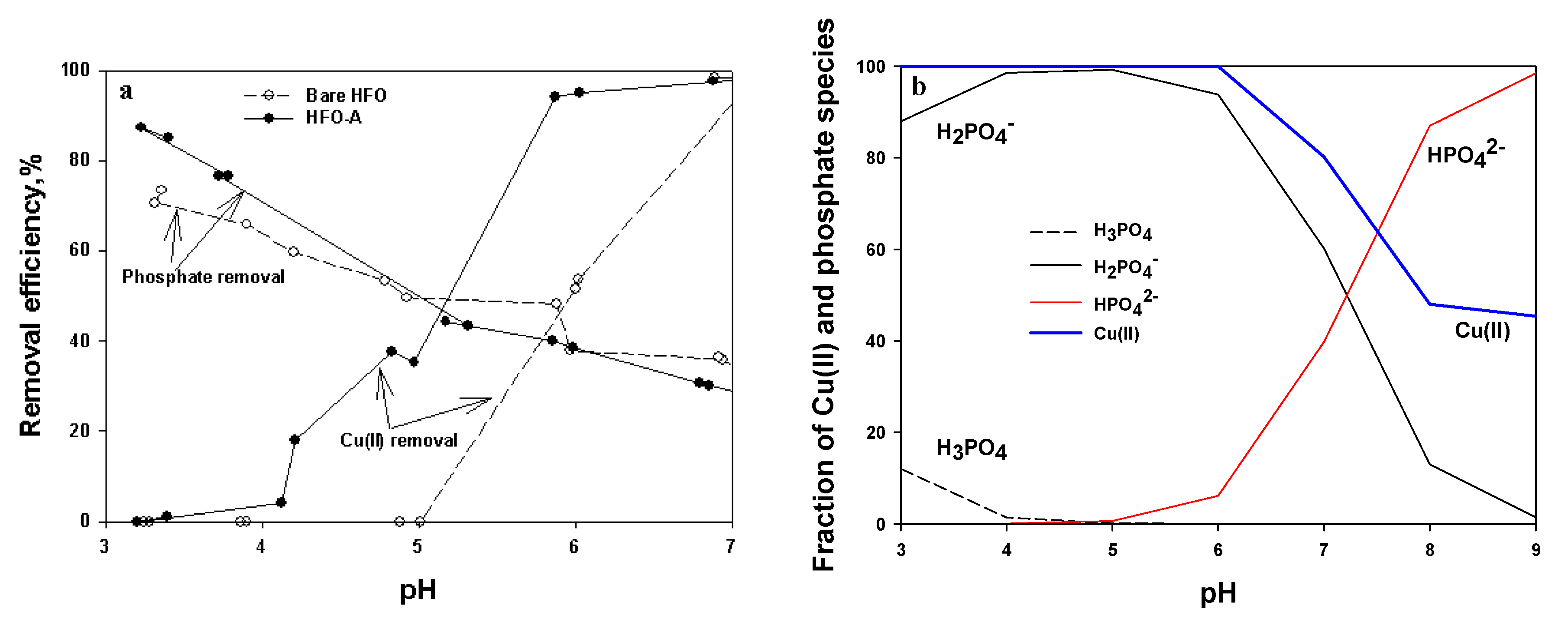
3.6. pH Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wilde, E.W.; Benemann, J.R. Bioremoval of heavy-metals by the use of microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef]
- An, B.; Son, H.; Chung, J.; Choi, J.W.; Lee, S.H.; Hong, S.W. Calcium and hydrogen effects during sorption of copper onto an alginate-based ion exchanger: Batch and fixed-bed column studies. Chem. Eng. J. 2013, 232, 51–58. [Google Scholar] [CrossRef]
- Paulino, A.T.; Minasse, F.A.S.; Guilherme, M.R.; Reis, A.V.; Muniz, E.C.; Nozaki, J. Novel adsorbent based on silkworm chrysalides for removal of heavy metals from wastewaters. J. Colloid Interface Sci. 2006, 301, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Abend, S.; Pandya, K.I.; Sparks, D. Kinetic controls on Cu and Pb sorption by ferrihydrite. Environ. Sci. Technol. 2001, 35, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Espana, J.S.; Pamo, E.L.; Pastor, E.S.; Andres, J.R.; Rubi, J.A.M. The removal of dissolved metals by hydroxysulphate precipitates during oxidation and neutralization of acid mine waters. Aquat. Geochem. 2006, 12, 269–298. [Google Scholar] [CrossRef]
- Lin, L.C.; Juang, R.S. Ion-exchange kinetics of Cu(II) and Zn(II) from aqueous solutions with two chelating resins. Chem. Eng. J. 2007, 132, 205–213. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.A.B.; Zainol, M.R.R.M.A.; Murshed, M.F.; Zain, H.; Faris, M.A.; Bayuaji, R. Review on adsorption of heavy metal in wastewater by using geopolymer. MATEC Web Conf. EDP Sci. 2017, 97, 01023. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Anastopoulos, L.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Lagoa, R.; Rodrigues, J.R. Kinetic analysis of metal uptake by dry and gel alginate particles. Biochem. Eng. J. 2009, 46, 320–326. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminbhavi, T.M. Novel sodium alginate-Na+ MMT hybrid composite membranes for pervaporation dehydration of isopropanol, 1,4-dioxane and tetrahydrofuran. Sep. Purif. Technol. 2006, 51, 85–94. [Google Scholar] [CrossRef]
- Schoubbn, A.; Blasi, P.; Glovagnoli, S.; Rossi, C.; Ricci, M. Development of a salable procedure for fine calcium alginate particle preparation. Chem. Eng. J. 2010, 160, 63–369. [Google Scholar]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.; Mallikarjuna, N.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Saha, B.; Bains, R.; Greenwood, F. Physicochemical characterization of granular ferric fydroxide (GFH) for arsenic(V) sorption from water. Sep. Sci. Technol. 2005, 40, 2909–2932. [Google Scholar] [CrossRef]
- Smith, E.; Ghiassi, K. Chromate removal by an iron sorbent: Mechanism and modeling. Water Environ. Res. 2006, 78, 84–93. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.; Huang, J. One-step synthesis of robust amine- and vinyl-capped magnetic iron oxide nanoparticles for polymer grafting, dye adsorption, and catalysis. Appl. Mater. Interfaces 2013, 5, 8678–8685. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ. Sci. Technol. 2005, 39, 6808–6818. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Katsoyiannis, I.A. Arsenic removal using iron oxide loaded alginate beads. Ind. Eng. Chem. Res. 2002, 41, 6149–6155. [Google Scholar] [CrossRef]
- An, B.; Lee, S.; Kim, H.G.; Zhao, D.; Park, J.; Choi, J.W. Organic/inorganic hybrid adsorbent for efficient phosphate removal from a reservoir affected by algae bloom. J. Ind. Eng. Chem. 2019, 69, 211–216. [Google Scholar] [CrossRef]
- Magdy, Y.H.; Altaher, H. Kinetic analysis of the adsorption of dyes from high strength wastewater on cement kiln dust. J. Environ. Chem. Eng. 2018, 6, 834–841. [Google Scholar] [CrossRef]
- An, B.; Zhao, D. Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles. J. Hazard. Mater. 2012, 211, 332–341. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indrasati, N.; Ismadji, S. Recent Progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. Clean Soil Air Water 2008, 36, 937–982. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Aisiri, A.M. Iron oxide nanoparticles. In Nanomaterials; Rahman, M., Ed.; IntechOpen: London, UK, 2011; pp. 43–66. [Google Scholar]
- Bumajdad, A.; Ali, S.; Mathew, A. Characterization of iron hydroxide/oxide nanaoparticles prepared in microemulsions stabilized with cationic/non-ionic surfactant mixtures. J. Colloid Interface Sci. 2011, 355, 282–292. [Google Scholar] [CrossRef]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Helmiyati; Aprilliza, M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 1. [Google Scholar]
- Li, Y.; Liu, F.; Xia, B.; Du, Q.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. J. Hazard. Mater. 2010, 177, 876–880. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate interactions in metal-alginate complex studied with FTIR spectroscopy. Carbhyd. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Filipiuk, D.; Fuks, L.; Majdan, M. Transition metal complexes with uronic acids. J. Mol. Struct. 2005, 744, 705–709. [Google Scholar] [CrossRef]
- Fuks, L.; Filipiuk, D.; Majdan, M. Transition metal complexes with alginate biosorbent. J. Mol. Struct. 2006, 792, 104–109. [Google Scholar] [CrossRef]
- Sigg, L.; Stumm, W. The interaction of anions and weak acids with the hydrous goethite (α-FeOOH) surface. Colloids Surf. 1981, 2, 101–117. [Google Scholar] [CrossRef]
- Goldberg, S.; Sposito, G. A chemical model of phosphate adsorption by soils: I. Reference oxide minerals. Soil Sci. Am. J. 1984, 48, 772–783. [Google Scholar] [CrossRef]
- Persson, P.; Nilsson, N.; SJöberg, S. Structure and bonding of orthophosphate ions at the iron oxide-aqueous interface. J. Colloid Interface Sci. 1996, 177, 263–275. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Equilibrium, kinetics, and thermodynamics of dye removal using Alginate in binary systems. J. Chem. Eng. Data 2011, 56, 2802–2811. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Peleka, E.N.; Lazaridis, N.K. Comparative study of phosphates removal from aqueous solutions by nanocrystalline akaganeite and hybrid surfactant-akaganeite. Sep. Purif. Technol. 2007, 52, 478–486. [Google Scholar] [CrossRef]
- Pan, B.; Wu, J.; Pan, B.; Lu, L.; Zhang, W.; Xiao, L.; Wang, X.; Tao, X.; Zheng, S. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents. Water Res. 2009, 43, 4421–4429. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y. Construction a hybrid biosorbent using scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): Kinetics and equilibrium studies. Bioresour. Technol. 2009, 100, 186–193. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Dursun, A.Z. A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated aspergillus niger. Biochem. Eng. J. 2006, 28, 187–195. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. ASCE 1963, 89, 31–59. [Google Scholar]
- Aroua, M.K.; Yin, C.Y.; Lim, F.N.; Kan, W.L.; Daud, W.M.A. Effect of impregnation of activated carbon with chelating polymer on adsorption kinetics of Pb2+. J. Hazard. Mater. 2009, 166, 1526–1529. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Toth, J. State equations of the solid gas interface layer. Acta Chem. Acad. Hung. 1971, 69, 311–317. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1026. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Kargi, F.; Cikla, S. Biosorption of zinc(II) ions onto powdered waste sludge (PWS): Kinetics and isotherms. Enzyme Microb. Technol. 2006, 38, 705–710. [Google Scholar] [CrossRef]
- An, B.; Lee, H.; Lee, S.; Lee, S.H.; Choi, J.W. Determining the selectivity of divalent metal cations for the carboxyl group of alginate hydrogel beads during competitive sorption. J. Hazard. Mater. 2015, 298, 11–18. [Google Scholar] [CrossRef]
- Onsøyen, E. Alginates. In Thickening and Gelling Agents for Food; Imeson, A., Ed.; Springer: Boston, MA, USA, 1997; pp. 22–44. [Google Scholar]
- Parks, G.A.; de Bruyn, P.L. PZC of iron oxide. J. Phys. Chem. 1962, 66, 967–973. [Google Scholar] [CrossRef]


| A-60 mg/L | A-120 mg/L | A-180 mg/L | |
|---|---|---|---|
| Initial alginate concentration in solution, mg/L | 60 | 120 | 180 |
| Final alginate concentration in solution, mg/L | 5.40 | 4.16 | 38.2 |
| Unused alginate, % | 9.00 | 3.47 | 21.4 |
| Amount of alginate on FHO, mg | 27.3 | 57.9 | 70.7 |
| k | R2 | C | q-exp. (1) | q-cal. (2) | ||
|---|---|---|---|---|---|---|
| Pseudo 1st | Cu(II) | 0.0174 | 0.926 | 3.29 | 26.8 | 84.8 |
| Phosphate | 0.0169 | 0.962 | 3.88 | 48.4 | 48.6 | |
| Pseudo 2nd | Cu(II) | 0.0107 | 0.999 | 0.0378 | 93.5 | 84.8 |
| Phosphate | 0.0581 | 0.986 | 1.27 | 17.2 | 48.6 | |
| Intraparticle | Cu(II) | 0.9124 | 0.822 | 76.5 | - | - |
| Phosphate | 2.1118 | 0.980 | 12.5 | - | - |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| Q | b | R2 | kf | n | R2 | ||
| Cu | Non-linear | 487 | 0.0678 | 0.966 | 40.1 | 1.44 | 0.977 |
| Linear | 350 | 0.117 | 0.704 | 49.1 | 1.71 | 0.924 | |
| Phosphate | Non-linear | 139 | 0.3905 | 0.983 | 58.9 | 3.73 | 0.988 |
| Linear | 136 | 0.401 | 0.986 | 62.6 | 4.28 | 0.944 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-G.; He, F.; An, B. The Application of Alginate Coated Iron Hydroxide for the Removal of Cu(II) and Phosphate. Appl. Sci. 2019, 9, 3835. https://doi.org/10.3390/app9183835
Kim H-G, He F, An B. The Application of Alginate Coated Iron Hydroxide for the Removal of Cu(II) and Phosphate. Applied Sciences. 2019; 9(18):3835. https://doi.org/10.3390/app9183835
Chicago/Turabian StyleKim, Hee-Gon, Feng He, and Byungryul An. 2019. "The Application of Alginate Coated Iron Hydroxide for the Removal of Cu(II) and Phosphate" Applied Sciences 9, no. 18: 3835. https://doi.org/10.3390/app9183835
APA StyleKim, H.-G., He, F., & An, B. (2019). The Application of Alginate Coated Iron Hydroxide for the Removal of Cu(II) and Phosphate. Applied Sciences, 9(18), 3835. https://doi.org/10.3390/app9183835





