Tomographic Diffractive Microscopy: A Review of Methods and Recent Developments
Abstract
1. Introduction
2. Theoretical Background
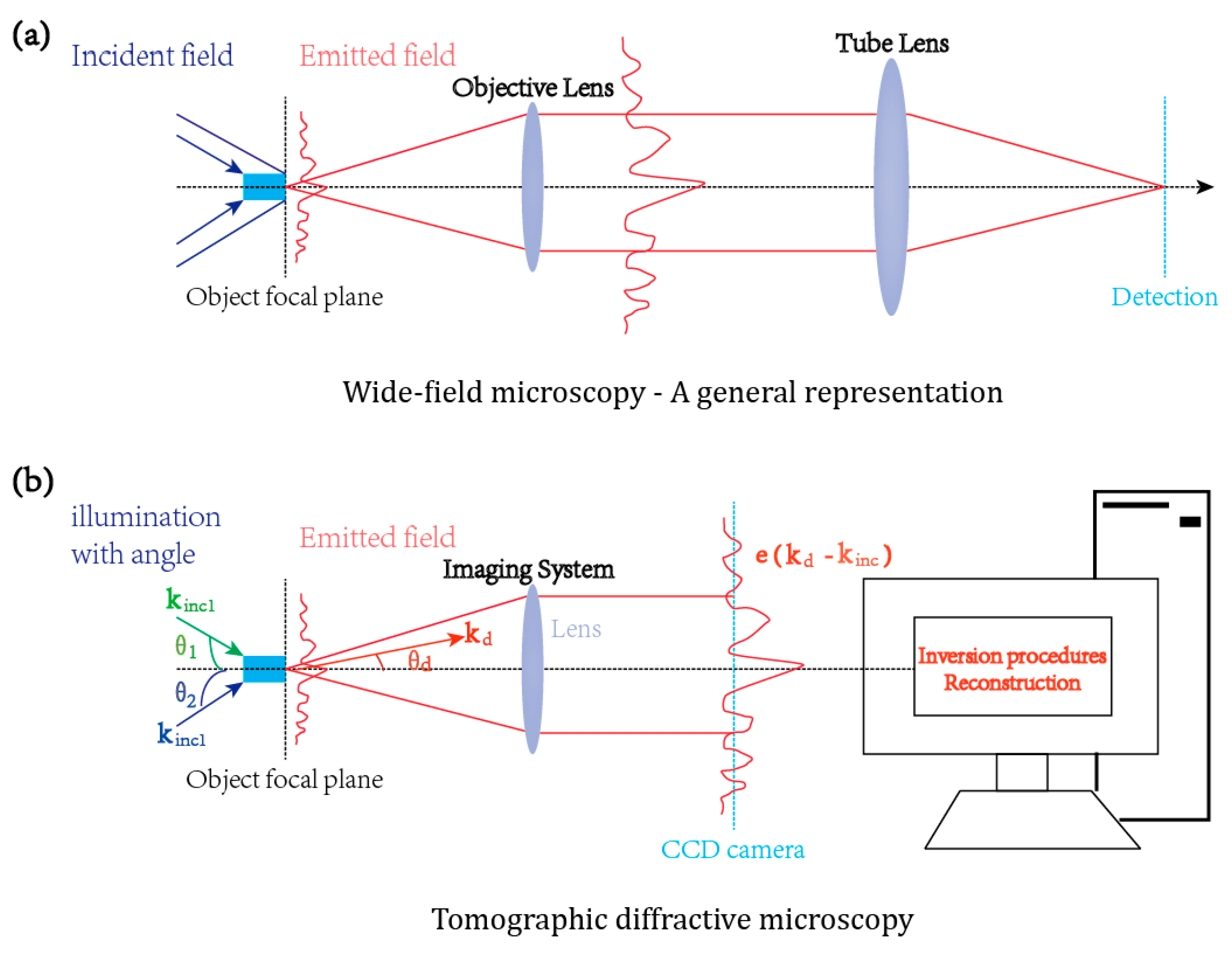
2.1. The Principle of Tomographic Diffractive Microscopy
2.2. Born Approximation with Linear Inversion
2.3. Rigorous Case with Non-linear Inversion
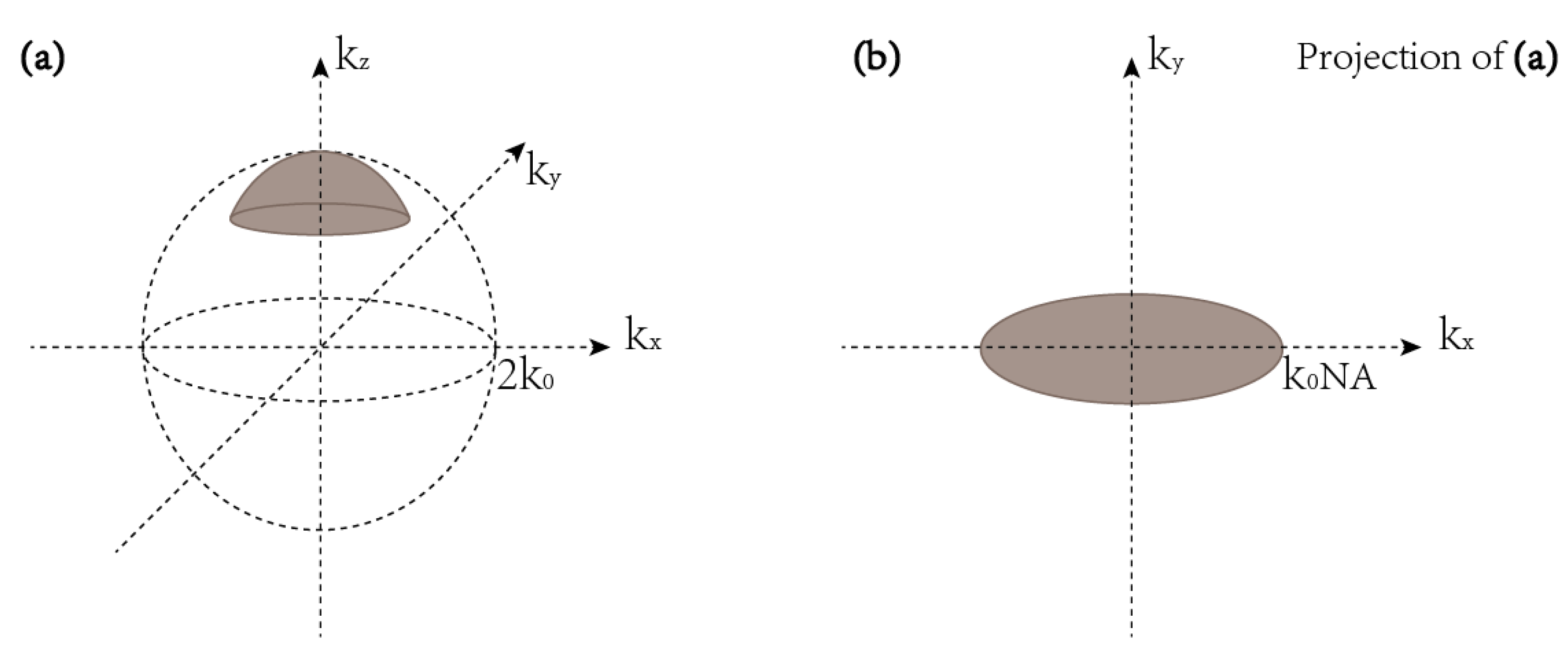
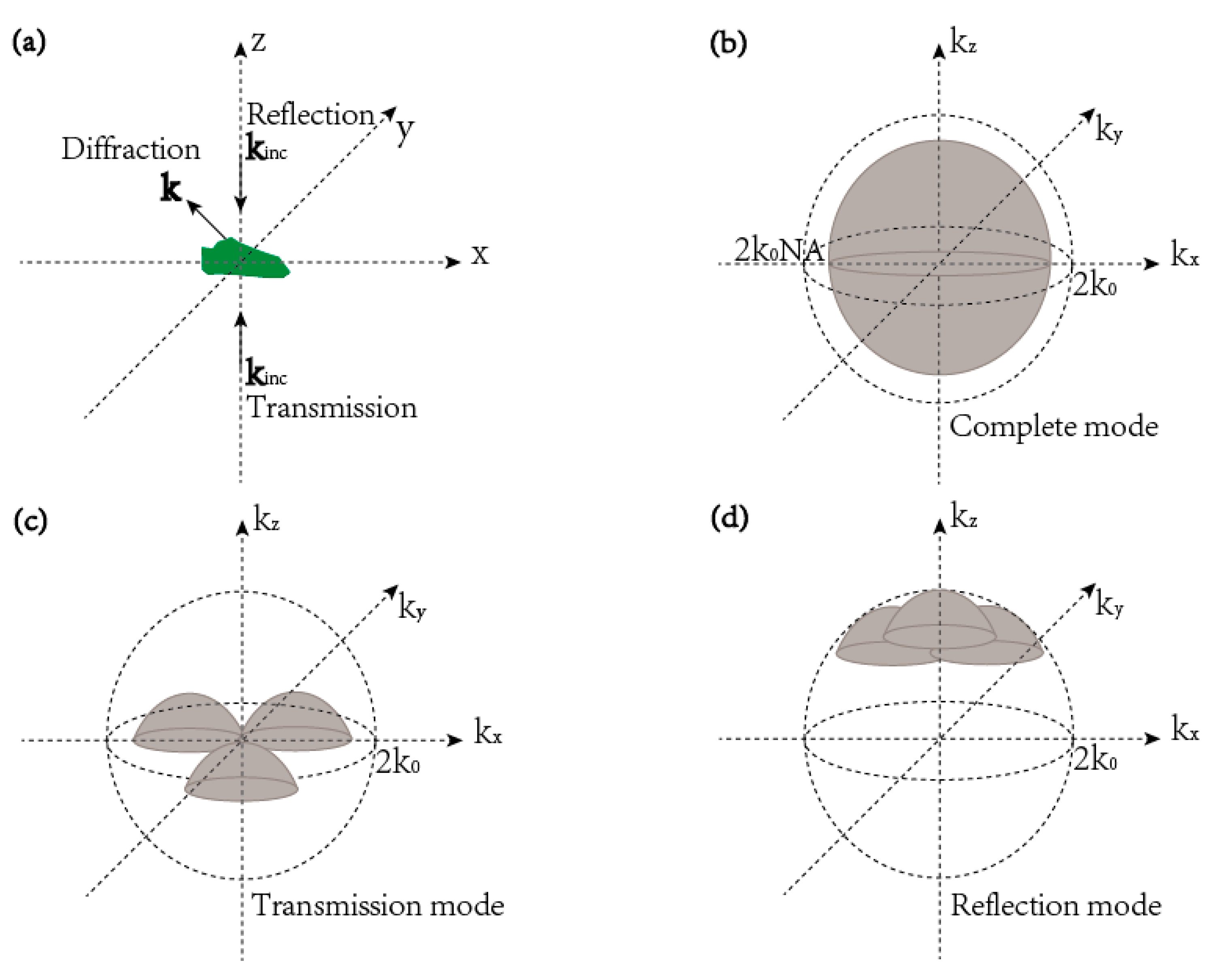
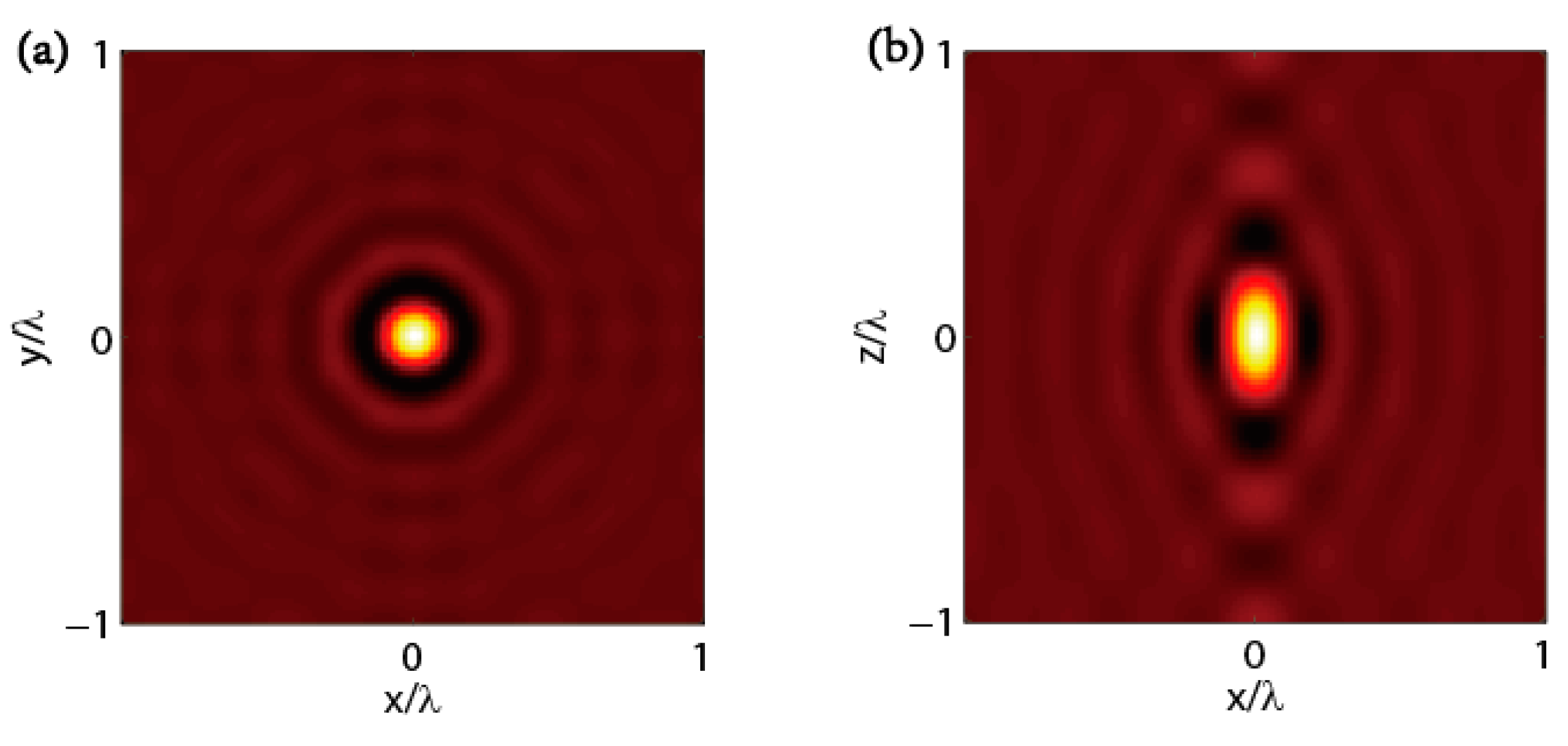
3. The Resolution of Tomographic Diffractive Microscopy
3.1. TDM in Complete Configuration
3.2. TDM in Transmission Configuration
3.3. TDM in Reflection Configuration
4. The Optical Setup of Tomographic Diffractive Microscopy
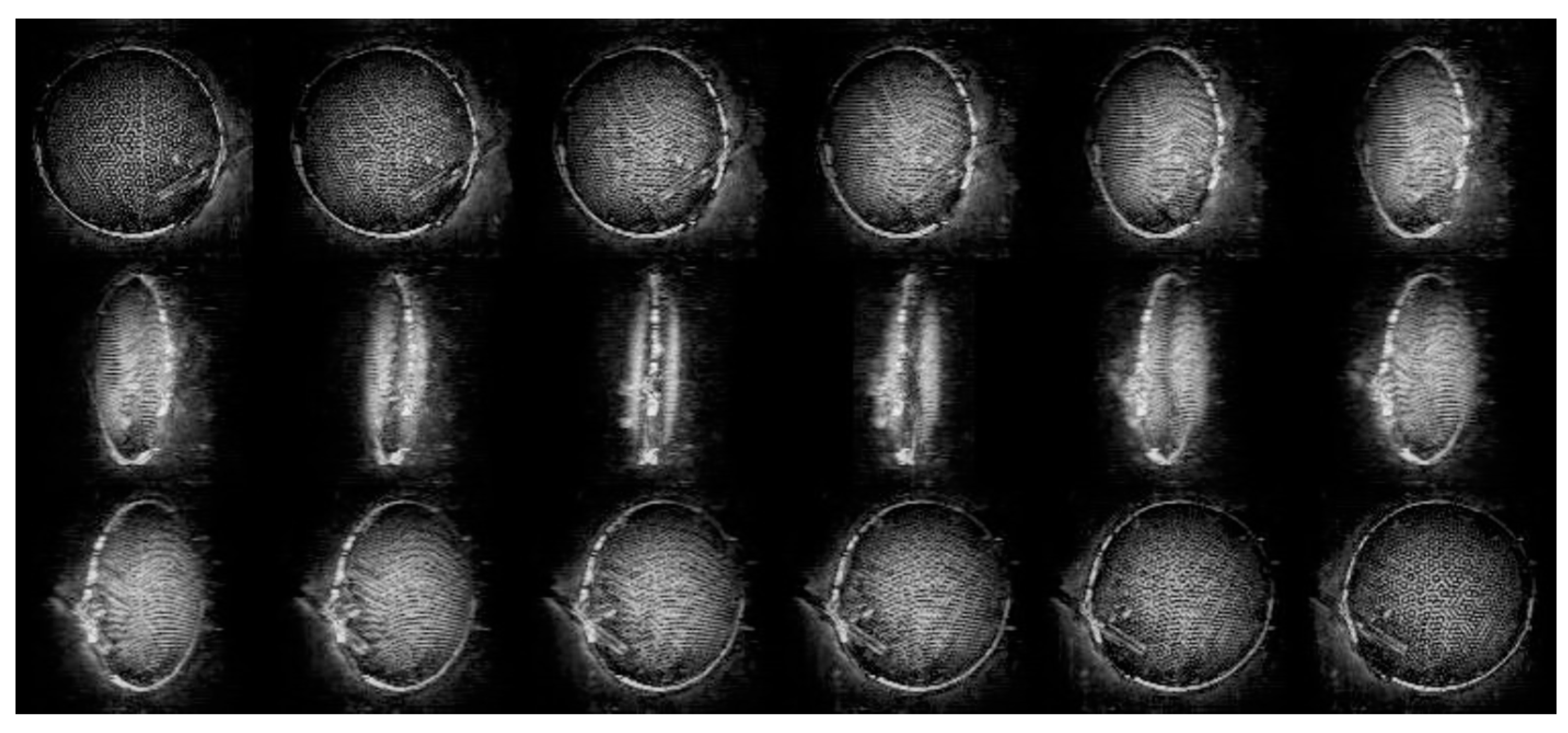
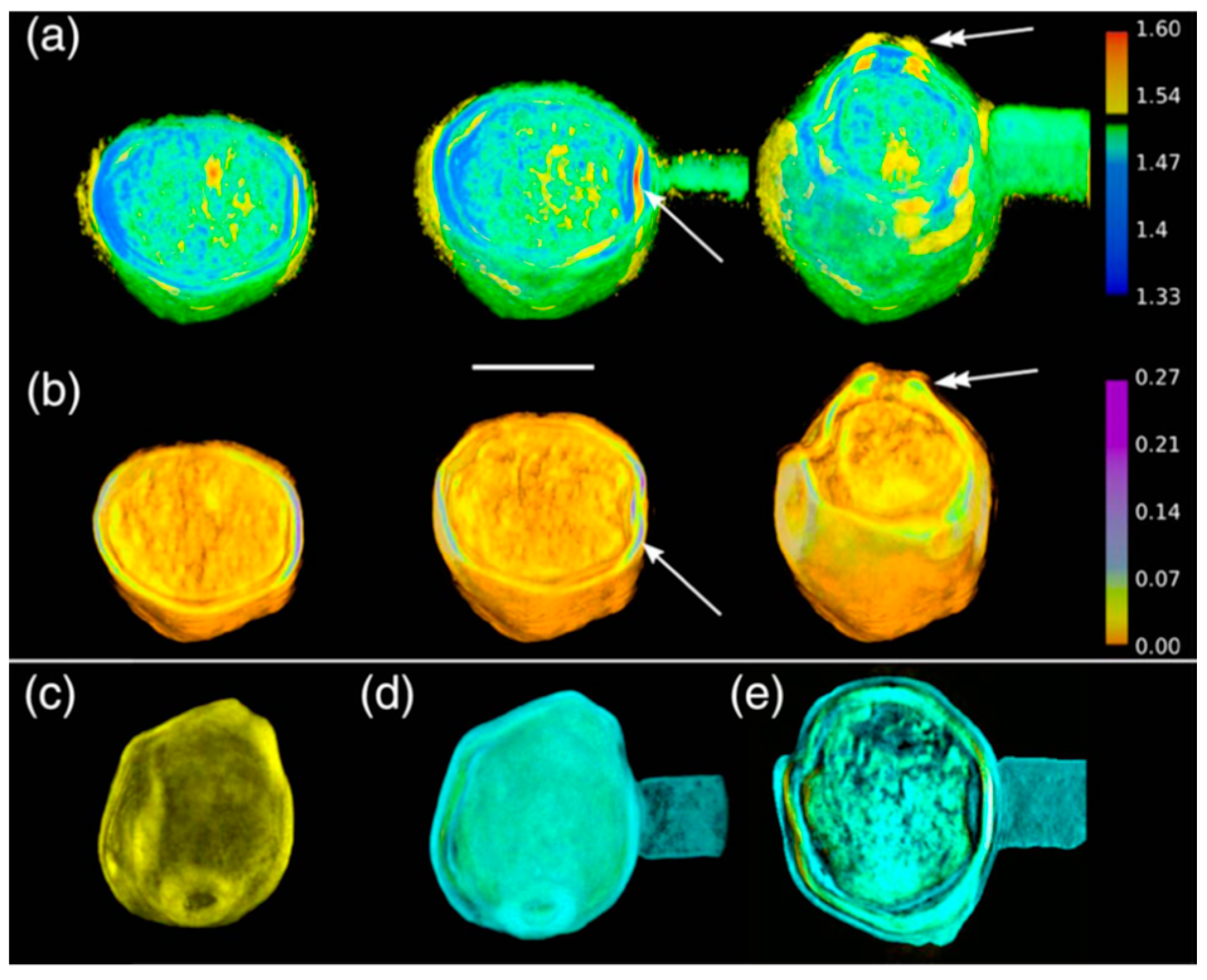
5. The Advancements of Tomographic Diffractive Microscopy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Henderson, R.; Unwin, P.N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature 1975, 257, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J.; Roots, L. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965, 27, A137. [Google Scholar]
- Meyer, G.; Amer, N.M. Novel optical approach to atomic force microscopy. Appl. Phys. Lett. 1988, 53, 1045–1047. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef]
- Betzig, E.; Trautman, J.K.; Harris, T.D.; Weiner, J.S.; Kostelak, R.L. Breaking the diffraction barrier: Optical microscopy on a nanometric scale. Science 1991, 251, 1468–1470. [Google Scholar] [CrossRef]
- Webb, R.H. Confocal optical microscopy. Rep. Prog. Phys. 1996, 59, 427–471. [Google Scholar] [CrossRef]
- Hecht, B.; Sick, B.; Wild, U.P.; Deckert, V.; Zenobi, R.; Martin, O.J.F.; Pohl, D.W. Scanning near-field optical microscopy with aperture probes: Fundamentals and applications. J. Chem. Phys. 2000, 112, 7761–7774. [Google Scholar] [CrossRef]
- Webb, D.J.; Brown, C.M. Epi-fluorescence microscopy. Methods Mol. Biol. (Clifton, N.J.) 2013, 931, 29–59. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Zhang, T.; Godavarthi, C.; Chaumet, P.C.; Maire, G.; Giovannini, H.; Talneau, A.; Allain, M.; Belkebir, K.; Sentenac, A. Far-field diffraction microscopy at λ/10 resolution. Optica 2016, 3, 609. [Google Scholar] [CrossRef]
- Evanko, D. Label-free microscopy. Nat. Methods 2009, 7, 36. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.; Gillette, J.M.; Patterson, G.H.; Shroff, H.; Hess, H.F.; Betzig, E.; Lippincott-Schwartz, J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 2008, 5, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Lauer, V. New approach to optical diffraction tomography yielding a vector equation of diffraction tomography and a novel tomographic microscope. J. Microsc. 2002, 205, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mazza, D.; Bianchini, P.; Caorsi, V.; Cella, F.; Mondal, P.P.; Ronzitti, E.; Testa, I.; Vicidomini, G.; Diaspro, A. Non-Linear Microscopy. In Biophotonics; Pavesi, L., Fauchet, P.M., Eds.; Springer: Berlin Germany, 2008. [Google Scholar]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, R.A.; Van Oven, C.H.; Gadella, T.W.J.; Dhonukshe, P.B.; Van Noorden, C.J.F.; Manders, E.M.M. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nat. Biotechnol. 2007, 25, 249–253. [Google Scholar] [CrossRef]
- Wachulak, P.; Bartnik, A.; Fiedorowicz, H. Optical coherence tomography (OCT) with 2 nm axial resolution using a compact laser plasma soft X-ray source. Sci. Rep. 2018, 8, 8494. [Google Scholar] [CrossRef]
- Bousi, E.; Pitris, C. Lateral resolution improvement in Optical Coherence Tomography (OCT) images. In Proceedings of the 2012 IEEE 12th International Conference on Bioinformatics & Bioengineering (BIBE), Larnaca, Cyprus, 11–13 November 2012; pp. 598–601. [Google Scholar]
- Cheng, J.X.; Xie, X.S. Coherent Anti-Stokes Raman Scattering Microscopy: Instrumentation, Theory, and Applications. J. Phys. Chem. B 2004, 108, 827–840. [Google Scholar] [CrossRef]
- Mann, C.; Yu, L.; Lo, C.M.; Kim, M. High-resolution quantitative phase-contrast microscopy by digital holography. Opt. Express 2005, 13, 8693–8698. [Google Scholar] [CrossRef]
- Zhang, H.L.; Monroy-Ramirez, F.A.; Lizana, A.; Iemmi, C.; Bennis, N.; Morawiak, P.; Piecek, W.; Campos, J. Wavefront imaging by using an inline holographic microscopy system based on a double-sideband filter. Opt. Laser. Eng. 2019, 113, 71–76. [Google Scholar] [CrossRef]
- Bailleul, J.; Simon, B.; Debailleul, M.; Haeberlé, O. An Introduction to Tomographic Diffractive Microscopy. In Micro- and Nanophotonic Technologies; Meyrueis, P., van de Voorde, M., Sakoda, K., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 425–442. [Google Scholar]
- Haeberlé, O. Tomographic diffractive microscopy: Principles and applications. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 24 May 2018. [Google Scholar]
- Dändliker, R.; Weiss, K. Reconstruction of the three-dimensional refractive index from scattered waves. Opt. Commun. 1970, 1, 323–328. [Google Scholar] [CrossRef]
- Kawata, S.; Nakamura, O.; Minami, S. Optical microscope tomography I Support constraint. J. Opt. Soc. Am. A 1987, 4, 292. [Google Scholar] [CrossRef]
- Vögeler, M. Reconstruction of the three-dimensional refractive index in electromagnetic scattering by using a propagation backpropagation method. Inverse Probl. 2003, 19, 739–753. [Google Scholar] [CrossRef]
- Park, Y.; Diez-Silva, M.; Popescu, G.; Lykotrafitis, G.; Choi, W.; Feld, M.S.; Suresh, S. Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2008, 105, 13730–13735. [Google Scholar] [CrossRef]
- Fiolka, R.; Wicker, K.; Heintzmann, R.; Stemmer, A. Simplified approach to diffraction tomography in optical microscopy. Opt. Express 2009, 17, 12407–12417. [Google Scholar] [CrossRef]
- Haeberlé, O.; Belkebir, K.; Giovaninni, H.; Sentenac, A. Tomographic diffractive microscopy: Basics, techniques and perspectives. J. Mod. Optic. 2010, 57, 686–699. [Google Scholar] [CrossRef]
- Sentenac, A.; Mertz, J. Unified description of three-dimensional optical diffraction microscopy: From transmission microscopy to optical coherence tomography: Tutorial. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2018, 35, 748–754. [Google Scholar] [CrossRef]
- Cole, E.S. Conventional Light Microscopy. Curr. Protoc. Essent. Lab. Tech. 2016, 12, 9.1.1–9.1.29. [Google Scholar] [CrossRef]
- Wolf, E. Three-dimensional structure determination of semi-transparent objects from holographic data. Opt. Commun. 1969, 1, 153–156. [Google Scholar] [CrossRef]
- Debailleul, M.; Simon, B.; Georges, V.; Haeberle, O.; Lauer, V. Holographic microscopy and diffractive microtomography of transparent samples. Meas. Sci. Technol. 2008, 19, 074009. [Google Scholar] [CrossRef]
- Maire, G.; Girard, J.; Drsek, F.; Giovannini, H.; Talneau, A.; Belkebir, K.; Chaumet, P.C.; Sentenac, A. Experimental inversion of optical diffraction tomography data with a nonlinear algorithm in the multiple scattering regime. J. Mod. Optic. 2010, 57, 746–755. [Google Scholar] [CrossRef]
- Ruan, Y.; Bon, P.; Mudry, E.; Maire, G.; Chaumet, P.C.; Giovannini, H.; Belkebir, K.; Talneau, A.; Wattellier, B.; Monneret, S.; et al. Tomographic diffractive microscopy with a wavefront sensor. Opt. Lett. 2012, 37, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Maire, G.; Ruan, Y.; Zhang, T.; Chaumet, P.C.; Giovannini, H.; Sentenac, D.; Talneau, A.; Belkebir, K.; Sentenac, A. High-resolution tomographic diffractive microscopy in reflection configuration. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2013, 30, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Debailleul, M.; Georges, V.; Simon, B.; Morin, R.; Haeberle, O. High-resolution three-dimensional tomographic diffractive microscopy of transparent inorganic and biological samples. Opt. Lett. 2009, 34, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Debailleul, M.; Beghin, A.; Tourneur, Y.; Haeberle, O. High-resolution tomographic diffractive microscopy of biological samples. J. Biophotonics 2010, 3, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Debailleul, M.; Georges, V.; Lauer, V.; Haeberle, O. Tomographic diffractive microscopy of transparent samples. Eur. Phys. J. Appl. Phys. 2008, 44, 29–35. [Google Scholar] [CrossRef]
- Sung, Y.; Choi, W.; Fang-Yen, C.; Badizadegan, K.; Dasari, R.R.; Feld, M.S. Optical diffraction tomography for high resolution live cell imaging. Opt. Express 2009, 17, 266–277. [Google Scholar] [CrossRef]
- Devaney, A.J. Inversion formula for inverse scattering within the Born approximation. Opt. Lett. 1982, 7, 111–112. [Google Scholar] [CrossRef]
- Simon, B.; Debailleul, M.; Houkal, M.; Ecoffet, C.; Bailleul, J.; Lambert, J.; Spangenberg, A.; Liu, H.; Soppera, O.; Haeberle, O. Tomographic diffractive microscopy with isotropic resolution. Optica 2017, 4, 460–463. [Google Scholar] [CrossRef]
- Charriere, F.; Pavillon, N.; Colomb, T.; Depeursinge, C.; Heger, T.J.; Mitchell, E.A.; Marquet, P.; Rappaz, B. Living specimen tomography by digital holographic microscopy: Morphometry of testate amoeba. Opt. Express 2006, 14, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Chaumet, P.C.; Sentenac, A.; Rahmani, A. Coupled dipole method for scatterers with large permittivity. Phys. Rev. E 2004, 70, 036606. [Google Scholar] [CrossRef] [PubMed]
- Belkebir, K.; C Chaumet, P.; Sentenac, A. Influence of multiple scattering on three-dimensional imagig with optical diffraction tomography. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2006, 23, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Charriere, F.; Marian, A.; Montfort, F.; Kuehn, J.; Colomb, T.; Cuche, E.; Marquet, P.; Depeursinge, C. Cell refractive index tomography by digital holographic microscopy. Opt. Lett. 2006, 31, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; Maire, G.; Giovannini, H.; Talneau, A.; Belkebir, K.; Chaumet, P.C.; Sentenac, A. Nanometric resolution using far-field optical tomographic microscopy in the multiple scattering regime. Phys. Rev. A 2010, 82, 061801. [Google Scholar] [CrossRef]
- Chaumet, P.C.; Belkebir, K. Three-dimensional reconstruction from real data using a conjugate gradient-coupled dipole method. Inverse Probl. 2009, 25, 024003. [Google Scholar] [CrossRef]
- Mudry, E.; Chaumet, P.C.; Belkebir, K.; Sentenac, A. Electromagnetic wave imaging of three-dimensional targets using a hybrid iterative inversion method. Inverse Probl. 2012, 28, 065007. [Google Scholar] [CrossRef]
- Maire, G.; Drsek, F.; Girard, J.; Giovannini, H.; Talneau, A.; Konan, D.; Belkebir, K.; Chaumet, P.C.; Sentenac, A. Experimental demonstration of quantitative imaging beyond Abbe’s limit with optical diffraction tomography. Phys. Rev. Lett. 2009, 102, 213905. [Google Scholar] [CrossRef]
- Zhang, T.; Chaumet, P.C.; Sentenac, A.; Belkebir, K. Improving three-dimensional target reconstruction in the multiple scattering regime using the decomposition of the time-reversal operator. J. Appl. Phys. 2016, 120, 243101. [Google Scholar] [CrossRef]
- Alexandrov, S.A.; Hillman, T.R.; Gutzler, T.; Sampson, D.D. Synthetic aperture fourier holographic optical microscopy. Phys. Rev. Lett. 2006, 97, 168102. [Google Scholar] [CrossRef]
- Kim, M.; Choi, Y.; Fang-Yen, C.; Sung, Y.; Dasari, R.R.; Feld, M.S.; Choi, W. High-speed synthetic aperture microscopy for live cell imaging. Opt. Lett. 2011, 36, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, Y.; Fang-Yen, C.; Sung, Y.; Kim, K.; Dasari, R.R.; Feld, M.S.; Choi, W. Three-dimensional differential interference contrast microscopy using synthetic aperture imaging. J. Biomed. Opt. 2012, 17, 026003. [Google Scholar] [CrossRef] [PubMed]
- Mudry, E.; Chaumet, P.C.; Belkebir, K.; Maire, G.; Sentenac, A. Mirror-assisted tomographic diffractive microscopy with isotropic resolution. Opt. Lett. 2010, 35, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Vertu, S.; Delaunay, J.-J.; Yamada, I.; Haeberlé, O. Diffraction microtomography with sample rotation: Influence of a missing apple core in the recorded frequency space. Cent. Eur. J. Phys. 2009, 7, 22–31. [Google Scholar] [CrossRef]
- Wei-Chen, H.; Jing-Wei, S.; Te-Yu, T.; Kung-Bin, S. Tomographic diffractive microscopy of living cells based on a common-path configuration. Opt. Lett. 2014, 39, 2210. [Google Scholar] [CrossRef]
- Ding, C.; Tan, Z. Improved longitudinal resolution in tomographic diffractive microscopy with an ellipsoidal mirror. J. Microsc. 2016, 262, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.C.; McLeod, R.R. Tomographic reconstruction of weak, replicated index structures embedded in a volume. Opt. Express 2007, 15, 14202–14212. [Google Scholar] [CrossRef] [PubMed]
- Berndt, F.; Shah, G.; Power, R.M.; Brugues, J.; Huisken, J. Dynamic and non-contact 3D sample rotation for microscopy. Nat. Commun. 2018, 9, 5025. [Google Scholar] [CrossRef]
- Leite, I.T.; Turtaev, S.; Jiang, X.; Siler, M.; Cuschieri, A.; Russell, P.S.; Cizmar, T. Three-dimensional holographic optical manipulation through a high-numerical-aperture soft-glass multimode fibre. Nat. Photonics 2018, 12, 33. [Google Scholar] [CrossRef]
- Kreysing, M.K.; Kiessling, T.; Fritsch, A.; Dietrich, C.; Guck, J.R.; Kas, J.A. The optical cell rotator. Opt. Express 2008, 16, 16984–16992. [Google Scholar] [CrossRef]
- Zhang, H.; Lizana, A.; Van Eeckhout, A.; Turpin, A.; Ramirez, C.; Iemmi, C.; Campos, J. Microparticle Manipulation and Imaging through a Self-Calibrated Liquid Crystal on Silicon Display. Appl. Sci. 2018, 8, 2310. [Google Scholar] [CrossRef]
- Lizana, A.; Zhang, H.; Turpin, A.; Van Eeckhout, A.; Torres-Ruiz, F.A.; Vargas, A.; Ramirez, C.; Pi, F.; Campos, J. Generation of reconfigurable optical traps for microparticles spatial manipulation through dynamic split lens inspired light structures. Sci. Rep. 2018, 8, 11263. [Google Scholar] [CrossRef] [PubMed]
- Vertu, S.; Flugge, J.; Delaunay, J.J.; Haeberle, O. Improved and isotropic resolution in tomographic diffractive microscopy combining sample and illumination rotation. Cent. Eur. J. Phys. 2011, 9, 969–974. [Google Scholar] [CrossRef]
- Sweeney, D.W.; Vest, C.M. Reconstruction of three-dimensional refractive index fields by holographic interferometry. Appl. Opt. 1972, 11, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, I.; Zhang, T. Phase-shifting digital holography. Opt. Lett. 1997, 22, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Awatsuji, Y.; Fujii, A.; Kubota, T.; Matoba, O. Parallel three-step phase-shifting digital holography. Appl. Opt. 2006, 45, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Ellis, J.D.; Verlaan, A.L.; Bergmans, R.H.; Spronck, J.W.; Schmidt, R.H.M. Toward interferometry for dimensional drift measurements with nanometer uncertainty. Meas. Sci. Technol. 2011, 22. [Google Scholar] [CrossRef]
- Lazar, J.; Číp, O.; Čížek, M.; Hrabina, J.; Buchta, Z. Suppression of Air Refractive Index Variations in High-Resolution Interferometry. Sensors 2011, 11, 7644. [Google Scholar] [CrossRef]
- Massig, J.H. Digital off-axis holography with a synthetic aperture. Opt. Lett. 2002, 27, 2179–2181. [Google Scholar] [CrossRef]
- Herrera Ramírez, J.A.; Garcia-Sucerquia, J. Digital off-axis holography without zero-order diffraction via phase manipulation. Opt. Commun. 2007, 277, 259–263. [Google Scholar] [CrossRef]
- Mico, V.; Zalevsky, Z.; Garcia-Martinez, P.; Garcia, J. Synthetic aperture superresolution with multiple off-axis holograms. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2006, 23, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Primot, J.; Guerineau, N. Extended hartmann test based on the pseudoguiding property of a hartmann mask completed by a phase chessboard. Appl. Opt. 2000, 39, 5715–5720. [Google Scholar] [CrossRef] [PubMed]
- Primot, J. Theoretical description of Shack-Hartmann wave-front sensor. Opt. Commun. 2003, 222, 81–92. [Google Scholar] [CrossRef]
- Zhang, H.L.; Lizana, A.; Iemmi, C.; Monroy-Ramirez, F.A.; Marquez, A.; Moreno, I.; Campos, J. LCoS display phase self-calibration method based on diffractive lens schemes. Opt. Laser. Eng. 2018, 106, 147–154. [Google Scholar] [CrossRef]
- Zhang, H.L.; Lizana, A.; Iemmi, C.; Monroy-Ramirez, F.A.; Marquez, A.; Moreno, I.; Campos, J. Self-addressed diffractive lens schemes for the characterization of LCoS displays. In Proceedings of the Emerging Liquid Crystal Technologies Xiii, San Francisco, CA, USA, 8 February 2018. [Google Scholar]
- Primot, J.; Sogno, L. Achromatic three-wave (or more) lateral shearing interferometer. J. Opt. Soc. Am. A 1995, 12, 2679–2685. [Google Scholar] [CrossRef]
- Bon, P.; Maucort, G.; Wattellier, B.; Monneret, S. Quadriwave lateral shearing interferometry for quantitative phase microscopy of living cells. Opt. Express 2009, 17, 13080–13094. [Google Scholar] [CrossRef]
- Zhang, T.; Ruan, Y.; Maire, G.; Sentenac, D.; Talneau, A.; Belkebir, K.; Chaumet, P.C.; Sentenac, A. Full-polarized tomographic diffraction microscopy achieves a resolution about one-fourth of the wavelength. Phys. Rev. Lett. 2013, 111, 243904. [Google Scholar] [CrossRef]
- Godavarthi, C.; Zhang, T.; Maire, G.; Chaumet, P.C.; Giovannini, H.; Talneau, A.; Belkebir, K.; Sentenac, A. Superresolution with full-polarized tomographic diffractive microscopy. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2015, 32, 287–292. [Google Scholar] [CrossRef]
- Habaza, M.; Gilboa, B.; Roichman, Y.; Shaked, N.T. Tomographic phase microscopy with 180 degrees rotation of live cells in suspension by holographic optical tweezers. Opt. Lett. 2015, 40, 1881–1884. [Google Scholar] [CrossRef]
- Schmidt, R.; Engelhardt, J.; Lang, M. 4Pi Microscopy. Methods Mol. Biol. 2013, 950, 27–41. [Google Scholar] [CrossRef]
- Zhang, T.; Godavarthi, C.; Chaumet, P.C.; Maire, G.; Giovannini, H.; Talneau, A.; Prada, C.; Sentenac, A.; Belkebir, K. Tomographic diffractive microscopy with agile illuminations for imaging targets in a noisy background. Opt. Lett. 2015, 40, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, R.; Garcia-Sucerquia, J. Single-shot 3D topography of reflective samples with digital holographic microscopy. Appl. Opt. 2018, 57, A12–A18. [Google Scholar] [CrossRef] [PubMed]
- Nicola, S.D.; Ferraro, P.; Finizio, A.; Grilli, S.; Coppola, G.; Iodice, M.; Natale, P.D.; Chiarini, M. Surface topography of microstructures in lithium niobate by digital holographic microscopy. Meas. Sci. Technol. 2004, 15, 961–968. [Google Scholar] [CrossRef]
- Nanolive Company. Available online: www.nanolive.ch (accessed on 8 September 2019).
- Tomocube Company. Available online: www.tomocube.com (accessed on 8 September 2019).
- Pollaro, L.; Equis, S.; Dalla Piazza, B.; Cotte, Y. Stain-free 3D Nanoscopy of Living Cells. Optik Photonik 2016, 11, 38–42. [Google Scholar] [CrossRef]
- Pedrini, G.; Osten, W.; Gusev, M.E. High-speed digital holographic interferometry for vibration measurement. Appl. Opt. 2006, 45, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Trivedi, V.; Vora, P.; Chhaniwal, V.; Javidi, B.; Anand, A. Highly stable digital holographic microscope using Sagnac interferometer. Opt. Lett. 2015, 40, 3743–3746. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Mohanty, S.K.; Gupta, P.K. Controlled rotation of biological microscopic objects using optical line tweezers. Biotechnol. Lett. 2003, 25, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, B.; Chalmond, B.; Yu, Y.; Trouve, A.; Renaud, O.; Shorte, S.L. Isotropic high-resolution three-dimensional confocal micro-rotation imaging for non-adherent living cells. J. Microsc. 2009, 233, 404–416. [Google Scholar] [CrossRef]
- Trattner, S.; Feigin, M.; Greenspan, H.; Sochen, N. Validity criterion for the Born approximation convergence in microscopy imaging: Reply to comment. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2011, 28, 665–666. [Google Scholar] [CrossRef]
- Chen, B.; Stamnes, J.J. Validity of diffraction tomography based on the first born and the first rytov approximations. Appl. Opt. 1998, 37, 2996–3006. [Google Scholar] [CrossRef]
- Maruyama, Y.; Iwata, K.; Nagata, R. Measurement of the refractive index distribution in the interior of a solid object from multi-directional interferograms. Jpn. J. Appl. Phys. 1977, 16, 1171–1176. [Google Scholar] [CrossRef]




| Optical Schema/Configuration | Illumination Wavelength | Measured Sample | Lateral Resolution | Inversion | Reference |
|---|---|---|---|---|---|
| Off-axis/Reflection | 475 nm | nanofabricated objects | 50 nm | Non-linear | [10] |
| Off-axis/Completeness | 475 nm | Bellis perennis pollen grain Betula pollen grain | 200 nm | Linear | [25] |
| On-axis/Reflection | 633 nm | nanofabricated objects | 400 nm | Non-linear | [38] |
| On-axis/Completeness | 633 nm | prolate spheroid | ~300 nm | Linear | [60] |
| Wavefront sensors/Reflection | 633 nm | nanofabricated objects | 1 µm | Non-linear | [37] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Li, K.; Godavarthi, C.; Ruan, Y. Tomographic Diffractive Microscopy: A Review of Methods and Recent Developments. Appl. Sci. 2019, 9, 3834. https://doi.org/10.3390/app9183834
Zhang T, Li K, Godavarthi C, Ruan Y. Tomographic Diffractive Microscopy: A Review of Methods and Recent Developments. Applied Sciences. 2019; 9(18):3834. https://doi.org/10.3390/app9183834
Chicago/Turabian StyleZhang, Ting, Kan Li, Charankumar Godavarthi, and Yi Ruan. 2019. "Tomographic Diffractive Microscopy: A Review of Methods and Recent Developments" Applied Sciences 9, no. 18: 3834. https://doi.org/10.3390/app9183834
APA StyleZhang, T., Li, K., Godavarthi, C., & Ruan, Y. (2019). Tomographic Diffractive Microscopy: A Review of Methods and Recent Developments. Applied Sciences, 9(18), 3834. https://doi.org/10.3390/app9183834






