Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite
Abstract
1. Introduction
2. Experimental
2.1. Materials Used
2.2. Preparation of Activated Carbon
2.3. Synthesis of Adsorbent (Fe3O4/AC Composite)
2.4. Batch Adsorption Experiments
3. Results and Discussion
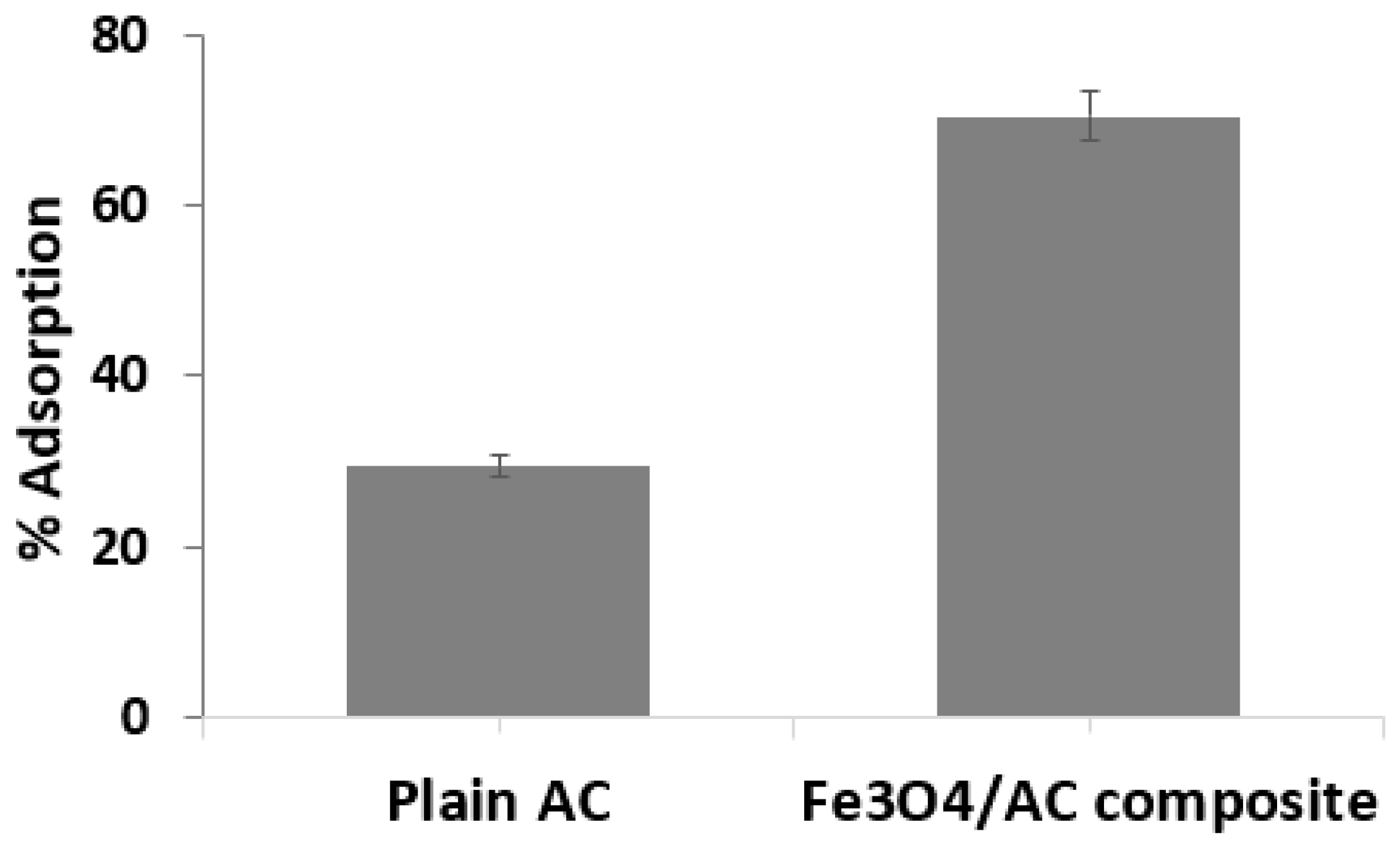
3.1. Comparisons of Percentage Adsorption
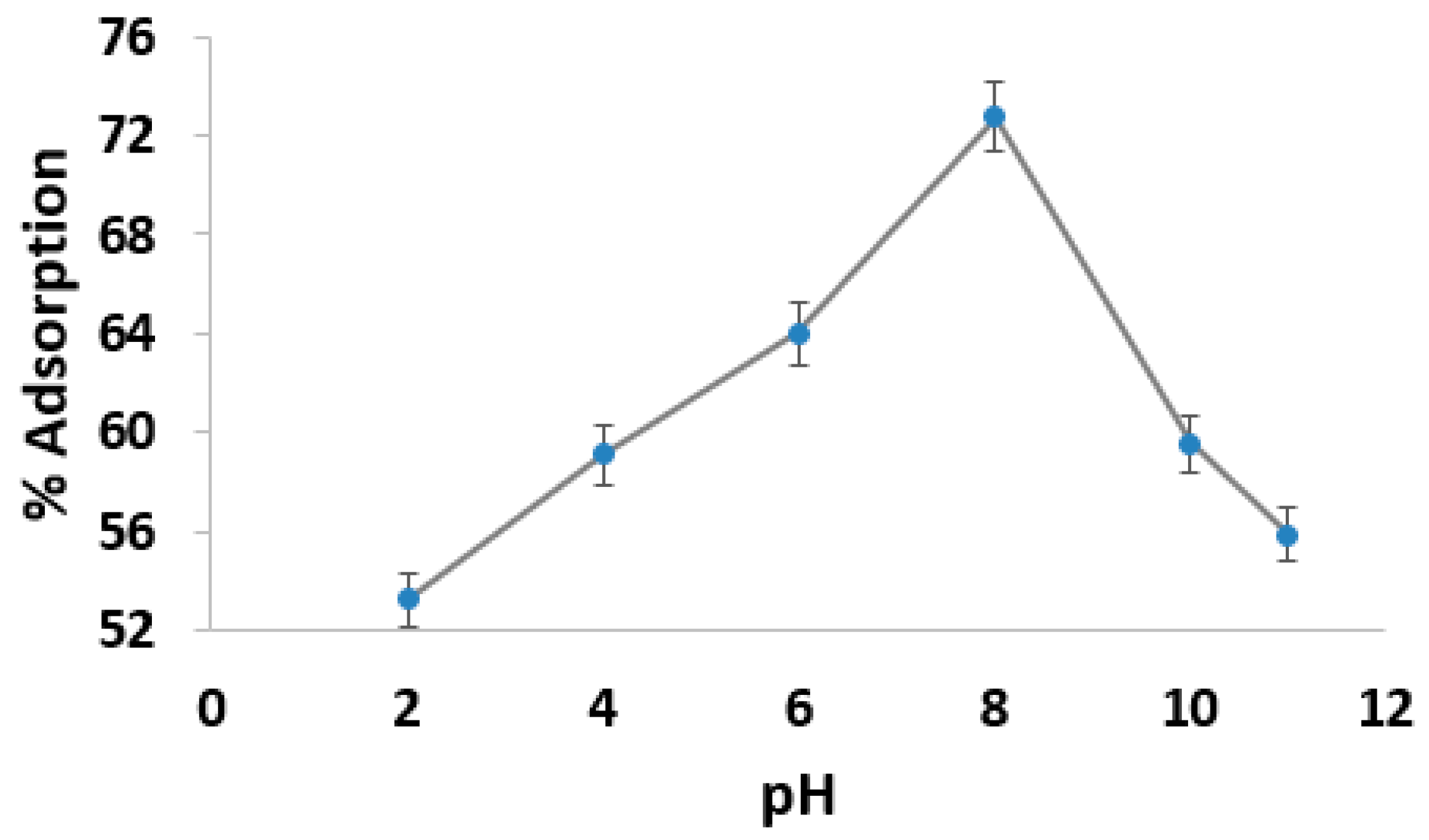
3.2. Effect of pH
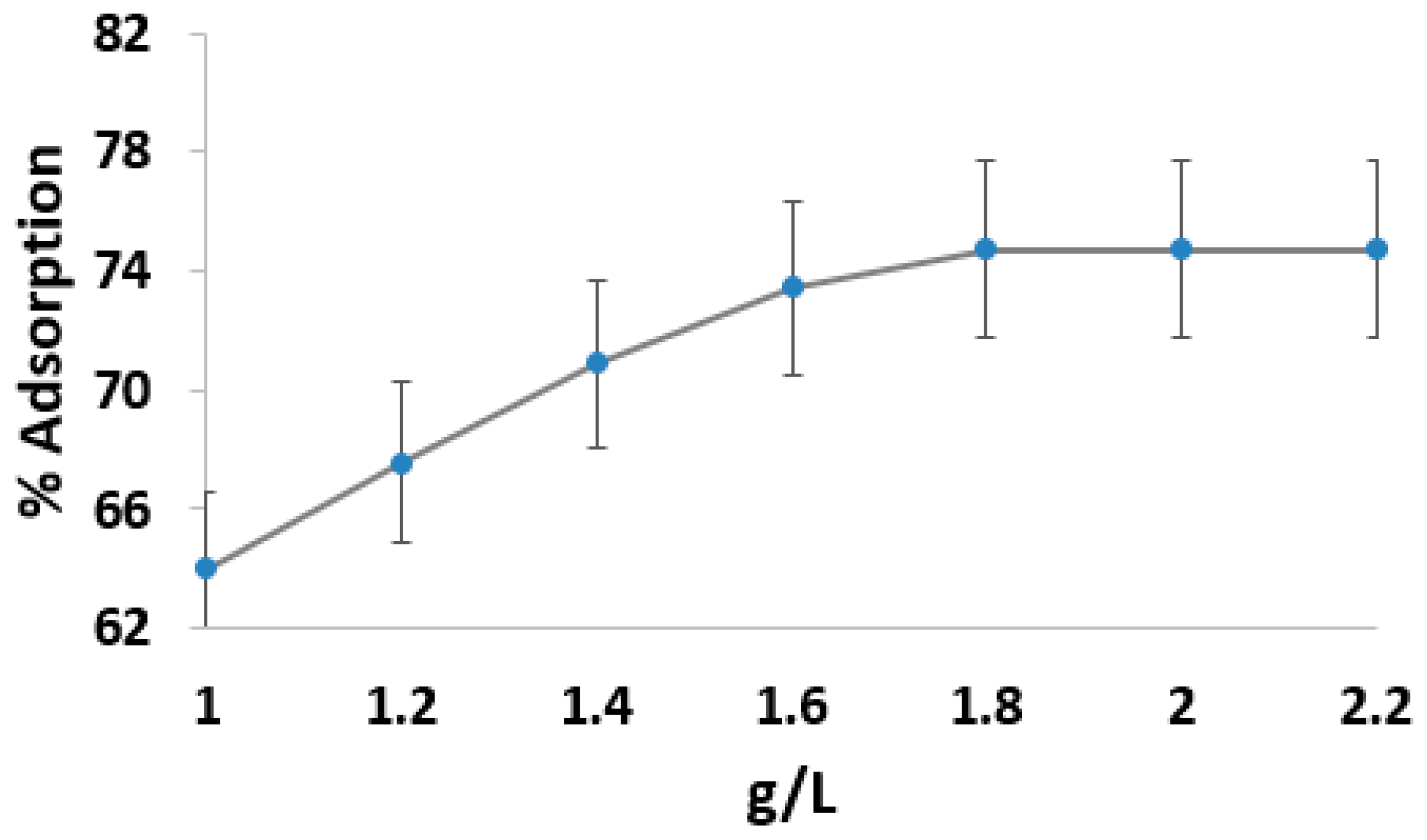
3.3. Effect of Adsorbent Dose
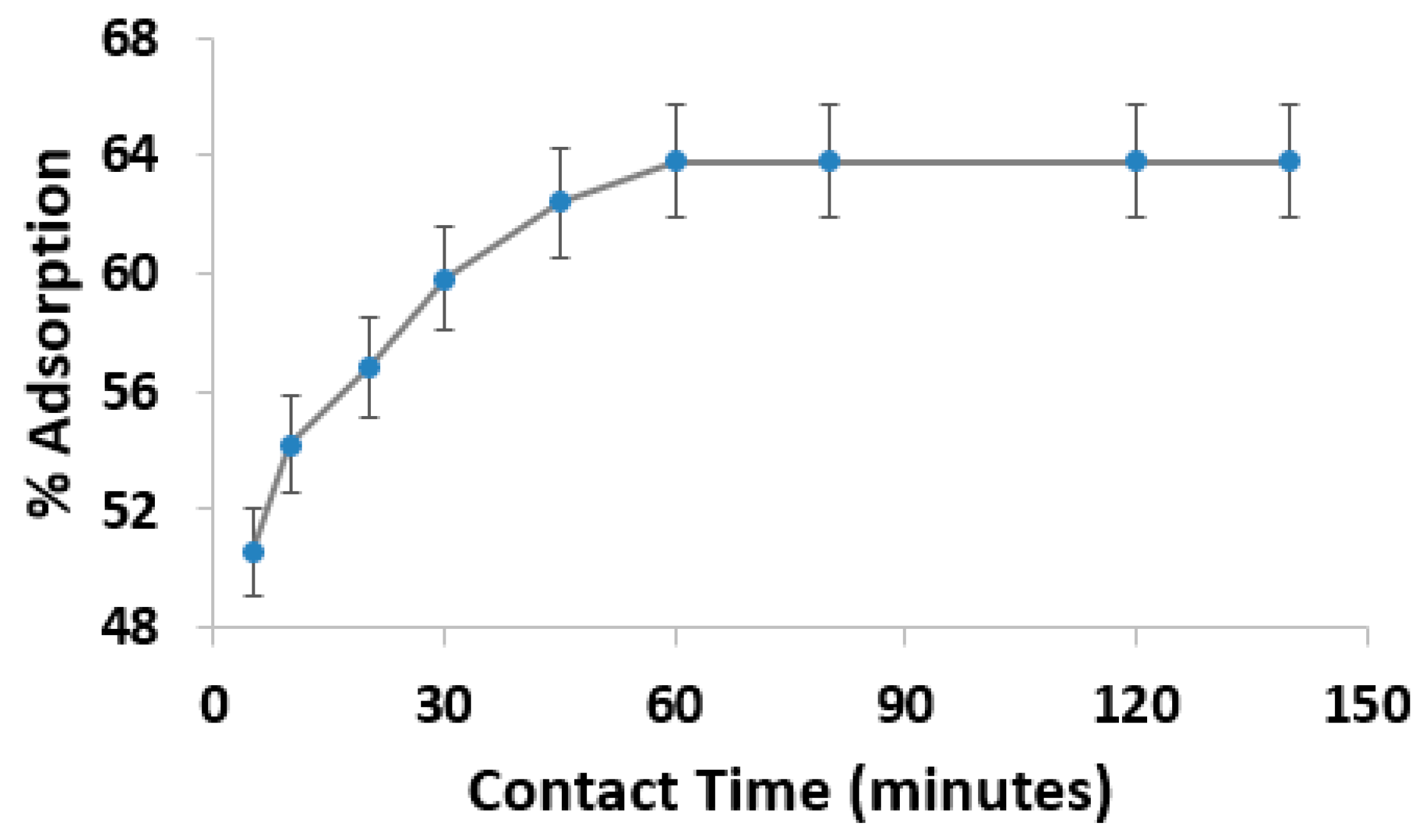
3.4. Effect of Contact Time
3.5. Adsorption Isotherm
3.6. Comparison of the Maximum Adsorption Capacity (qm) for As (III)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shankar, S.; Shikha, U. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 304524, 1–18. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 1996; Volume 2, pp. 940–949. [Google Scholar]
- Huq, S.M.I.; Joardar, J.C.; Parvin, S.; Correll, R.; Naidu, R. Arsenic Contamination in Food Chain: Transfer of Arsenic into Food Materials through Groundwater Irrigation. J. Healthpopul. Nutr. 2006, 24, 305–316. [Google Scholar]
- Pokhrel, D.; Bhandari, B.S.; Viraraghavan, T. Arsenic contamination of groundwater in the Terai region of Nepal: An overview of health concerns and treatment options. Environ. Int. 2009, 35, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.K.; Regmi, D.; Kafle, B.P. Seasonal variation of arsenic concentration in groundwater of Nawalparasi district of Nepal. Int. J. Appl. Sci. Biotechnol. 2014, 2, 59–63. [Google Scholar] [CrossRef]
- Thakur, J.K.; Thakur, R.K.; Ramanathan, A.L.; Kumar, M.; Singh, S.K. Review Arsenic Contamination of Groundwater in Nepal—An Overview. Water 2011, 3, 1–20. [Google Scholar] [CrossRef]
- Mueller, B. Preliminary trace element analysis of arsenic in Nepalese groundwater may pinpoint its origin. Environ. Earth Sci. 2018, 77, 1–6. [Google Scholar] [CrossRef]
- Jang, Y.C.; Somann, S.; Kim, H. Distribution, Toxicity and Remediation of Arsenic in the Environment—A review. Int. J. Appl. Environ. Sci. 2016, 11, 559–581. [Google Scholar]
- Mueller, B. Ground Water Contamination by Arsenic in Nepal: Lessons to be Learned from Geology. Austin Chem. Eng. 2019, 6, 1064. [Google Scholar]
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef]
- Bose, P.; Sharma, A. Role of iron in controlling speciation and mobilization of arsenic in subsurface environment. Water Res. 2002, 36, 4916–4926. [Google Scholar] [CrossRef]
- Masue, Y.; Loeppert, R.H.; Kramer, T.A. Arsenate and arsenite adsorption and desorption behavior on coprecipitated aluminum: Iron hydroxides. Environ. Sci. Technol. 2007, 41, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Amy, G.L.; Prevost, M. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Sperlich, A.; Werner, A.; Genz, A.; Amy, G.; Worch, E.; Jekel, M. Breakthrough behavior of granular ferric hydroxide (GFH) fixed-bed adsorption filters: Modeling and experimental approaches. Water Res. 2005, 39, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Amy, G.; Chung, J.; Sohn, J.; Yoon, Y. Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere 2009, 77, 228–235. [Google Scholar] [CrossRef]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. J. Environ. Health Sci. Eng. 2014, 12, 58. [Google Scholar] [CrossRef]
- Medina-Ramirez, A.; Beatriz, R.C.P.; Minchaca-Mojica, J.I.; Romero-Toledo, R.; Gamero-Vega, K.Y. Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite. Water 2019, 11, 281. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Aredes, S.; Klein, B.; Pawlik, M. The removal of arsenic from water using natural iron oxide minerals. J. Clean. Prod. 2012, 29–30, 208–213. [Google Scholar] [CrossRef]
- Polowczyk, I.; Cyganowski, P.; Ulatowska, J.; Sawiński, W.; Bastrzyk, A. Synthetic Iron Oxides for Adsorptive Removal of Arsenic. Water Air Soil Pollut. 2018, 229, 203. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Gao, N.Y.; Lin, Y.C.; Xu, B.; Le, L.S. Removal of Arsenic(V) from Aqueous Solutions Using Iron-Oxide-Coated Modified Activated Carbon. Water Environ. Res. 2007, 79, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Ciminelli, V.S.T.; Dantas, M.S.S.; Willscher, S. A comparative study of As(III) and As(V) in aqueous solutions and adsorbed on iron oxyhydroxides by Raman Spectroscopy. Water Res. 2010, 44, 5660–5672. [Google Scholar] [CrossRef] [PubMed]
- Wannahari, R.; Sannasi, P.; Nordin, M.F.M.; Mukhtar, H. Sugarcane bagasse derived nano magnetic adsorbent composite (SCB-NMAC) for removal of Cu2+ from aqueous solution. ARPN J. Eng. Appl. Sci. 2018, 13, 1–9. [Google Scholar]
- Kakavandi, B.; Kalantary, R.R.; Farzadkia, M.; Mahvi, A.H.; Esrafili, A.; Azari, A.; Yari, A.R.; Javid, A.B. Enhanced chromium (VI) removal using activated carbonmodified by zero valent iron and silver bimetallic nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Gholamvaisi, D.; Azizian, S.; Cheraghi, M. Preparation of magnetic-activated carbon nanocomposite and its application for dye removal from aqueous solution. J. Dispers. Sci. Technol. 2014, 35, 1264–1269. [Google Scholar] [CrossRef]
- Affam, A.C.; Wong, C.C.; Seyam, M.A.B.; Matt, C.A.A.F.; Sumbai, J.L.A.; Evuti, A.M. Preparation, characterization and adsorption study of granular activatedcarbon/iron oxide composite for the removal of boron and organics from wastewater. E3S Web Conf. 2018, 34, 02006. [Google Scholar] [CrossRef]
- Yao, S.; Sun, S.; Wang, S.; Shi, Z. Adsorptive removal of lead ion from aqueous solution by activated carbon/iron oxide magnetic composite. Indian J. Chem. Technol. 2016, 23, 146–152. [Google Scholar]
- Liang, S.Z.; Mei, L.F.; Hua, Y.S. Adsorptive removal of phosphate from aqueous solutions using activated carbon loaded with Fe(III) oxide. New Carbon Mater. 2011, 26, 299–306. [Google Scholar]
- Castro, C.S.; Guerreiro, M.S.; Goncalves, M.; Oliveira, L.C.A.; Anastácio, A.S. Activated carbon/iron oxide composites for the removal of atrazine from aqueousmedium. J. Hazard. Mater. 2009, 164, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants inwater. Carbon 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Faulconer, E.K.; Hoogesteijn, N.V.V.R.; Mazyck, D.W. Optimization of magnetic powdered activated carbon for aqueous Hg(II) removal and magnetic recovery. J. Hazard. Mater. 2012, 199–200, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.; Morad, N.; Teng, T.T.; Norli, I.; Panneerselvam, P. Removal of cationic dye by magnetic nanoparticle (Fe3O4) impregnated onto activated maize cobpowder and kinetic study of dye waste adsorption. APCBEE Procedia 2012, 1, 83–89. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the valorization of lignocellulosic materials by biotechnology: An overview. Bioresources 2013, 8, 2. [Google Scholar] [CrossRef]
- Debalina, B.; Reddy, R.B.; Vinu, R. Production of carbon nanostructures in biochar, bio-oil and gases from bagasse via microwave assisted pyrolysis using Fe and Co as susceptors. J. Anal. Appl. Pyrolysis 2017, 24, 310–318. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Dumbre, D.K.; Inamuddin, A.M.A.; Bhutto, A.W.; Srinivasan, M.; Griffin, G.J. Synthesis of magnetic carbon nanocomposites by hydrothermal carbonization and pyrolysis. Environ. Chem. Lett. 2018, 16, 821–844. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, S.; Zhang, D.; Xu, S. Synthesis and properties of magnetic Fe3O4-activated carbon nanocomposite particles for dye removal. Mater. Lett. 2008, 62, 645–647. [Google Scholar] [CrossRef]
- Seabra, J.E.A.; Tao, L.; Chum, H.L.; Macedo, I.C. A techno-economic evaluation of the effects of centralized cellulosic ethanol and co-products refinery options with sugarcane mill clustering. Biomass Bioenergy 2010, 34, 1065–1078. [Google Scholar] [CrossRef]
- Neupane, P.R.; Maraseni, T.N.; Koehl, M. The sugarcane industry in Nepal: Opportunities and challenges. Environ. Dev. 2017, 24, 86–98. [Google Scholar] [CrossRef]
- Sandell, E.B. Colorimetric Determination of Traces of Metals; Interscience Publisher: New York, NY, USA, 1959; pp. 1906–1984. [Google Scholar]
- Mahmood, T.; Aslam, M.; Naeem, A.; Siddique, T.; Din, S.U. Adsorption of As(III) from aqueous solution onto iron impregnated used tea activated carbon: Equilibrium, kinetic and thermodynamic study. J. Chil. Chem. Soc. 2018, 63, 3855–3866. [Google Scholar] [CrossRef]
- Agrawal, S.; Singh, N.B. Removal of arsenic from aqueous solution by an adsorbent nickel ferrite-polyaniline nanocomposite. Indian J. Chem. Technol. 2016, 5, 374–383. [Google Scholar]
- Thakuri, H.C.; Pokhrel, M.R.; Ghimire, K.N.; Khadka, D.B. Removal of Arsenic from Aqueous Solution Using Iron(III)-loaded Sugarcane Bagasse. Res. J. Chem. Sci. 2015, 11, 51–58. [Google Scholar]
- Thapa, S.; Pokhrel, M.R. Removal of As(III) from Aqueous Solution Using Fe(III) Loaded Pomegranate Waste. J. Nepal Chem. Soc. 2012, 30, 29–36. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Das, B.; Mondal, N.K.; Bhaumik, R.; Roy, P. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int. J. Environ. Sci. Technol. 2014, 11, 1101–1114. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Rajbhandari, R. Preparation and Characterization of Activated Carbon from Lapsi (Choerospondias axillaris) Seed Stone for the Removal of Arsenic (iii) from Water. Ph.D. Thesis, Department of Engineering Science and Humanities, Pulchowk Campus, Tribhuvan University, Lalitpur, Nepal, 2014. [Google Scholar]
- Alarcon, M.; Lopez, M. Technical feasibility of using magnetic nanoparticles obtained from metallic wool for arsenite (As (III)) removal from aqueous solutions. J. Nanosci. Technol. 2016, 4, 35–43. [Google Scholar]
- Rahman, S.; Yanful, E. Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 11, 2238–2247. [Google Scholar]
- Joshi, S.; Kumari, A.; Banjara, A.; Sharma, M. Use of iron oxide/activated carbon magnetic composite for adsorptive removal of arsenic from water. Int. J. Adv. Eng. 2019, 1, 9–16. [Google Scholar]
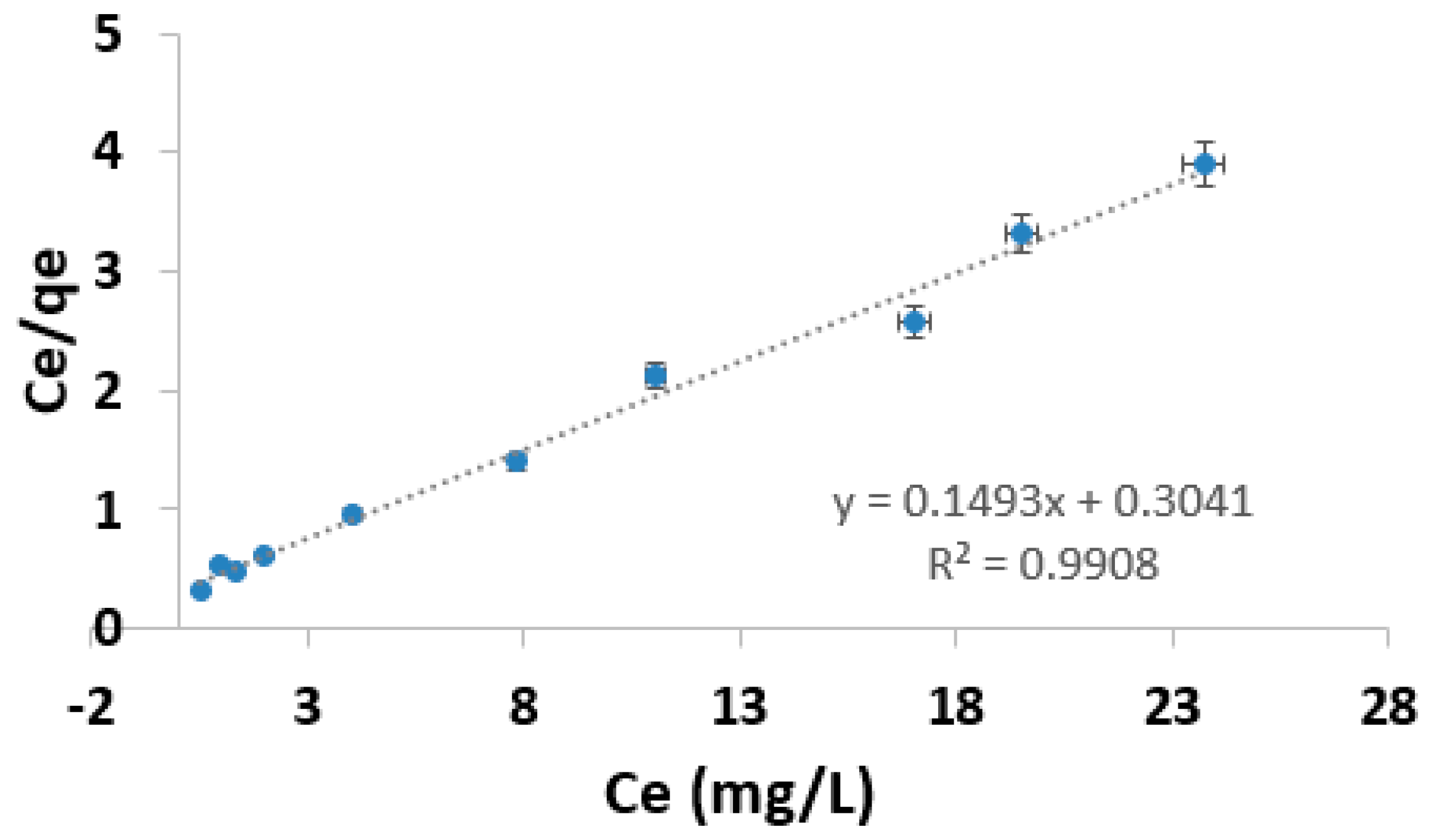
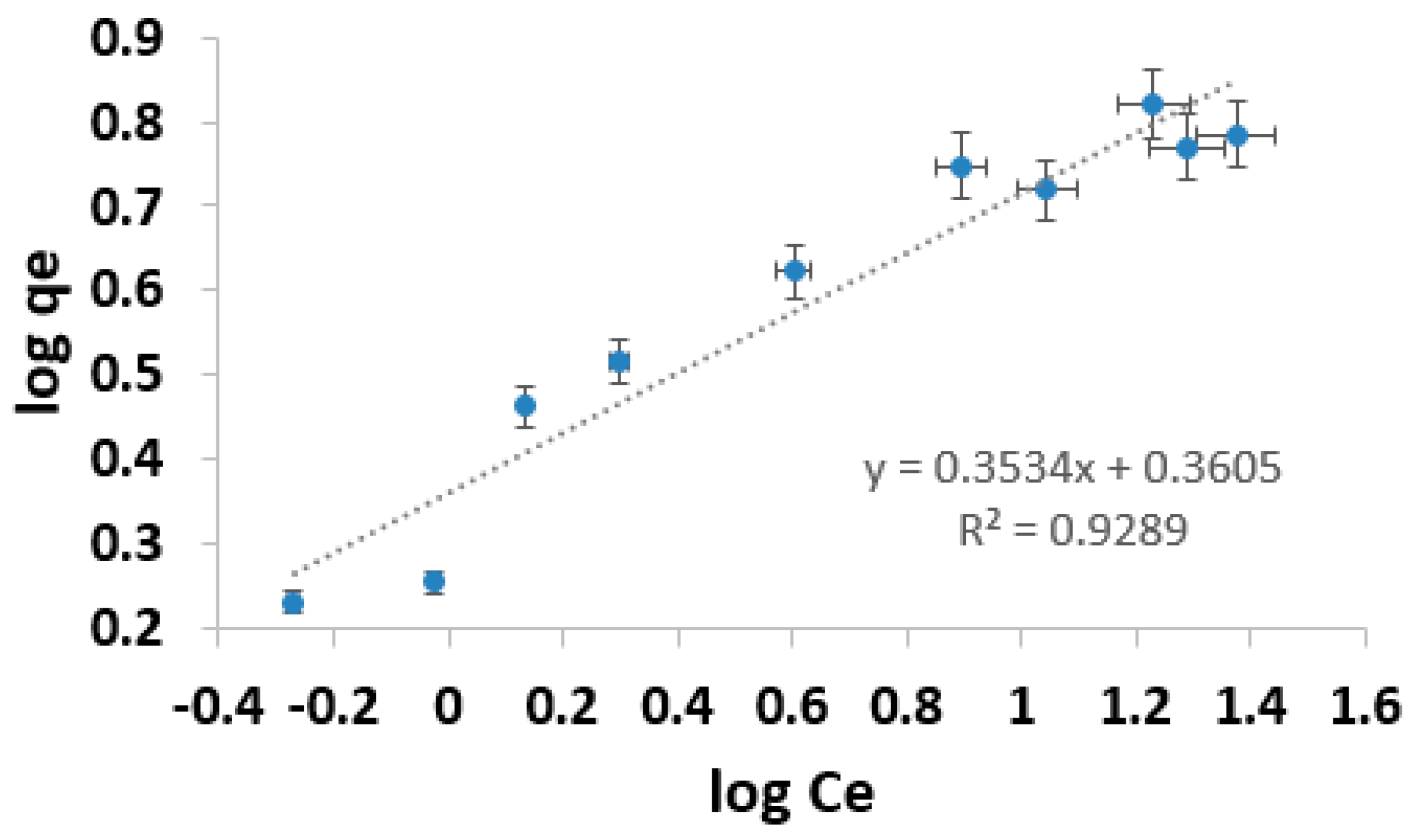
| Species | Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | K (mg/g) | 1/n | R2 | ||
| As (III) | AC composite | 6.69 | 0.491 | 0.991 | 2.293 | 0.353 | 0.929 |
| Adsorbents | Maximum Adsorption Capacity (qm) | References |
|---|---|---|
| Iron impregnated AC from Lapsi seed stone | 2 mg/g | Rajbhandari R. [48] |
| Magnetic nanoparticles obtained from metallic wool | 2.2 mg/g. | Alarcon M. and Lopez M. [49] |
| Magnetite-maghemite nanoparticles | 3.69 mg/g | Rahman S. and Yanful E. [50] |
| Iron oxide/commercial activated carbon composite | 7.5 mg/g | Previous study [51] |
| Magnetic Fe3O4/sugarcane Bagasse activated carbon Composite | 6.69 mg/g | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. https://doi.org/10.3390/app9183732
Joshi S, Sharma M, Kumari A, Shrestha S, Shrestha B. Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Applied Sciences. 2019; 9(18):3732. https://doi.org/10.3390/app9183732
Chicago/Turabian StyleJoshi, Sahira, Manobin Sharma, Anshu Kumari, Surendra Shrestha, and Bhanu Shrestha. 2019. "Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite" Applied Sciences 9, no. 18: 3732. https://doi.org/10.3390/app9183732
APA StyleJoshi, S., Sharma, M., Kumari, A., Shrestha, S., & Shrestha, B. (2019). Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Applied Sciences, 9(18), 3732. https://doi.org/10.3390/app9183732






