Contrasting Effects of Sediment Microbial Fuel Cells (SMFCs) on the Degradation of Macrophyte Litter in Sediments from Different Areas of a Shallow Eutrophic Lake
Abstract
1. Introduction
2. Material and Methods
2.1. Sediment, Lake Water and Macrophyte Sampling
2.2. Experimental Design
2.3. Chemical Analyses
2.4. Microbial Diversity Analysis with High-Throughput Pyrosequencing
2.5. Calculation and Statistical Analysis
3. Results and Discussion
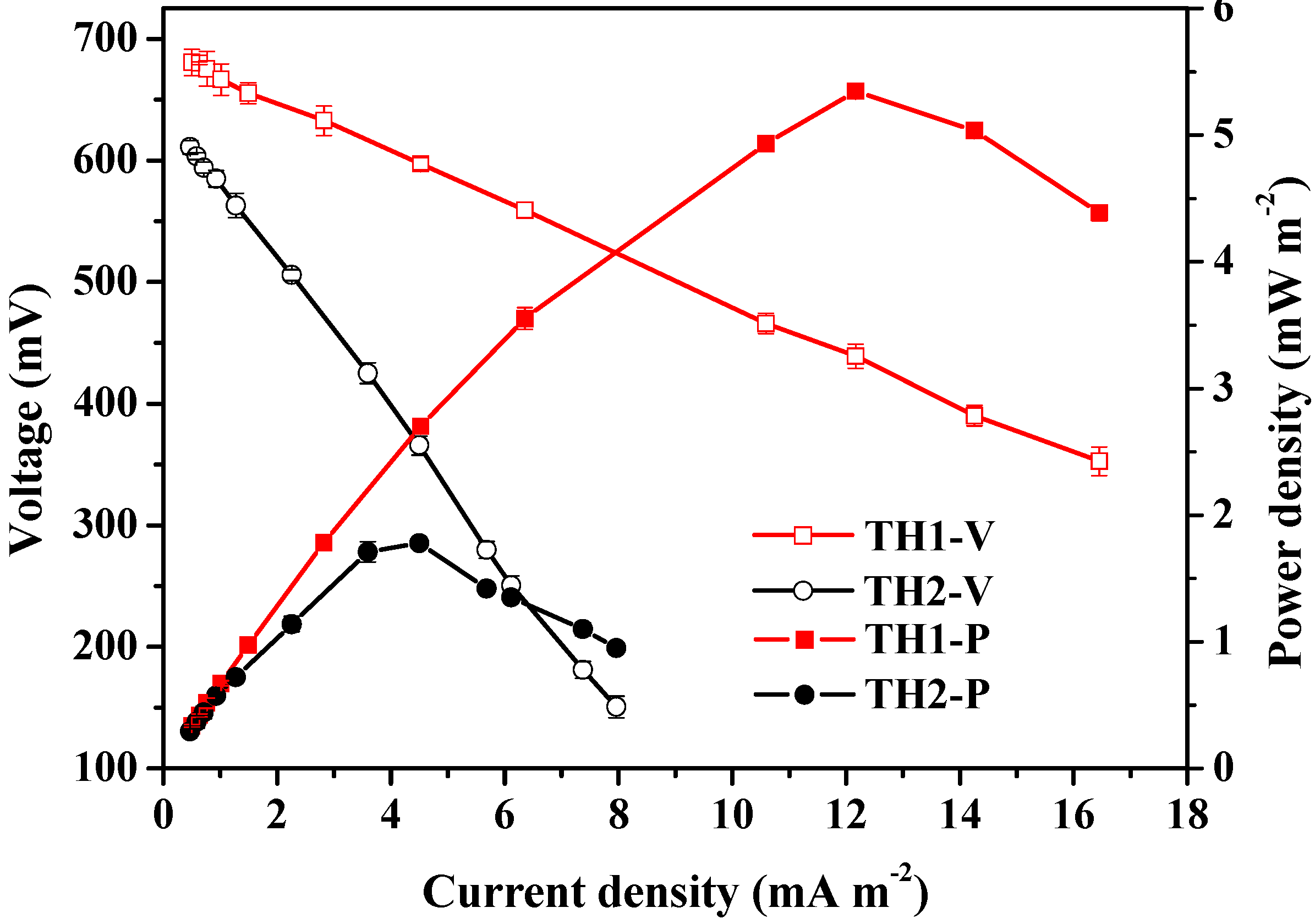
3.1. Chemical Properties of the Original Sediments and Lake Water and Electrochemical Performance from SMFCs
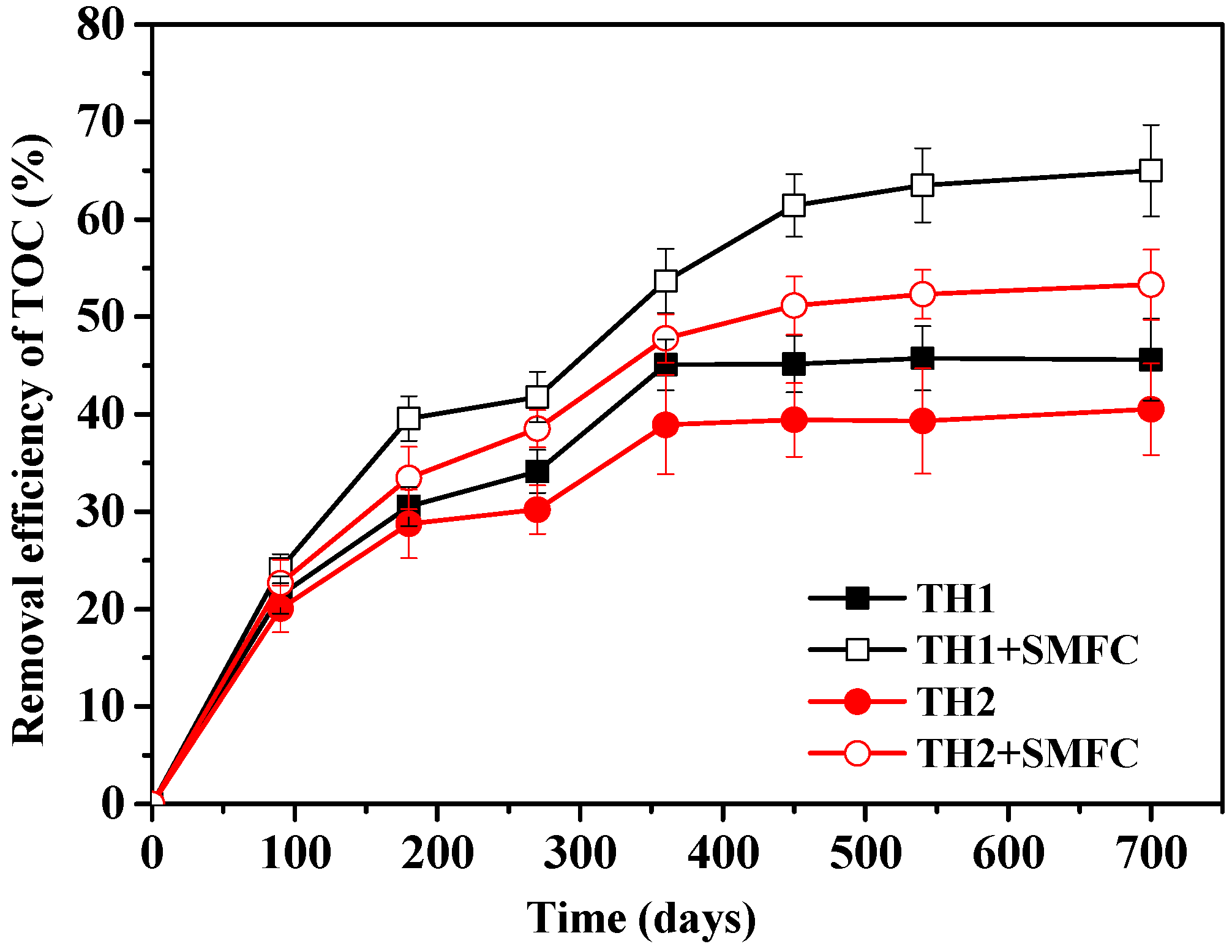
3.2. Changing of TOC Removal Efficiencies during SMFC Operation
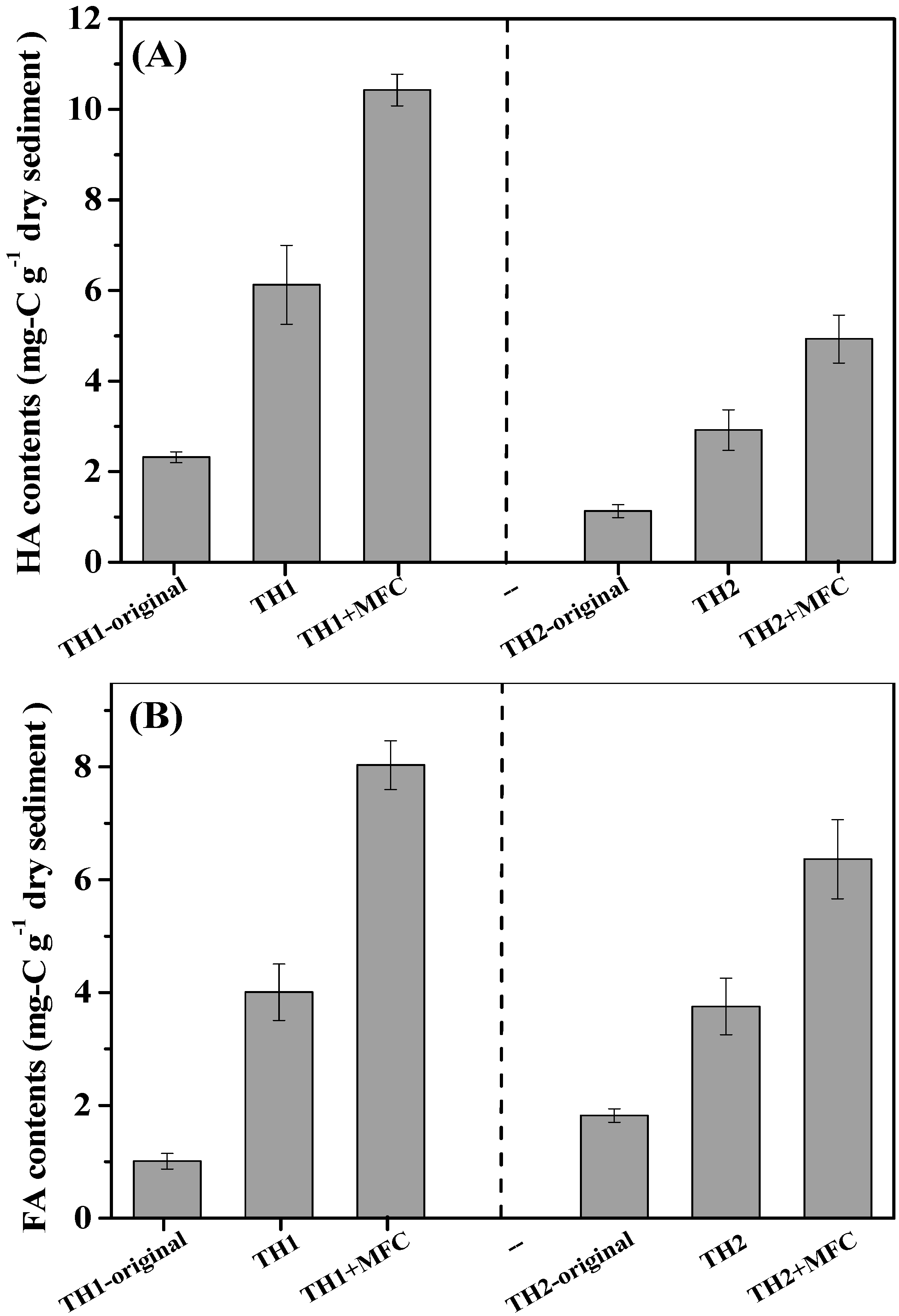
3.3. Alternation of Sediment Humic Substances in SMFCs
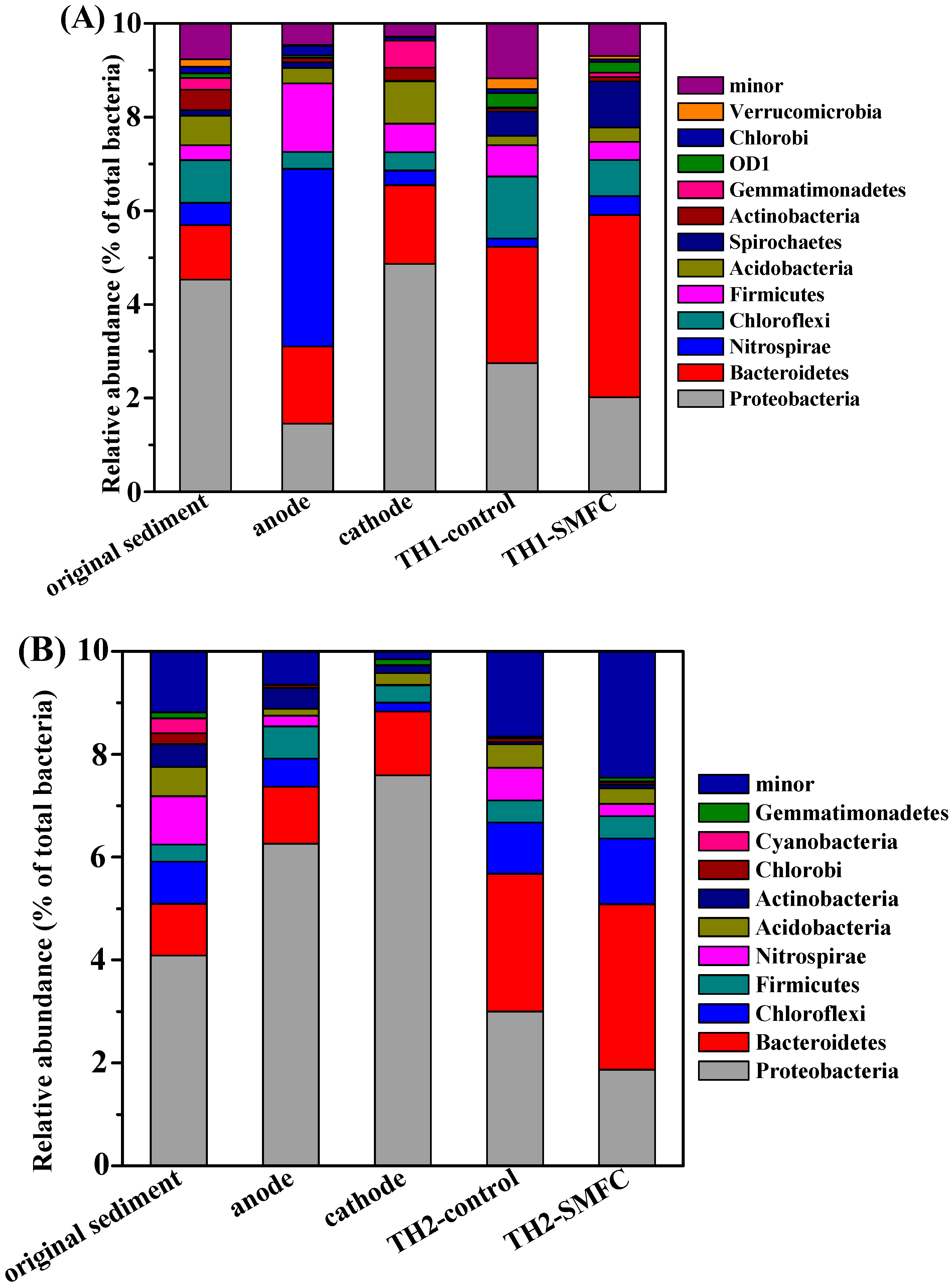
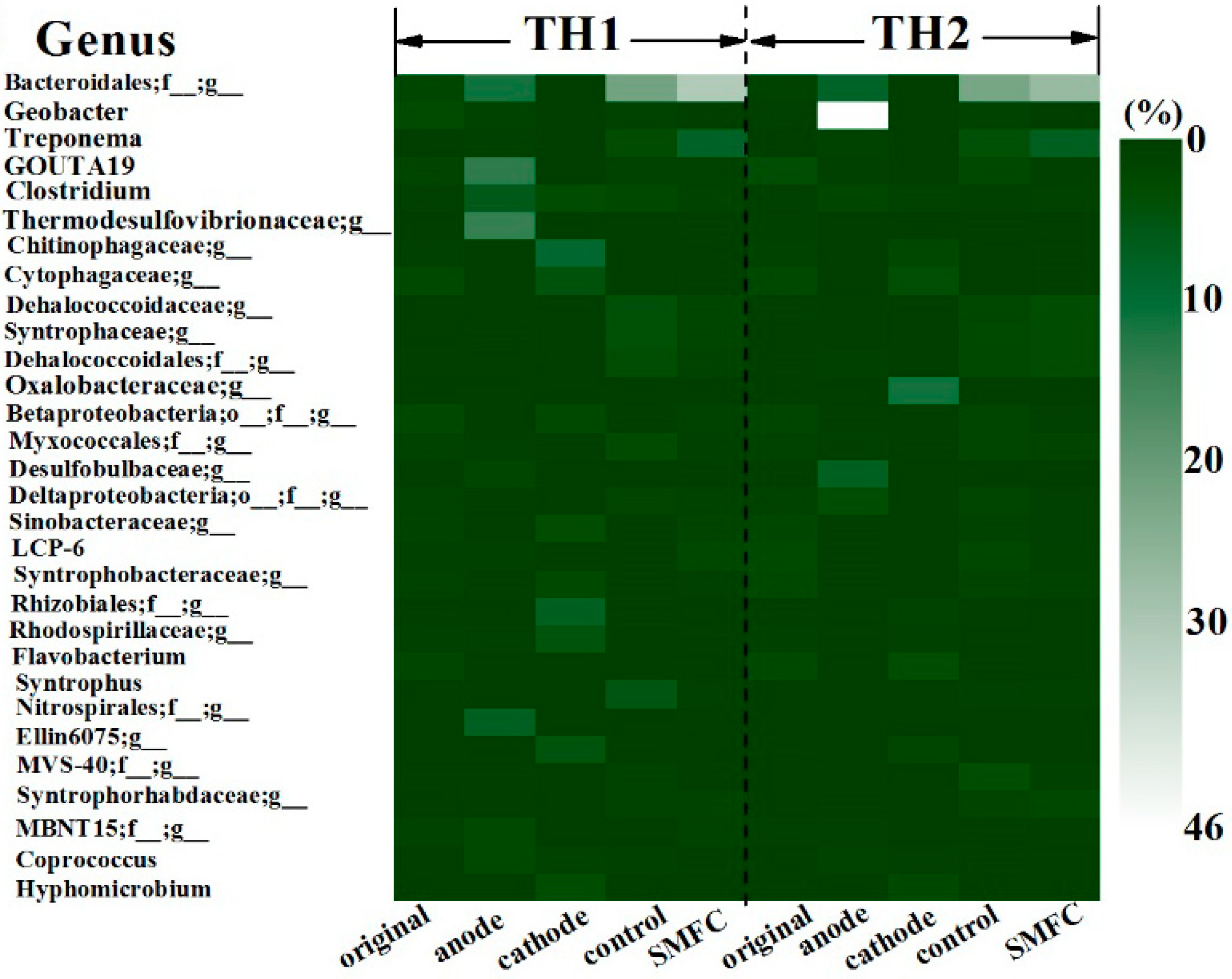
3.4. Microbial Communities Diversity in Sediments and on the Surfaces of Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCrackin, M.L.; Jones, H.P.; Jones, P.C.; Moreno-Mateos, D. Recovery of lakes and coastal marine ecosystems from eutrophication: A global meta-analysis. Limnol. Oceanogr. 2017, 62, 507–518. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Chimney, M.J.; Pietro, K.C. Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecol. Eng. 2006, 27, 301–321. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; Smolders, A.J.P.; Banach, A.M.; de Graaf, J.P.M.V.; Roelofs, J.G.M.; Lamers, L.P.M. The interaction between decomposition, net N and P mineralization and their mobilization to the surface water in fens. Water Res. 2010, 44, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.R.; Wu, Z.B.; Liu, B.Y.; Deng, J.Q.; Fu, G.P.; He, F. The restoration of aquatic macrophytes for improving water quality in a hypertrophic shallow lake in Hubei Province, China. Ecol. Eng. 2001, 18, 147–156. [Google Scholar] [CrossRef]
- Li, X.; Cui, B.S.; Yang, Q.C.; Lan, Y.; Wang, T.T.; Han, Z. Effects of plant species on macrophyte decomposition under three nutrient conditions in a eutrophic shallow lake, North China. Ecol. Model. 2013, 252, 121–128. [Google Scholar] [CrossRef]
- Carmichael, M.J.; Helton, A.M.; White, J.C.; Smith, W.K. Standing dead trees are a conduit for the atmospheric flux of CH4 and CO2 from wetlands. Wetlands 2018, 38, 133–143. [Google Scholar] [CrossRef]
- Nyenje, P.M.; Foppen, J.W.; Uhlenbrook, S.; Kulabako, R.; Muwanga, A. Eutrophication and nutrient release in urban areas of sub-Saharan Africa—A review. Sci. Total Environ. 2010, 408, 447–455. [Google Scholar] [CrossRef]
- Sygmund, C.; Kracher, D.; Scheiblbrandner, S.; Zahma, K.; Felice, A.K.G.; Harreither, W.; Kittl, R.; Ludwig, R. Characterization of the two neurospora crassa cellobiose dehydrogenases and their connection to oxidative cellulose degradation. Appl. Environ. Microbiol. 2012, 78, 6161–6171. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.S.; He, Y.H.; Cai, H.Y.; Nostrand, J.D.V.; He, Z.L.; Zhou, J.Z.; Krumholz, L.R.; Jiang, H.L. Interconnection of key microbial functional genes for enhanced Benzo[a] pyrene biodegradation in sediments by microbial electrochemistry. Environ. Sci Technol. 2017, 51, 8519–8529. [Google Scholar] [CrossRef] [PubMed]
- Drendel, G.; Mathews, E.R.; Semenec, L.; Franks, A.E. Microbial fuel cells, related technologies, and their applications. Appl. Sci. 2018, 8, 2384. [Google Scholar] [CrossRef]
- Hong, S.W.; Kim, H.S.; Chung, T.H. Alteration of sediment organic matter in sediment microbial fuel cells. Environ. Pollut. 2010, 158, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, K.P.; Becker, J.G. Design and characterization of a microbial fuel cell for the conversion of a lignocellulosic crop residue to electricity. Bioresour. Technol. 2012, 119, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Sajana, T.K.; Ghangrekar, M.M.; Mitra, A. Effect of presence of cellulose in the freshwater sediment on the performance of sediment microbial fuel cell. Bioresour. Technol. 2014, 155, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Jiang, H.L.; Cai, H.Y.; Yan, Z.S.; Zhou, Y.L. Beyond enhancement of macrophyte litter decomposition in sediments from a terrestrializated shallow lake through bioanode employment. Chem. Eng. J. 2015, 279, 433–441. [Google Scholar] [CrossRef]
- Song, N.; Yan, Z.S.; Cai, H.Y.; Jiang, H.L. Effect of temperature on submerged macrophyte litter decomposition within sediments from a large shallow and subtropical freshwater lake. Hydrobiologia 2013, 714, 131–144. [Google Scholar] [CrossRef]
- Jiang, H.R.; Shyy, W.; Wu, M.C.; Wei, L.; Zhao, T.S. Highly active, bi-functional and metal-free B4C-nanoparticle-modified graphite felt electrodes for vanadium redox flow batteries. J. Power Sources 2017, 365, 34–42. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Song, N.; Jiang, H.L. Effects of initial sediment properties on start-up times for sediment microbial fuel cells. Int. J. Hydrogen Energy 2018, 43, 10082–10093. [Google Scholar] [CrossRef]
- Thakuria, D.; Schmidt, O.; Mac Siurtain, M.; Egan, D.; Doohan, F.M. Importance of DNA quality in comparative soil microbial community structure analyses. Soil Biol. Biochem. 2008, 40, 1390–1403. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Callaghan, T.M.; Douglas, B.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Yang, Y.; Chen, M.; Zhao, Z.W.; Jiang, H.L. To improve the performance of sediment microbial fuel cell through amending colloidal iron oxyhydroxide into freshwater sediments. Bioresour. Technol. 2014, 159, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Yan, Z.S.; Xu, H.C.; Yao, Z.B.; Wang, C.H.; Chen, M.; Zhao, Z.W.; Peng, Z.L.; Wang, C.L.; Jiang, H.L. Development of a sediment microbial fuel cell-based biosensor for sinultaneous online monitoring of dissolved oxygen. Sci. Total Environ. 2019, 673, 272–280. [Google Scholar] [CrossRef]
- Hong, S.; Chang, I.; Choi, Y.; Kim, B.; Chung, T. Responses from freshwater sediment during electricity generation using microbial fuel cells. Bioproc. Biosyst. Eng. 2009, 32, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Babanova, S.; Hubenova, Y.; Mitov, M.; Mandjukov, P. Uncertainties of yeast-based biofuel cell operational characteristics. Fuel Cells 2011, 11, 824–837. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Early-stage performance evaluation of flowing microbial fuel cells using chemically treated carbon felt and yeast biocatalyst. Appl. Energy 2018, 222, 369–382. [Google Scholar] [CrossRef]
- Mitov, M.; Bardarov, I.; Mandjukov, P.; Hubenova, Y. Chemometrical assessment of the electrical parameters obtained by long-term operating freshwater sediment microbial fuel cells. Bioelectrochemistry 2015, 106, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Richard, T.L.; Brennan, R.A.; Logan, B.E. Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ. Sci. Technol. 2007, 41, 4053–4058. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, E.I.; Avendaño, K.A.; Balagurusamy, N.; Arriaga, S.; Nieto-Delgado, C.; Thalasso, F.; Cervantes, F.J. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci. Total Environ. 2019, 650, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.S.; Song, N.; Cai, H.Y.; Tay, J.H.; Jiang, H.L. Enhanced degradation of phenanthrene and pyrene in freshwater sediments by combined employment of sediment microbial fuel cell and amorphous ferric hydroxide. J. Hazard. Mater. 2012, 199, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; He, Y.H.; Jiang, H.L. Inferior adaptation of bay sediments in a eutrophic shallow lake to winter season for organic matter decomposition. Environ. Pollut. 2016, 219, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.S.; Jiang, H.L.; Cai, H.Y.; Zhou, Y.L.; Krumholz, L.R. Complex interactions between the macrophyte acorus calamus and microbial fuel cells during Pyrene and Benzo[a]Pyrene Degradation in sediments. Sci. Rep. 2015, 5, 10709. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Gao, Y.M.; Wang, Y.J.; Liu, Q.; Sun, Z.Y.; Fu, B.R.; Wen, X.; Cui, Z.J.; Wang, W.D. Diversity of a mesophilic lignocellulolytic microbial consortium which is useful for enhancement of biogas production. Bioresour. Technol. 2012, 111, 49–54. [Google Scholar] [CrossRef]
- Lucey, K.S.; Leadbetter, J.R. Catechol 2,3-dioxygenase and other meta-cleavage catabolic pathway genes in the ‘anaerobic’ termite gut spirochete Treponema primitia. Mol. Ecol. 2014, 23, 1531–1543. [Google Scholar] [CrossRef]
- Tulupova, Y.R.; Parfenova, V.V.; Sitnikova, T.Y.; Sorokovnikova, E.G.; Khanaev, I.B. First report on bacteria of the family Spirochaetaceae from digestive tract of endemic gastropods from Lake Baikal. Microbiology 2012, 81, 460–467. [Google Scholar] [CrossRef]
- Gu, Y.; Ding, Y.; Ren, C.; Sun, Z.; Rodionov, D.A.; Zhang, W.W.; Yang, S.; Yang, C.; Jiang, W.H. Reconstruction of xylose utilization pathway and regulons in Firmicutes. BMC Genom. 2010, 11, 255. [Google Scholar] [CrossRef]
- Dos Passos, V.F.; Marcilio, R.; Aquino-Neto, S.; Santana, F.B.; Dias, A.C.F.; Andreote, F.D.; de Andrade, A.R.; Reginatto, V. Hydrogen and electrical energy co-generation by a cooperative fermentation system comprising Clostridium and microbial fuel cell inoculated with port drainage sediment. Bioresour. Technol. 2019, 277, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bardarov, I.; Mitov, M.; Ivanova, D.; Hubenova, Y. Light-dependent processes on the cathode enhance the electrical outputs of sediment microbial fuel cells. Bioelectrochemistry 2018, 122, 1–10. [Google Scholar] [CrossRef] [PubMed]

| Samples | Number of Sequences a | OTUs b | Shannon c | Coverage d | |
|---|---|---|---|---|---|
| Site TH1 | original | 12,503 | 1329 | 7.94 | 92.1% |
| anode | 5671 | 567 | 5.63 | 90.2% | |
| cathode | 3257 | 351 | 4.36 | 88.5% | |
| control | 10,276 | 1026 | 7.68 | 91.3% | |
| SMFC | 9876 | 756 | 6.65 | 90.9% | |
| Site TH2 | original | 11,165 | 1021 | 7.78 | 91.5% |
| anode | 3524 | 452 | 5.21 | 89.3% | |
| cathode | 2367 | 254 | 4.02 | 89.1% | |
| control | 9056 | 956 | 7.25 | 88.6% | |
| SMFC | 7862 | 636 | 6.01 | 90.2% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, N.; Jiang, H.; Yan, Z. Contrasting Effects of Sediment Microbial Fuel Cells (SMFCs) on the Degradation of Macrophyte Litter in Sediments from Different Areas of a Shallow Eutrophic Lake. Appl. Sci. 2019, 9, 3703. https://doi.org/10.3390/app9183703
Song N, Jiang H, Yan Z. Contrasting Effects of Sediment Microbial Fuel Cells (SMFCs) on the Degradation of Macrophyte Litter in Sediments from Different Areas of a Shallow Eutrophic Lake. Applied Sciences. 2019; 9(18):3703. https://doi.org/10.3390/app9183703
Chicago/Turabian StyleSong, Na, Helong Jiang, and Zaisheng Yan. 2019. "Contrasting Effects of Sediment Microbial Fuel Cells (SMFCs) on the Degradation of Macrophyte Litter in Sediments from Different Areas of a Shallow Eutrophic Lake" Applied Sciences 9, no. 18: 3703. https://doi.org/10.3390/app9183703
APA StyleSong, N., Jiang, H., & Yan, Z. (2019). Contrasting Effects of Sediment Microbial Fuel Cells (SMFCs) on the Degradation of Macrophyte Litter in Sediments from Different Areas of a Shallow Eutrophic Lake. Applied Sciences, 9(18), 3703. https://doi.org/10.3390/app9183703




