Mineralogical and Geochemical Constraints of the REE Accumulation in the Almásfüzitő Red Mud Depository in Northwest Hungary
Abstract
Featured Application
Abstract
1. Introduction
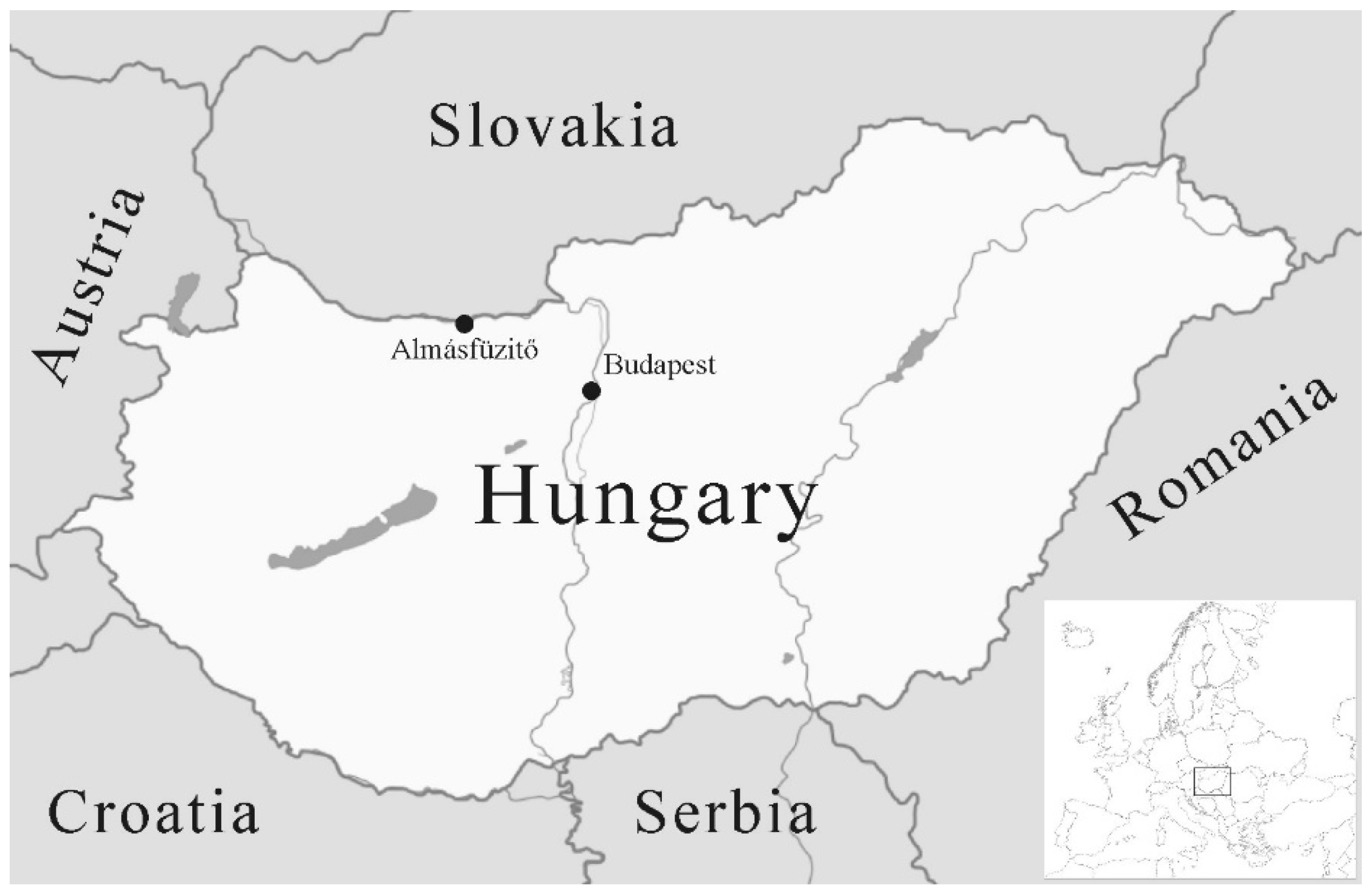
2. Materials and Methods
3. Results
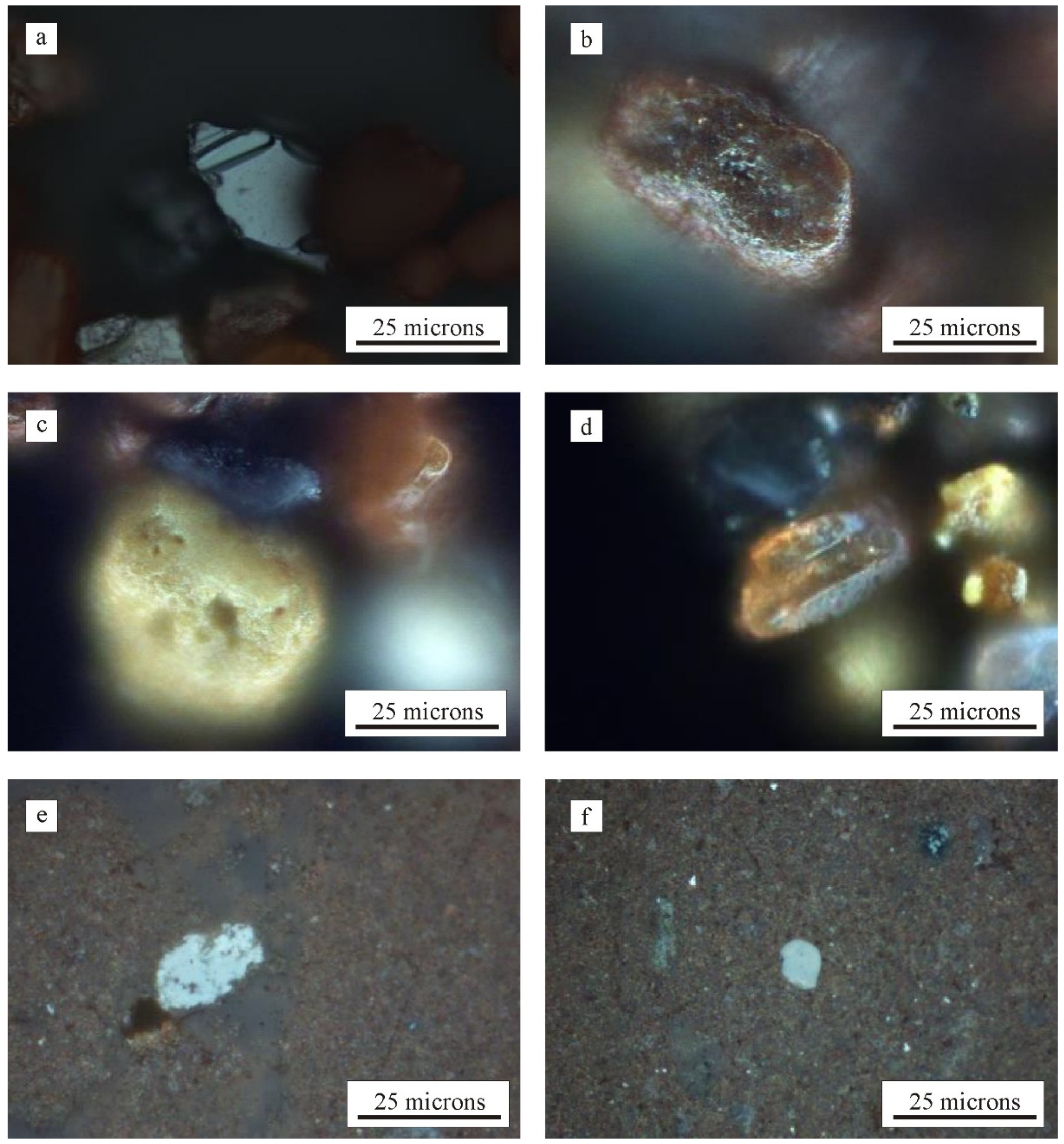
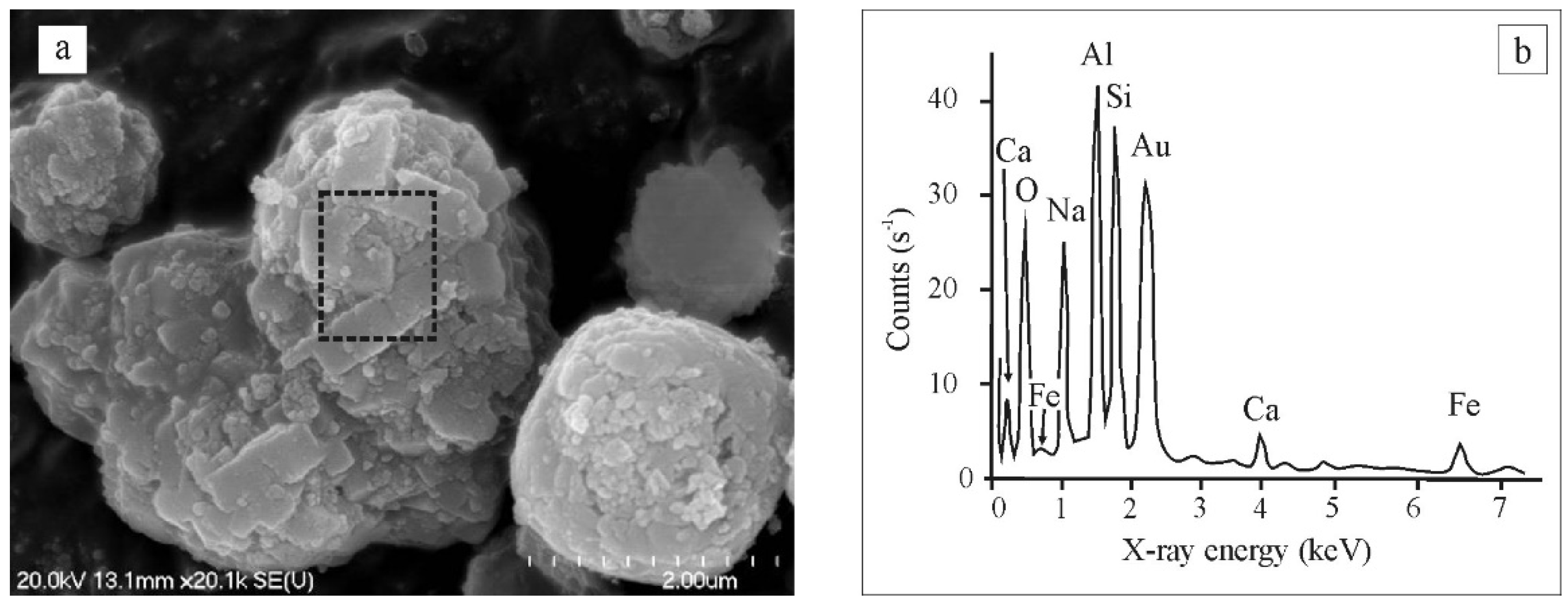
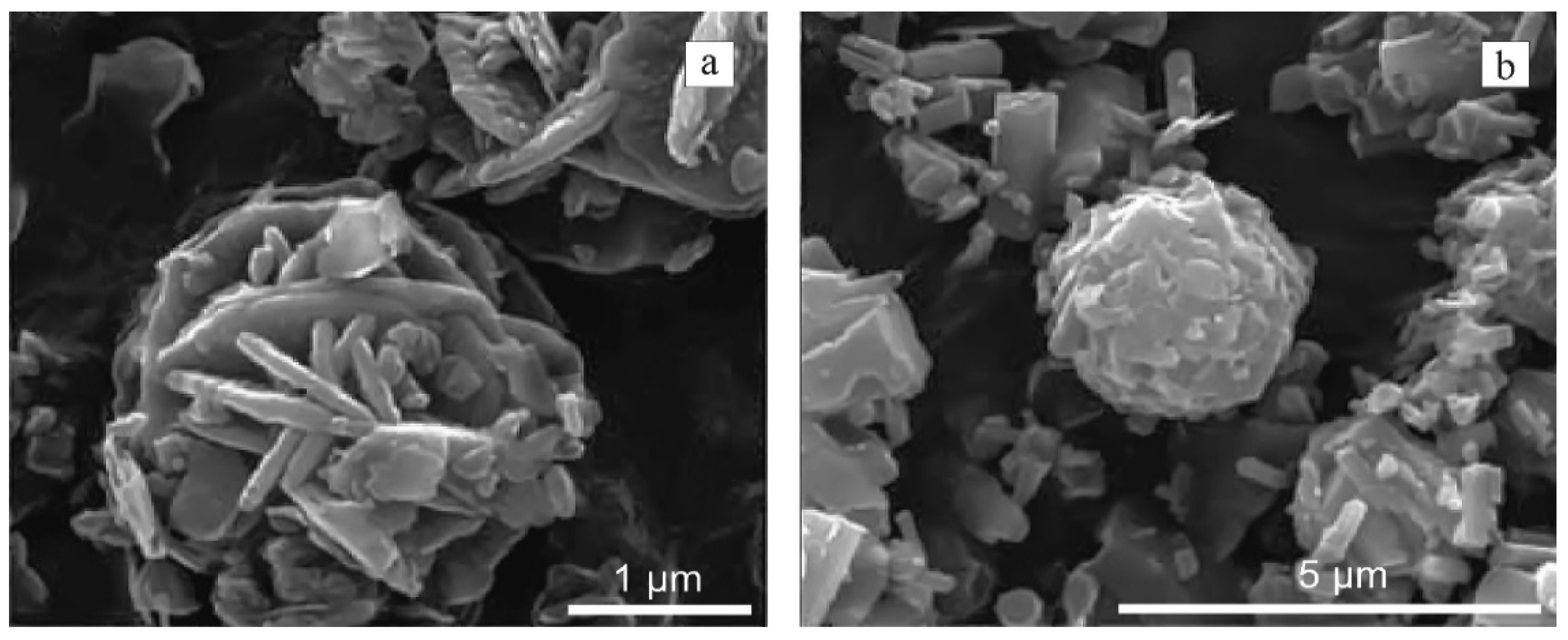
3.1. Mineralogy, Microtextures
3.2. Geochemical Data
4. Discussion
4.1. Statistical Evaluation of Geochemical Data
4.2. Origin of the Major Mineral Phases
4.3. Spatial Variability
4.4. Distribution of REEs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, A.S.; Suri, N.M.; Kant, S. Applications of bauxite residue: A mini-review. Waste Manag. Res. 2017, 35, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Cardenia, C.; Balomenos, E.; Panias, D. Iron Recovery from Bauxite Residue Through Reductive Roasting and Wet Magnetic Separation. J. Sustain. Metall. 2019, 5, 9–19. [Google Scholar] [CrossRef]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.A.; Lymperopoulou, T.; Ochsenkuehn, K.M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef]
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Čížková, M.; Mezricky, D.; Rucki, M.; Tóth, T.M.; Náhlík, V.; Lanta, V.; Bišová, K.; Zachleder, V.; Vítová, M. Bio-mining of Lanthanides from Red Mud by Green Microalgae. Molecules 2019, 24, 1356. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; Walter De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Goldschmidt, V. The principles of distribution of chemical elements in minerals and rocks. The seventh Hugo Müller Lecture, delivered before the Chemical Society. J. Chem. Soc. 1937, 655–673. [Google Scholar] [CrossRef]
- Mordberg, L.E.; Stanley, C.J.; Germann, K. Mineralogy and geochemistry of trace elements in bauxites: The Devonian Schugorsk deposit, Russia. Mineral. Mag. 2001, 65, 81–101. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Du, Y.; Ling, W.; Wu, L.; Cui, T.; Zhou, Q.; Jin, Z.; Lei, Z.; Weng, S. REE mobility and Ce anomaly in bauxite deposit of WZD area, Northern Guizhou, China. J. Geochem. Explor. 2013, 133, 103–117. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1997; p. 387. [Google Scholar]
- Nash, W.P.; Crecraft, H.R. Partition coefficients for trace elements in silicic magmas. Geochim. Cosmochim. Acta 1985, 49, 2309–2322. [Google Scholar] [CrossRef]
- Klemme, S.; Prowatke, S.; Hametner, K.; Günther, D. The partitioning of trace elements between rutile and silicate melts: Implications for subduction zones. Geochim. Cosmochim. Acta 2005, 69, 2361–2371. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Boni, M.; Rollinson, G.; Mondillo, N.; Balassone, G.; Santoro, L. Quantitative Mineralogical Characterization of Karst Bauxite Deposits in the Southern Apennines, Italy. Econ. Geol. 2013, 108, 813–833. [Google Scholar] [CrossRef]
- Reinhardt, N.; Proenza, J.A.; Villanova-de-Benavent, C.; Aiglsperger, T.; Bover-Arnal, T.; Torró, L.; Salas, R.; Dziggel, A. Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain. Minerals 2018, 8, 562. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in Greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Stetzenbach, K.J.; Hodge, V.F.; Berry Lyons, W. Rare earth element complexation behaviour in circumneutral pH groundwaters: Assessing the role of carbonate and phosphate ions. Earth Planet. Sci. Lett. 1996, 139, 305–319. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.E.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef]
- Littlewood, J.; Shaw, S.; Bots, P.; Peacock, C.L.; Trivedi, D.; Burke, I.T. Effect of solution composition on the recrystallization of kaolinite to feldspathoids in hyperalkaline conditions: Limitations of pertechnetate incorporation by ion competition effects. Mineral. Mag. 2015, 79, 1379–1388. [Google Scholar] [CrossRef]
- Sirbescu, M.; Jenkins, D.M. Experiments on the stability of cancrinite in the system Na2O-CaO-Al2O3-SiO2-CO2-H2O. Am. Mineral. 1999, 84, 1850–1860. [Google Scholar] [CrossRef]
- Renforth, P.; Mayes, W.M.; Jarvis, A.P.; Burke, I.T.; Manning, D.A.C.; Gruiz, K. Contaminant mobility and carbon sequestration downstream of the Ajka (Hungary) red mud spill: The effects of gypsum dosing. Sci. Total Environ. 2012, 421, 253–259. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Liu, F.; Liang, X.; Feng, X.; Tan, W.; Zheng, L.; Yin, H. Effects of Al3+ doping on the structure and properties of goethite and its adsorption behaviour towards phosphate. J. Environ. Sci. 2016, 45, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S.; Fendorf, M. Sorption mechanisms of lanthanum on oxide minerals. Clays Clay Miner. 1996, 44, 220–227. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts. Microporous Mesoporous Mater. 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Vaughan, D.E.W.; Yennawar, H.P.; Perrotta, A.J.; Benesi, A.J. Structure comparison of co-crystallized 6- and 12-sided large cancrinite crystals. Microporous Mesoporous Mater. 2009, 123, 274–279. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, Y.; Harsh, J.B.; Flury, M.; Boyle, J.S. Alteration of kaolinite to cancrinite and sodalite by simulated Hanford tank waste and its impact on cesium retention. Clays Clay Miner. 2004, 52, 1–13. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
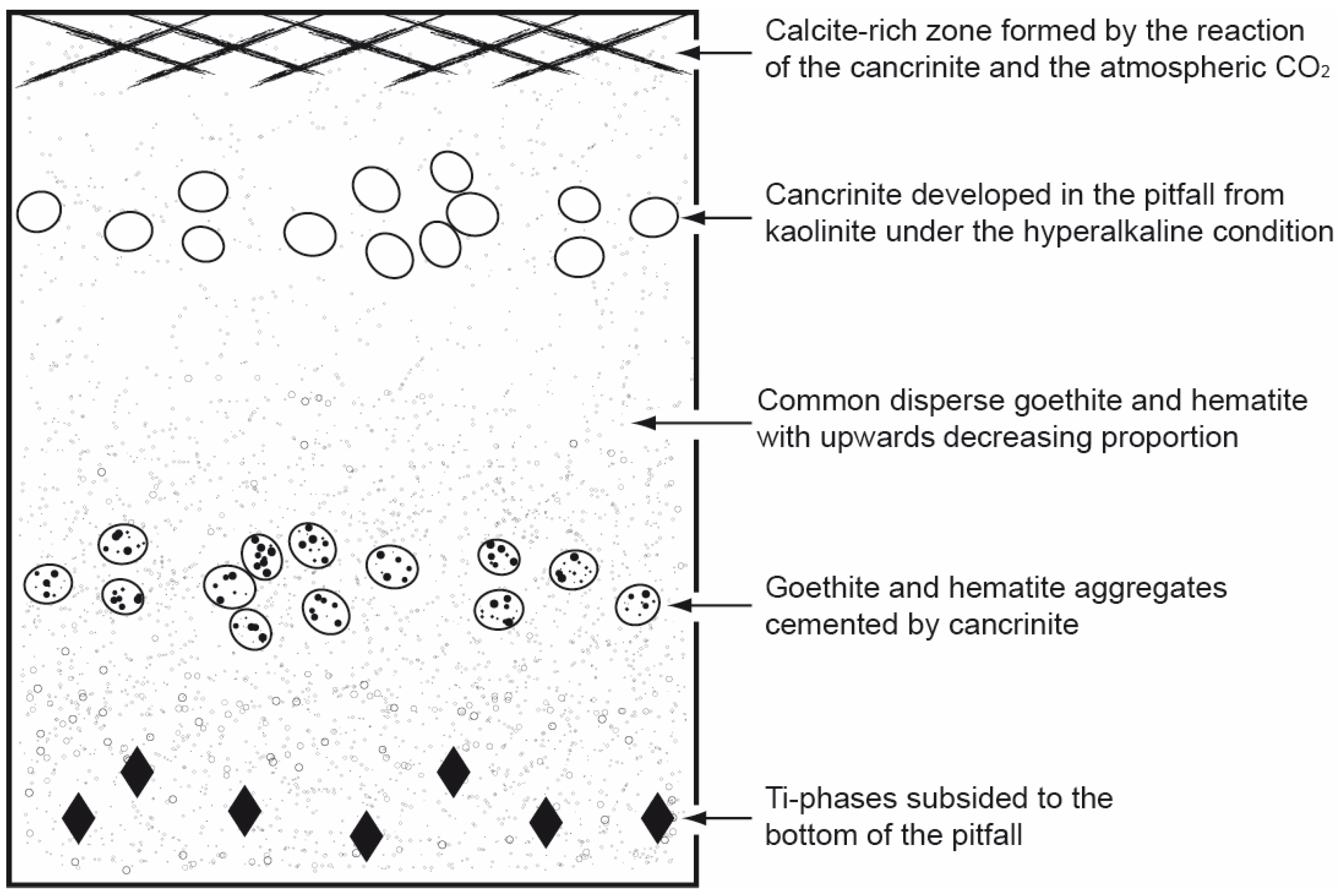
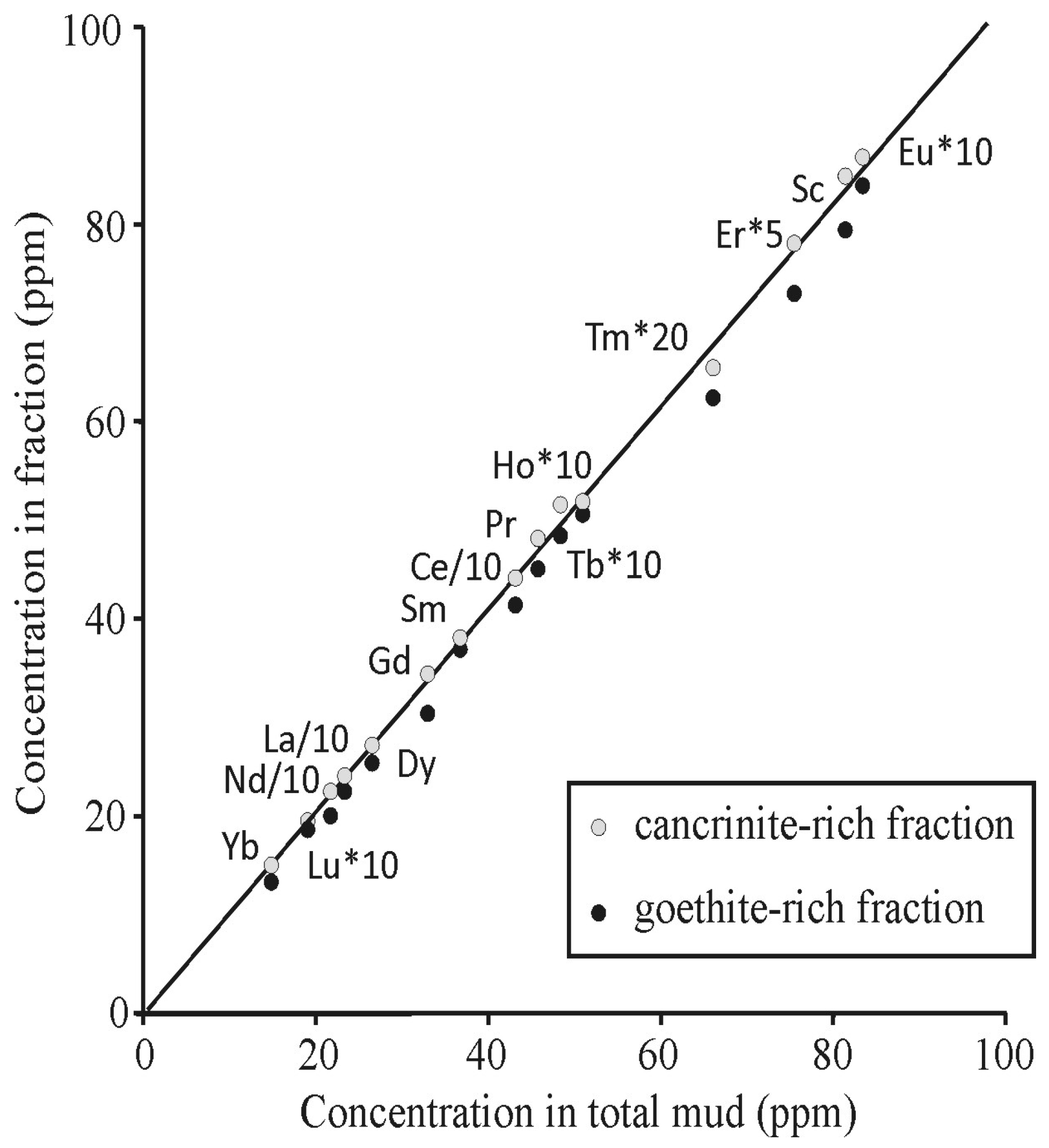
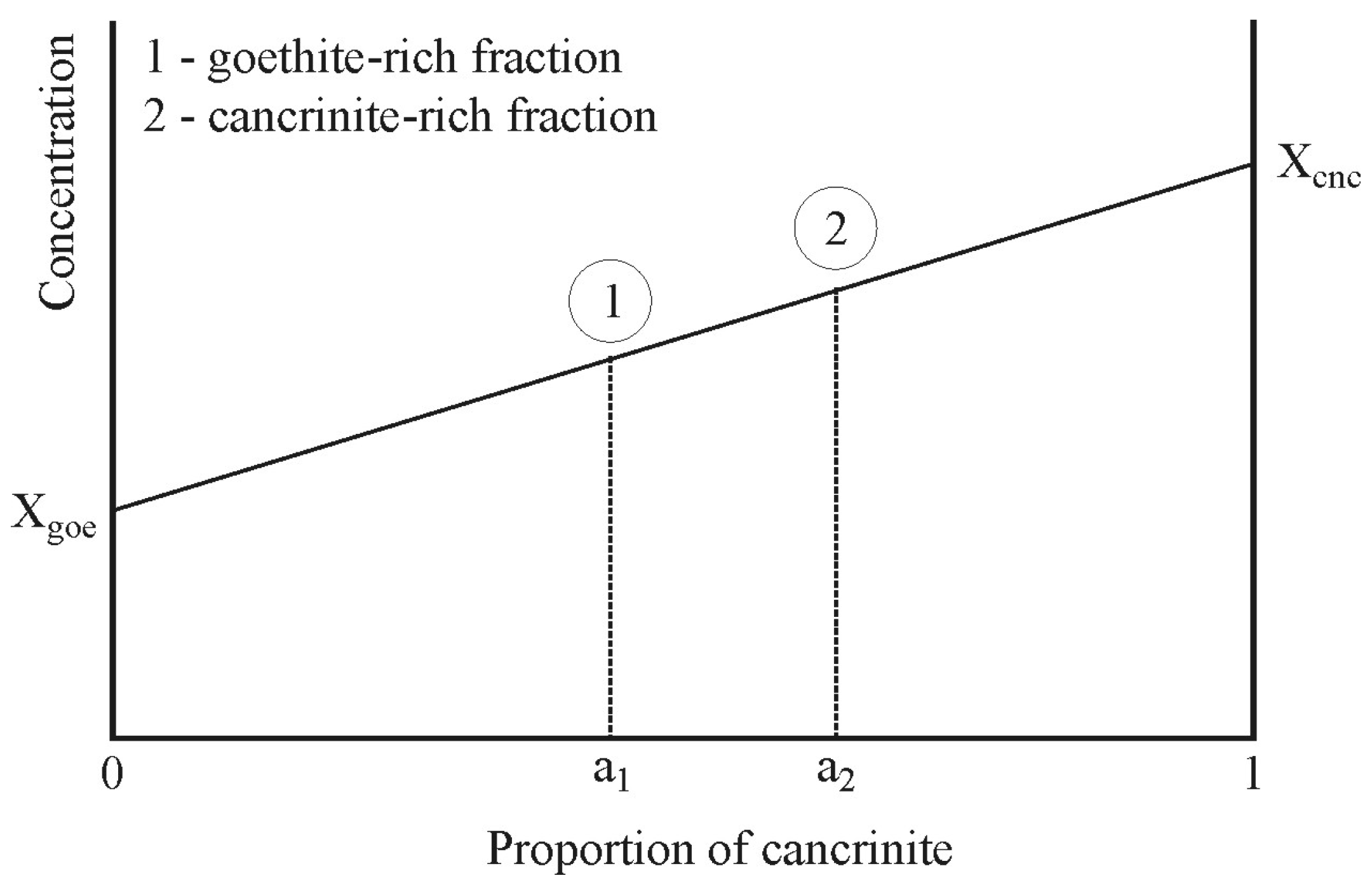
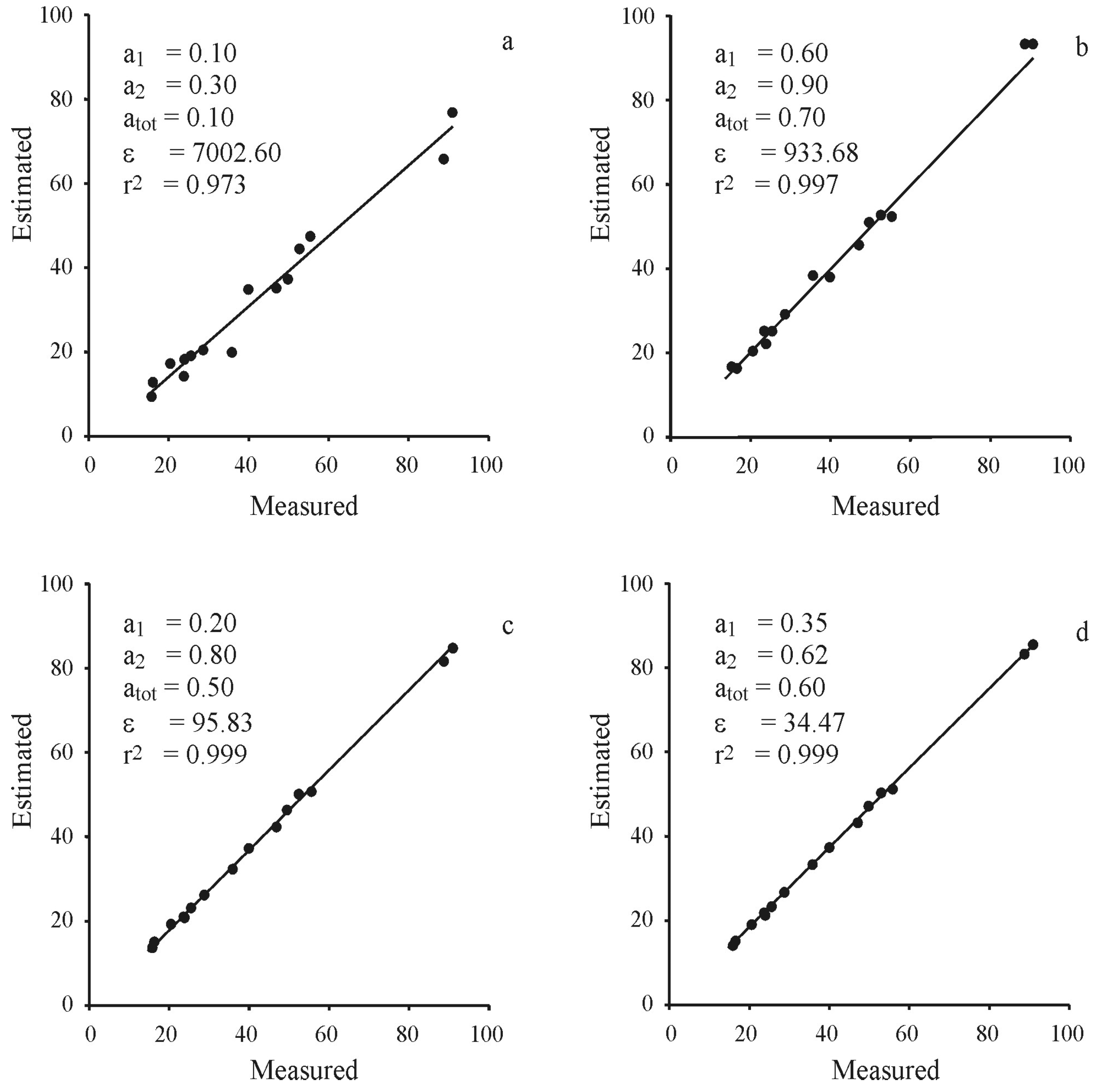
| Deep (Depth > 4.0 m) | Shallow (Depth < 4.0 m) | Total Mud | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | St. Dev. | Min | Max | Mean | Median | St. Dev. | Min | Max | Mean | Median | St. Dev. | Min | Max | |
| Ti | 1.5 | 1.5 | 0.2 | 1.1 | 1.9 | 1.3 | 1.3 | 0.3 | 0.7 | 1.8 | 1.4 | 1.4 | 0.3 | 0.7 | 1.9 |
| Al | 7.1 | 7.1 | 0.8 | 4.5 | 9.0 | 7.0 | 7.1 | 0.7 | 5.3 | 8.4 | 7.0 | 7.1 | 0.7 | 4.5 | 9.0 |
| Fe | 22.3 | 22.3 | 2.5 | 18.6 | 29.5 | 19.5 | 19.4 | 3.0 | 11.8 | 25.4 | 20.7 | 20.4 | 3.1 | 11.8 | 29.5 |
| Mg | 0.5 | 0.5 | 0.3 | 0.3 | 1.9 | 0.5 | 0.5 | 0.2 | 0.2 | 1.4 | 0.5 | 0.5 | 0.2 | 0.2 | 1.9 |
| Ca | 3.5 | 3.7 | 1.5 | 0.4 | 6.8 | 4.1 | 4.0 | 2.0 | 0.6 | 12.5 | 3.9 | 4.0 | 1.8 | 0.4 | 12.5 |
| K | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 | 0.4 |
| P | 0.2 | 0.2 | 0.0 | 0.1 | 0.3 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.3 |
| Rb | 4.6 | 4.4 | 1.2 | 2.3 | 6.8 | 4.9 | 4.5 | 1.7 | 1.9 | 9.0 | 4.8 | 4.5 | 1.5 | 1.9 | 9.0 |
| Sr | 1092.1 | 1106.2 | 247.7 | 684.7 | 1485.2 | 1011.2 | 1009.9 | 300.6 | 355.2 | 1507.7 | 1046.1 | 1019.7 | 280.1 | 355.2 | 1507.7 |
| Sc | 76.3 | 76.2 | 5.5 | 65.3 | 89.0 | 68.5 | 68.6 | 9.4 | 39.6 | 84.9 | 71.9 | 72.6 | 8.8 | 39.6 | 89.0 |
| V | 1033.2 | 1018.0 | 135.3 | 721.0 | 1486.0 | 904.4 | 909.0 | 156.9 | 546.0 | 1190.0 | 959.8 | 980.0 | 160.4 | 546.0 | 1486.0 |
| Ni | 295.5 | 293.2 | 28.0 | 240.9 | 352.7 | 259.4 | 264.6 | 45.6 | 148.3 | 358.0 | 274.9 | 277.9 | 42.8 | 148.3 | 358.0 |
| Cu | 100.5 | 94.9 | 23.5 | 76.0 | 194.1 | 96.1 | 94.6 | 17.9 | 65.5 | 173.3 | 98.0 | 94.8 | 20.5 | 65.5 | 194.1 |
| Pb | 168.8 | 166.6 | 16.1 | 141.6 | 221.1 | 149.2 | 151.1 | 21.4 | 86.8 | 191.6 | 157.6 | 158.5 | 21.5 | 86.8 | 221.1 |
| Zn | 244.6 | 244.1 | 72.0 | 134.7 | 456.6 | 198.0 | 197.1 | 70.6 | 89.9 | 499.7 | 218.1 | 211.0 | 74.4 | 89.9 | 499.7 |
| Cd | 3.0 | 2.9 | 0.9 | 1.6 | 5.6 | 2.6 | 2.5 | 0.7 | 1.3 | 4.3 | 2.7 | 2.7 | 0.8 | 1.3 | 5.6 |
| As | 127.9 | 132.4 | 35.3 | 70.7 | 216.2 | 100.2 | 93.9 | 29.8 | 50.3 | 200.4 | 112.2 | 102.0 | 34.9 | 50.3 | 216.2 |
| Y | 108.6 | 110.7 | 14.9 | 79.3 | 134.1 | 90.7 | 90.3 | 17.5 | 45.8 | 126.5 | 98.4 | 99.3 | 18.6 | 45.8 | 134.1 |
| Zr | 242.3 | 248.6 | 95.3 | 78.8 | 401.9 | 181.2 | 142.6 | 95.9 | 61.7 | 390.4 | 207.5 | 191.7 | 99.7 | 61.7 | 401.9 |
| Nb | 10.2 | 9.2 | 5.8 | 2.5 | 28.1 | 8.3 | 7.1 | 5.3 | 2.1 | 21.6 | 9.1 | 8.3 | 5.5 | 2.1 | 28.1 |
| Hf | 3.7 | 2.8 | 2.9 | 0.4 | 9.9 | 2.5 | 1.1 | 2.5 | 0.5 | 8.6 | 3.0 | 2.0 | 2.7 | 0.4 | 9.9 |
| Ta | 0.2 | 0.2 | 0.1 | 0.1 | 0.6 | 0.2 | 0.2 | 0.1 | 0.1 | 0.4 | 0.2 | 0.2 | 0.1 | 0.1 | 0.6 |
| W | 2.1 | 1.6 | 1.3 | 0.4 | 5.5 | 1.7 | 1.1 | 1.5 | 0.3 | 7.3 | 1.9 | 1.5 | 1.4 | 0.3 | 7.3 |
| La | 185.2 | 186.4 | 36.1 | 94.6 | 247.0 | 154.8 | 160.6 | 36.3 | 59.3 | 233.3 | 167.9 | 168.2 | 39.0 | 59.3 | 247.0 |
| Ce | 328.5 | 350.7 | 65.0 | 177.5 | 443.6 | 275.5 | 262.9 | 81.2 | 117.6 | 411.3 | 298.3 | 320.2 | 78.7 | 117.6 | 443.6 |
| Pr | 40.8 | 41.8 | 8.3 | 21.6 | 54.6 | 33.4 | 34.3 | 8.4 | 12.0 | 51.1 | 36.6 | 35.5 | 9.1 | 12.0 | 54.6 |
| Nd | 149.0 | 147.2 | 28.7 | 73.8 | 194.7 | 124.3 | 125.8 | 29.7 | 43.2 | 190.2 | 134.9 | 130.0 | 31.6 | 43.2 | 194.7 |
| Sm | 27.9 | 27.9 | 4.8 | 15.7 | 35.7 | 23.7 | 23.4 | 5.3 | 9.1 | 35.1 | 25.5 | 24.7 | 5.5 | 9.1 | 35.7 |
| Eu | 6.3 | 6.1 | 1.0 | 3.8 | 7.9 | 5.3 | 5.2 | 1.2 | 2.2 | 7.7 | 5.7 | 5.7 | 1.2 | 2.2 | 7.9 |
| Gd | 23.1 | 22.6 | 3.4 | 15.3 | 30.1 | 19.6 | 19.1 | 4.0 | 9.4 | 27.2 | 21.1 | 21.1 | 4.1 | 9.4 | 30.1 |
| Tb | 3.7 | 3.7 | 0.5 | 2.8 | 4.7 | 3.1 | 3.1 | 0.6 | 1.5 | 4.6 | 3.4 | 3.3 | 0.7 | 1.5 | 4.7 |
| Dy | 21.0 | 21.0 | 2.9 | 15.9 | 27.1 | 17.7 | 17.7 | 3.4 | 9.2 | 24.2 | 19.1 | 19.3 | 3.6 | 9.2 | 27.1 |
| Ho | 4.1 | 4.2 | 0.6 | 3.0 | 5.4 | 3.4 | 3.3 | 0.7 | 1.8 | 5.2 | 3.7 | 3.7 | 0.8 | 1.8 | 5.4 |
| Er | 11.5 | 11.5 | 1.4 | 9.2 | 14.5 | 9.8 | 10.0 | 1.9 | 5.4 | 14.0 | 10.5 | 10.8 | 1.9 | 5.4 | 14.5 |
| Tm | 1.8 | 1.8 | 0.3 | 1.3 | 2.3 | 1.5 | 1.5 | 0.3 | 0.8 | 2.1 | 1.6 | 1.6 | 0.3 | 0.8 | 2.3 |
| Yb | 11.3 | 11.0 | 1.3 | 8.1 | 13.6 | 9.7 | 10.2 | 1.7 | 5.2 | 13.2 | 10.4 | 10.6 | 1.8 | 5.2 | 13.6 |
| Lu | 1.8 | 1.8 | 0.3 | 1.3 | 2.2 | 1.5 | 1.4 | 0.3 | 0.7 | 2.3 | 1.6 | 1.6 | 0.3 | 0.7 | 2.3 |
| SumREE | 816.0 | 826.9 | 146.8 | 444.5 | 1046.2 | 683.1 | 690.4 | 169.1 | 277.5 | 1021.3 | 740.3 | 770.1 | 172.1 | 277.5 | 1046.2 |
| Principal Component | Total Variance% | Elements with a Correlation Coefficient > 0.5 | Geochemical Process |
|---|---|---|---|
| PC1-1 | 37.7 | La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Sc, Ni, Fe, V, U, Th, Sr, -Ca, Ti, Al | Accumulation of REE in different mineral phases |
| PC1-2 | 51.1 | Cu, Pb, Zn, As, Cd, Fe | Chalcophile elements |
| PC1-3 | 70.1 | Zr, Nb, Ta, Hf, Ce | HFSE elements |
| PC1-4 | 76.5 | Mg, K, Rb | Illite |
| PC1-5 | 81.3 | As, P, W | AsO43−, PO42−, WO42− phases |
| Principal Component | Total Variance% | Elements with a Correlation Coefficient > 0.5 | Geochemical Process |
|---|---|---|---|
| PC2-1 | 27.8 | Ti, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy | Accumulation of Ti-phases and the LREE |
| PC2-2 | 55.2 | Ni, Fe, V, Sc, Ho, Er, Tm, Yb, Lu | Accumulation of the Fe-phases and the HREE and Sc |
| PC2-3 | 80.1 | Sr, -Ca, Al, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu | Accumulation of cancrinite and selected REEs; no calcite |
| Element | Total Sample | Goethite-Rich Fraction | Cancrinite-Rich Fraction | Coarse-Grained Magnetic Fraction | Coarse-Grained Non-Magnetic Fraction |
|---|---|---|---|---|---|
| La | 237.90 | 226.20 | 239.40 | 46.60 | 47.30 |
| Sc | 82.90 | 79.20 | 84.40 | 47.50 | 61.80 |
| Ce | 438.90 | 414.00 | 437.90 | 115.70 | 117.80 |
| Pr | 49.26 | 48.41 | 51.04 | 11.98 | 13.25 |
| Nd | 193.63 | 189.56 | 197.13 | 49.00 | 55.93 |
| Sm | 37.29 | 37.07 | 37.83 | 10.84 | 13.58 |
| Eu | 8.49 | 8.35 | 8.61 | 2.45 | 3.43 |
| Gd | 33.48 | 30.34 | 34.33 | 10.92 | 14.59 |
| Tb | 4.64 | 4.50 | 4.79 | 1.72 | 2.31 |
| Dy | 27.01 | 25.49 | 27.30 | 10.90 | 14.68 |
| Ho | 5.18 | 5.05 | 5.17 | 2.31 | 3.18 |
| Er | 15.36 | 14.58 | 15.52 | 6.44 | 9.13 |
| Tm | 2.24 | 2.08 | 2.17 | 1.04 | 1.46 |
| Yb | 14.84 | 13.49 | 14.98 | 6.88 | 10.81 |
| Lu | 22.10 | 2.02 | 2.25 | 1.11 | 1.71 |
| Element | Measured | Estimated | Element | Measured | Estimated |
|---|---|---|---|---|---|
| La | 237.90 | 236.96 | Tb | 4.64 | 4.74 |
| Sc | 82.90 | 83.44 | Dy | 27.01 | 26.96 |
| Ce | 438.90 | 433.47 | Ho | 5.18 | 5.15 |
| Pr | 49.26 | 50.71 | Er | 15.36 | 15.35 |
| Nd | 193.63 | 195.73 | Tm | 2.24 | 2.15 |
| Sm | 37.29 | 37.69 | Yb | 14.84 | 14.70 |
| Eu | 8.49 | 8.56 | Lu | 2.21 | 2.21 |
| Gd | 33.48 | 33.59 |
| Element | Original Sample | Xgoethite | Xcancrinite | Xcoarse | Ecnc/goe | Ecnc/coarse |
|---|---|---|---|---|---|---|
| La | 237.90 | 207.62 | 256.51 | 46.95 | 1.24 | 5.46 |
| Sc | 82.90 | 71.88 | 91.14 | 54.65 | 1.27 | 1.67 |
| Ce | 438.90 | 380.36 | 468.88 | 116.75 | 1.23 | 4.02 |
| Pr | 49.26 | 46.75 | 53.35 | 12.62 | 1.14 | 4.23 |
| Nd | 193.63 | 178.91 | 206.94 | 52.47 | 1.16 | 3.94 |
| Sm | 37.29 | 36.00 | 38.82 | 12.21 | 1.08 | 3.18 |
| Eu | 8.49 | 7.98 | 8.95 | 2.94 | 1.12 | 3.04 |
| Gd | 33.48 | 24.72 | 39.50 | 12.76 | 1.60 | 3.10 |
| Tb | 4.64 | 4.09 | 5.17 | 2.02 | 1.26 | 2.56 |
| Dy | 27.01 | 22.94 | 29.65 | 12.79 | 1.29 | 2.32 |
| Ho | 5.18 | 4.88 | 5.33 | 2.75 | 1.09 | 1.94 |
| Er | 15.36 | 13.26 | 16.74 | 7.79 | 1.26 | 2.15 |
| Tm | 2.24 | 1.95 | 2.29 | 1.25 | 1.17 | 1.83 |
| Yb | 14.84 | 11.39 | 16.91 | 8.85 | 1.48 | 1.91 |
| Lu | 2.21 | 1.70 | 2.55 | 1.41 | 1.50 | 1.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Tóth, T.; Schubert, F.; Raucsik, B.; Fintor, K. Mineralogical and Geochemical Constraints of the REE Accumulation in the Almásfüzitő Red Mud Depository in Northwest Hungary. Appl. Sci. 2019, 9, 3654. https://doi.org/10.3390/app9183654
M. Tóth T, Schubert F, Raucsik B, Fintor K. Mineralogical and Geochemical Constraints of the REE Accumulation in the Almásfüzitő Red Mud Depository in Northwest Hungary. Applied Sciences. 2019; 9(18):3654. https://doi.org/10.3390/app9183654
Chicago/Turabian StyleM. Tóth, Tivadar, Félix Schubert, Béla Raucsik, and Krisztián Fintor. 2019. "Mineralogical and Geochemical Constraints of the REE Accumulation in the Almásfüzitő Red Mud Depository in Northwest Hungary" Applied Sciences 9, no. 18: 3654. https://doi.org/10.3390/app9183654
APA StyleM. Tóth, T., Schubert, F., Raucsik, B., & Fintor, K. (2019). Mineralogical and Geochemical Constraints of the REE Accumulation in the Almásfüzitő Red Mud Depository in Northwest Hungary. Applied Sciences, 9(18), 3654. https://doi.org/10.3390/app9183654






