Major Depressive Disorder and Oxidative Stress: In Silico Investigation of Fluoxetine Activity against ROS
Abstract
Featured Application
Abstract
1. Introduction
1.1. Background
1.2. Aim of This Work
2. Materials and Methods
2.1. Calculation of ΔG°HAT/RAF
2.2. Calculation of ΔG‡HAT
3. Results
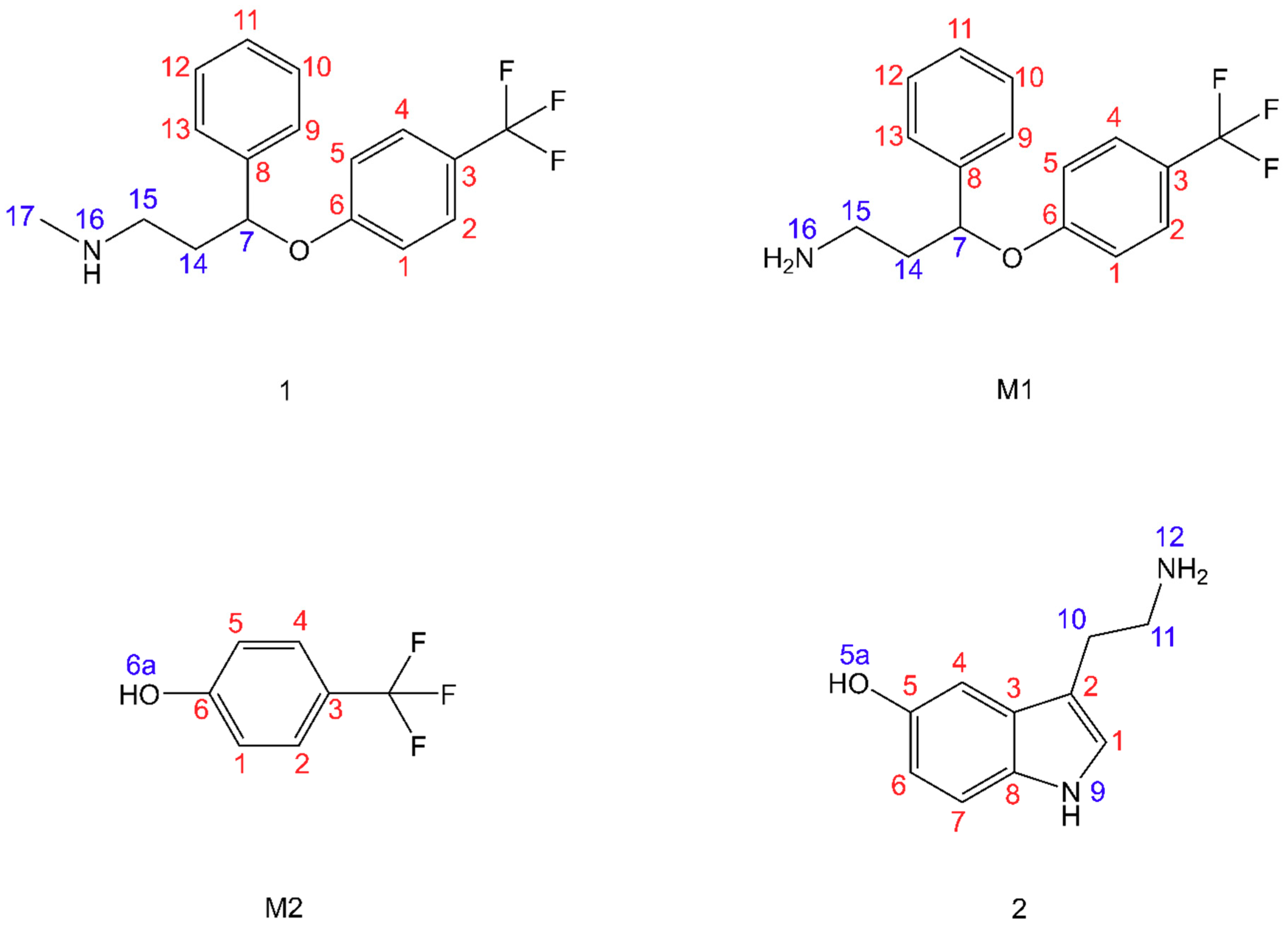
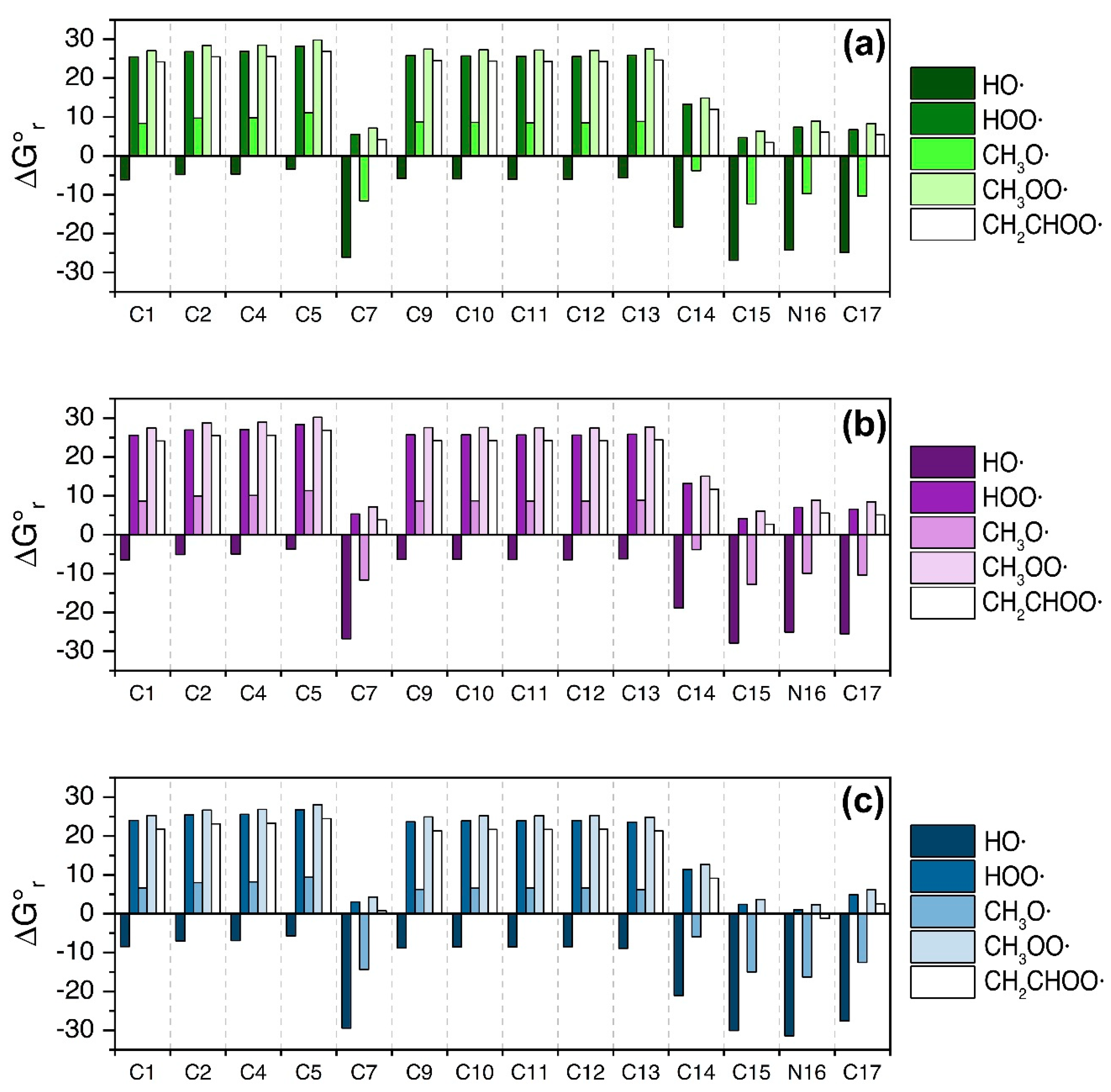
3.1. Fluoxetine
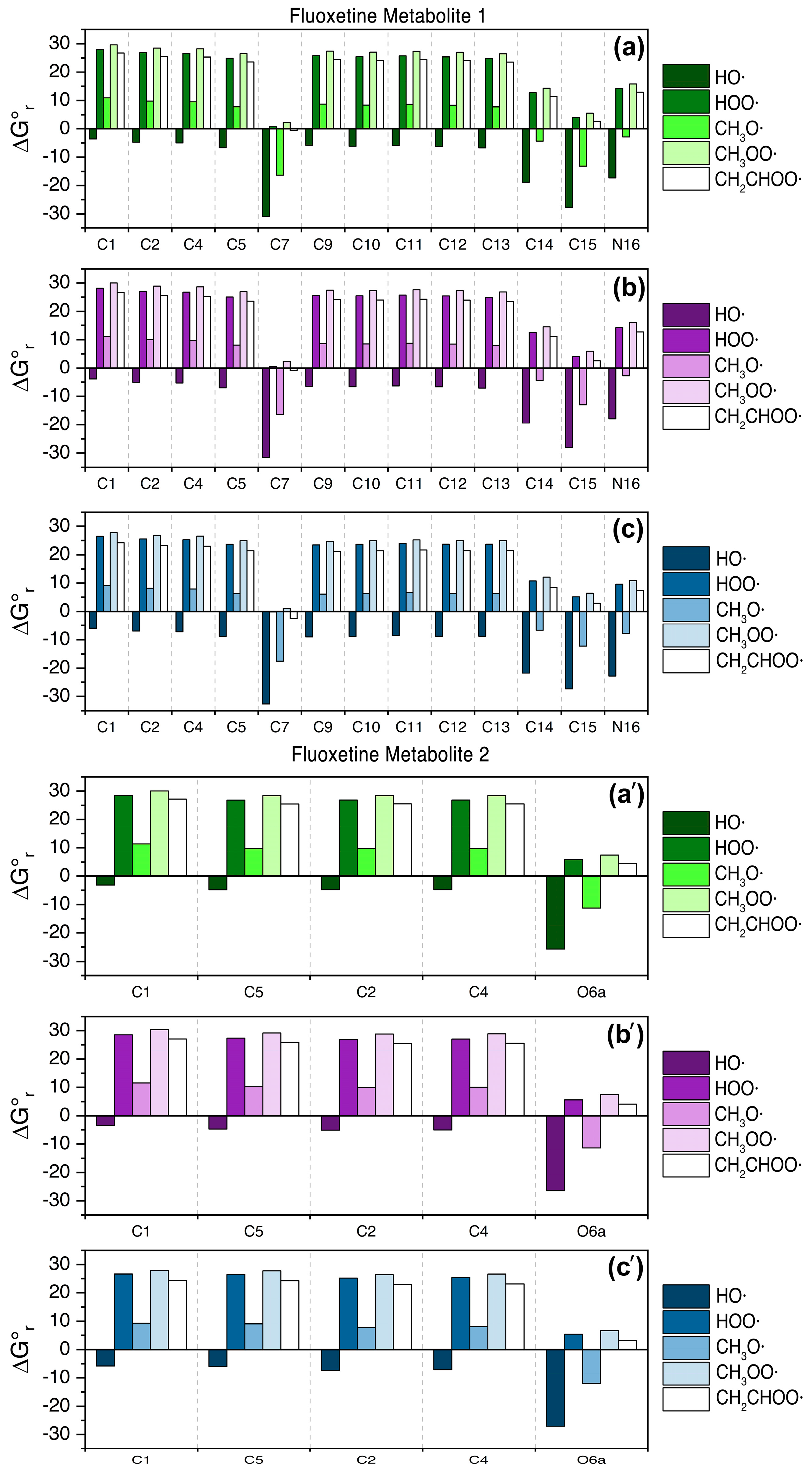
3.2. Fluoxetine Metabolites
3.3. Serotonin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wong, D.T.; Horng, J.S.; Bymaster, F.P.; Hauser, K.L.; Molloy, B.B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-Trifluoromethylphenoxy)-n-methyl-3-phenylpropylamine. Life Sci. 1974, 15, 471–479. [Google Scholar] [CrossRef]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The Discovery of Fluoxetine Hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Härtter, S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef]
- Fuller, R.W.; Beasley, C.M. Fluoxetine mechanism of action. J. Am. Acad. Child Adolesc. Psychiatry 1991, 30, 849. [Google Scholar] [PubMed]
- Mandrioli, R.; Forti, G.; Raggi, M. Fluoxetine metabolism and pharmacological interactions: The role of cytochrome P450. Curr. Drug Metab. 2006, 7, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Cârcu-Dobrin, M.; Budău, M.; Rusu, A. Analytical methodologies for the stereoselective determination of fluoxetine: An overview. Biomed. Chromatogr. 2018, 32, e4040. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.G.; Miledi, R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac). Proc. Natl. Acad. Sci. USA 1997, 94, 2036–2040. [Google Scholar] [CrossRef]
- Wood, A.J.J.; Gram, L.F. Fluoxetine. N. Engl. J. Med. 1994, 331, 1354–1361. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Law, C.J.; Reith, M.E.A.; Wang, D.-N. Antidepressant specificity of serotonin transporter suggested by three LeuT–SSRI structures. Nat. Struct. Mol. Biol. 2009, 16, 652–657. [Google Scholar] [CrossRef]
- Spinks, D.; Spinks, G. Serotonin reuptake inhibition: An update on current research strategies. Curr. Med. Chem. 2002, 9, 799–810. [Google Scholar] [CrossRef]
- Ciribassi, J.; Luescher, A.; Pasloske, K.S.; Robertson-Plouch, C.; Zimmerman, A.; Kaloostian-Whittymore, L. Comparative bioavailability of fluoxetine after transdermal and oral administration to healthy cats. Am. J. Vet. Res. 2003, 64, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Tascedda, F.; Merlo, S.; Benatti, C.; Spampinato, S.F.; Munafò, A.; Leggio, G.M.; Nicoletti, F.; Brunello, N.; Drago, F.; et al. Fluoxetine prevents Aβ1-42-induced toxicity via a paracrine signaling mediated by transforming-growth-factor-β1. Front. Pharmacol. 2016, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and mood: Mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Novío, S.; Núñez, M.J.; Amigo, G.; Freire-Garabal, M. Effects of fluoxetine on the oxidative status of peripheral blood leucocytes of restraint-stressed mice. Basic Clin. Pharmacol. Toxicol. 2011, 109, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Matias, B.I.; Maciel, A.L.; Abelaira, H.M.; Ignácio, Z.M.; de Moura, A.B.; Matos, D.; Danielski, L.G.; Petronilho, F.; Carvalho, A.F.; et al. Mechanism of synergistic action on behavior, oxidative stress and inflammation following co-treatment with ketamine and different antidepressant classes. Pharmacol. Rep. 2017, 69, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Robertson, O.D.; Coronado, N.G.; Sethi, R.; Berk, M.; Dodd, S. Putative neuroprotective pharmacotherapies to target the staged progression of mental illness. Early Interv. Psychiatry 2019. [Google Scholar] [CrossRef] [PubMed]
- Herbet, M.; Gawrońska-Grzywacz, M.; Izdebska, M.; Piątkowska-Chmiel, I. Effect of the interaction between atorvastatin and selective serotonin reuptake inhibitors on the blood redox equilibrium. Exp. Ther. Med. 2016, 12, 3440–3444. [Google Scholar] [CrossRef] [PubMed]
- Caiaffo, V.; Oliveira, B.D.R.; De Sá, F.B.; Evêncio Neto, J. Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine. Pharmacol. Res. Perspect. 2016, 4, e00231. [Google Scholar] [CrossRef] [PubMed]
- Behr, G.A.; Moreira, J.C.F.; Frey, B.N. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: Implications for the pathophysiology of major depressive disorder. Oxid. Med. Cell. Longev. 2012, 2012, 609421. [Google Scholar] [CrossRef]
- Erman, H.; Guner, I.; Yaman, M.O.; Uzun, D.D.; Gelisgen, R.; Aksu, U.; Yelmen, N.; Sahin, G.; Uzun, H. The effects of fluoxetine on circulating oxidative damage parameters in rats exposed to aortic ischemia–reperfusion. Eur. J. Pharmacol. 2015, 749, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kolla, N.; Wei, Z.; Richardson, J.S.; Li, X.M. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J. Psychiatry Neurosci. 2005, 30, 196–201. [Google Scholar] [PubMed]
- Safhi, M.M.; Qumayri, H.M.; Masmali, A.U.M.; Siddiqui, R.; Alam, M.F.; Khan, G.; Anwer, T. Thymoquinone and fluoxetine alleviate depression via attenuating oxidative damage and inflammatory markers in type-2 diabetic rats. Arch. Physiol. Biochem. 2019, 125, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Dalmizrak, O.; Teralı, K.; Asuquo, E.B.; Ogus, I.H.; Ozer, N. The relevance of glutathione reductase inhibition by fluoxetine to human health and disease: Insights derived from a combined kinetic and docking study. Protein J. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kalogiannis, M.; Delikatny, E.J.; Jeitner, T.M. Serotonin as a putative scavenger of hypohalous acid in the brain. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Côté, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and membrane binding properties of serotonin protect lipids from oxidation. Biophys. J. 2017, 112, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Lobayan, R.M.; Schmit, M.C.P. Conformational and NBO studies of serotonin as a radical scavenger. Changes induced by the OH group. J. Mol. Graph. Model. 2018, 80, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, A.M.; Kist, L.W.; Almeida, E.A.; Silva, D.G.H.; Bonan, C.D.; Altenhofen, S.; Kaufmann, C.G.; Bogo, M.R.; Barros, D.M.; Oliveira, S.; et al. Neurotoxicity in zebrafish exposed to carbon nanotubes: Effects on neurotransmitters levels and antioxidant system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, S.B.O.; Gulcin, I. Radical scavenging and antioxidant capacity of serotonin. Curr. Bioact. Compd. 2013, 9, 143–152. [Google Scholar] [CrossRef]
- Orian, L.; Mauri, P.; Roveri, A.; Toppo, S.; Benazzi, L.; Bosello-Travain, V.; De Palma, A.; Maiorino, M.; Miotto, G.; Zaccarin, M.; et al. Selenocysteine oxidation in glutathione peroxidase catalysis: An MS-supported quantum mechanics study. Free Radic. Biol. Med. 2015, 87, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Torsello, M.; Bickelhaupt, F.M.; Orian, L. Role of the Chalcogen (S, Se, Te) in the oxidation mechanism of the glutathione peroxidase active site. ChemPhysChem 2017, 18, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.; Bosello-Travain, V.; Cozza, G.; Miotto, G.; Orian, L.; Roveri, A.; Toppo, S.; Zaccarin, M.; Ursini, F. Glutathione Peroxidase 4 from Selenium: Its Molecular Biology and Role in Human Health, 4th ed.; Springer International Publisher: New York, NY, USA, 2016; ISBN 9783319412832. [Google Scholar]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: A long story of a promise still unfulfilled. Free Radic. Biol. Med. 2014, 66, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wolters, L.P.; Orian, L. Peroxidase activity of organic selenides: Mechanistic insights from quantum chemistry. Curr. Org. Chem. 2016, 20, 189–197. [Google Scholar] [CrossRef]
- Ribaudo, G.; Bellanda, M.; Menegazzo, I.; Wolters, L.P.; Bortoli, M.; Ferrer-Sueta, G.; Zagotto, G.; Orian, L. Mechanistic insight into the oxidation of organic phenylselenides by H2O2. Chem. A Eur. J. 2017, 23, 2405–2422. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Zaccaria, F.; Dalla Tiezza, M.; Bruschi, M.; Fonseca Guerra, C.; Bickelhaupt, F.M.; Orian, L. Oxidation of organic diselenides and ditellurides by H2O2 for bioinspired catalyst design. Phys. Chem. Chem. Phys. 2018, 20, 20874–20885. [Google Scholar] [CrossRef] [PubMed]
- Dalla Tiezza, M.; Ribaudo, G.; Orian, L. Organodiselenides: Organic catalysis and drug design learning from glutathione peroxidase. Curr. Org. Chem. 2019. [Google Scholar] [CrossRef]
- Bortoli, M.; Dalla Tiezza, M.; Muraro, C.; Pavan, C.; Ribaudo, G.; Rodighiero, A.; Tubaro, C.; Zagotto, G.; Orian, L. Psychiatric disorders and oxidative injury: Antioxidant effects of zolpidem therapy disclosed in silico. Comput. Struct. Biotechnol. J. 2019, 17, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Exploration of chemical compound, conformer, and reaction space with meta-dynamics simulations based on tight-binding quantum chemical calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Gaussian 16, Revision B.01. Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.V.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, J.L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J.A., Jr.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.J.; Heyd, J.J.; Brothers, E.N.; Kudin, K.N.; Staroverov, V.N.; Keith, T.A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.P.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Millam, J.M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B.; Fox, D.J. Gaussian Inc.: Wallingford, CT, USA, 2016.
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Sure, R.; Grimme, S. Using dispersion-corrected density functional theory to understand supramolecular binding thermodynamics. Chem. Commun. 2015, 51, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Galano, A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Wolters, L.P.; Orian, L.; Bickelhaupt, F.M. Addition-elimination or nucleophilic substitution? Understanding the energy profiles for the reaction of chalcogenolates with dichalcogenides. J. Chem. Theory Comput. 2016, 12, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; Russo, N.; Grand, A.; Galano, A. A physicochemical examination of the free radical scavenging activity of trolox: Mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 2013, 15, 4642. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Galano, A.; Martínez, A. Capsaicin, a tasty free radical scavenger: Mechanism of action and kinetics. J. Phys. Chem. B 2012, 116, 1200–1208. [Google Scholar] [CrossRef]
- Van Harten, J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin. Pharmacokinet. 1993, 24, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Strauss, W.L.; Layton, M.E.; Hayes, C.E.; Dager, S.R. 19F magnetic resonance spectroscopy investigation in vivo of acute and steady-state brain fluvoxamine levels in obsessive-compulsive disorder. Am. J. Psychiatry 1997, 154, 516–522. [Google Scholar] [PubMed]
- Benfield, P.; Heel, R.C.; Lewis, S.P. Fluoxetine. Drugs 1986, 32, 481–508. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J.M.; O’Donnell, J.P.; Mankowski, D.C.; Ekins, S.; Obach, R.S. (R)-, (S)-, and Racemic fluoxetine n-demethylation by human cytochrome P450 enzymes. Drug Metab. Dispos. 2000, 28, 1187–1191. [Google Scholar] [PubMed]
- Zarghi, A.; Kebriaeezadeh, A.; Ahmadkhaniha, R.; Akhgari, M.; Rastkari, N. Selective liquid chromatographic method for determination of fluoxetine in plasma. J. AOAC Int. 2001, 84, 1735–1737. [Google Scholar] [PubMed]
- Gupta, R.N.; Steiner, M. Determination of fluoxetine and norfluoxetine in serum by liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 1990, 13, 3785–3797. [Google Scholar] [CrossRef]
- Rohrig, T.P.; Prouty, R.W. A nortriptyline death with unusually high tissue concentrations. J. Anal. Toxicol. 1989, 13, 303–304. [Google Scholar] [CrossRef] [PubMed]

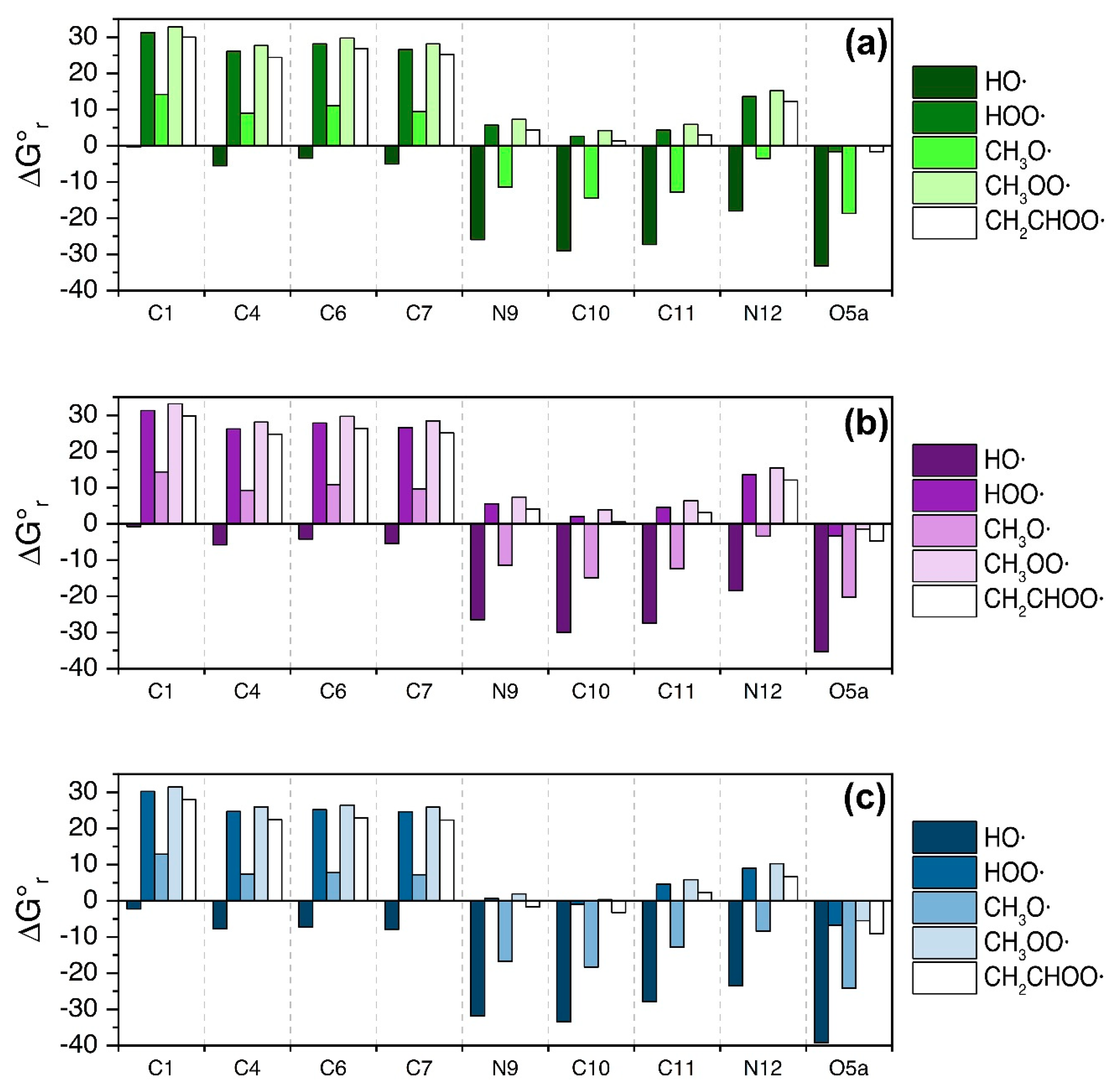

| Site | Gas Phase | Benzene | Water | Gas Phase | Benzene | Water |
|---|---|---|---|---|---|---|
| HO• | CH3O• | |||||
| 7 | 6.57 | 8.58 | 10.03 | 13.69 | 16.07 | 16.45 |
| 14 | 7.48 | 8.91 | 9.54 | 14.95 | 16.72 | 16.26 |
| 15 | 5.39 | 5.95 | 4.24 | 11.67 | 12.35 | 9.74 |
| 16 | 3.68 | 4.01 | 0.85 | 8.34 | 9.01 | 6.18 |
| 17 | 7.84 | 8.35 | 5.50 | 14.43 | 15.28 | 12.19 |
| Site | Gas Phase | Benzene | Water | Gas Phase | Benzene | Water |
|---|---|---|---|---|---|---|
| HO• | CH3O• | |||||
| 7 | 5.40 | 7.99 | 11.58 | 12.23 | 15.19 | 17.63 |
| 14 | 5.36 | 7.33 | 10.44 | 13.64 | 15.96 | 18.05 |
| 15 | 4.35 | 5.42 | 6.69 | 9.79 | 11.07 | 11.55 |
| 16 | 3.84 | 4.87 | 5.71 | 11.37 | 12.61 | 13.07 |
| 6a 1 | 6.21 | 8.26 | 12.40 | 1.83 | 3.11 | 5.38 |
| Site | Gas Phase | Benzene | Water | Gas Phase | Benzene | Water |
|---|---|---|---|---|---|---|
| HO• | CH3O• | |||||
| 9 | 5.61 | 6.87 | 0.08 | 11.97 | 14.14 | 11.12 |
| 10 | 6.86 | 8.02 | 7.40 | 14.43 | 15.73 | 14.08 |
| 11 | 6.35 | 6.91 | 6.46 | 12.12 | 12.92 | 11.44 |
| 12 | 5.40 | 6.06 | 5.14 | 13.13 | 14.10 | 13.10 |
| 5a | 5.02 | 6.14 | 11.23 | 7.29 | 8.50 | 5.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraro, C.; Dalla Tiezza, M.; Pavan, C.; Ribaudo, G.; Zagotto, G.; Orian, L. Major Depressive Disorder and Oxidative Stress: In Silico Investigation of Fluoxetine Activity against ROS. Appl. Sci. 2019, 9, 3631. https://doi.org/10.3390/app9173631
Muraro C, Dalla Tiezza M, Pavan C, Ribaudo G, Zagotto G, Orian L. Major Depressive Disorder and Oxidative Stress: In Silico Investigation of Fluoxetine Activity against ROS. Applied Sciences. 2019; 9(17):3631. https://doi.org/10.3390/app9173631
Chicago/Turabian StyleMuraro, Cecilia, Marco Dalla Tiezza, Chiara Pavan, Giovanni Ribaudo, Giuseppe Zagotto, and Laura Orian. 2019. "Major Depressive Disorder and Oxidative Stress: In Silico Investigation of Fluoxetine Activity against ROS" Applied Sciences 9, no. 17: 3631. https://doi.org/10.3390/app9173631
APA StyleMuraro, C., Dalla Tiezza, M., Pavan, C., Ribaudo, G., Zagotto, G., & Orian, L. (2019). Major Depressive Disorder and Oxidative Stress: In Silico Investigation of Fluoxetine Activity against ROS. Applied Sciences, 9(17), 3631. https://doi.org/10.3390/app9173631