Adsorption and Sensing Behaviors of Pd-Doped InN Monolayer upon CO and NO Molecules: A First-Principles Study
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
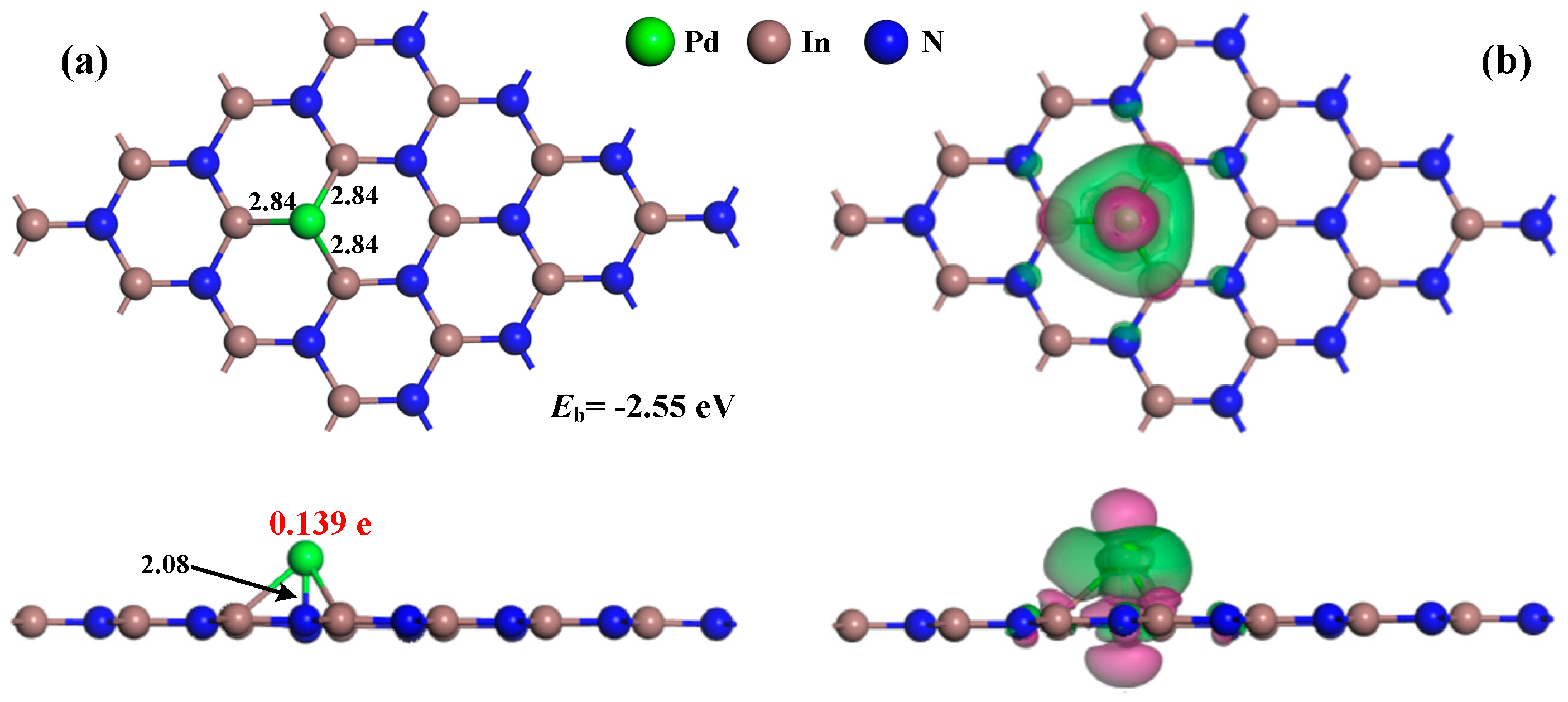
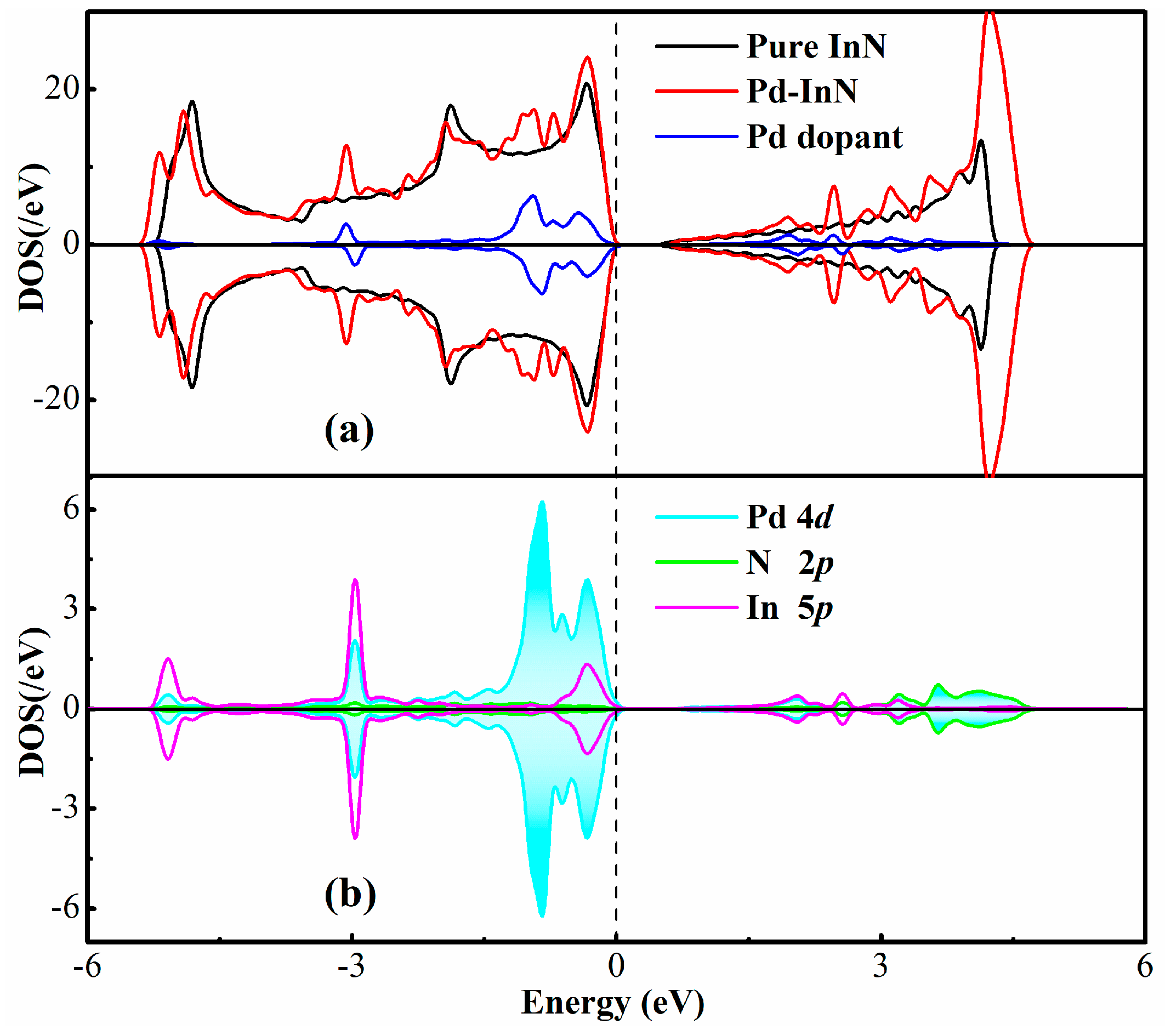
3.1. Analysis of Pd-InN Monolayer
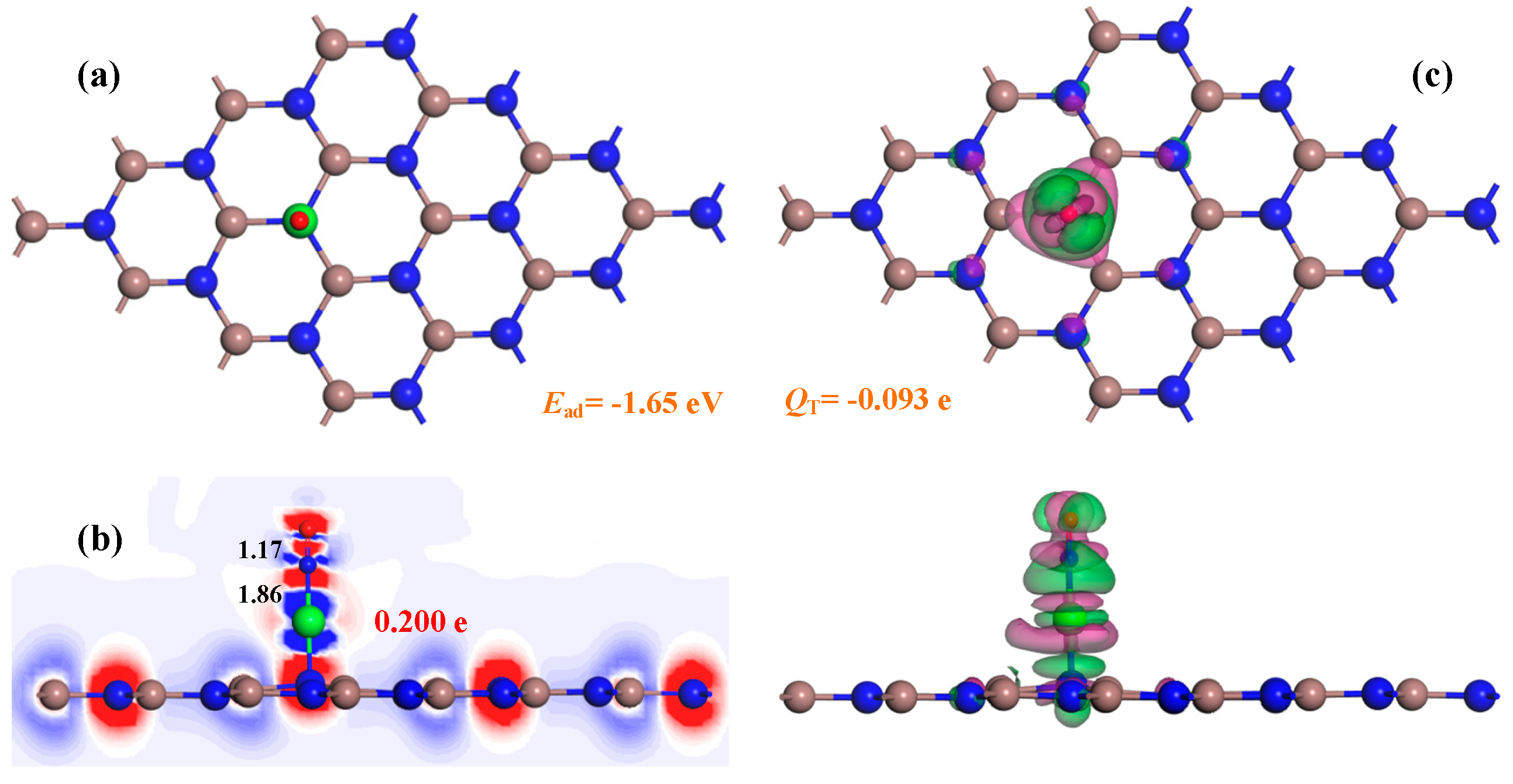
3.2. Adsorption Behavior of the Pd-InN Monolayer
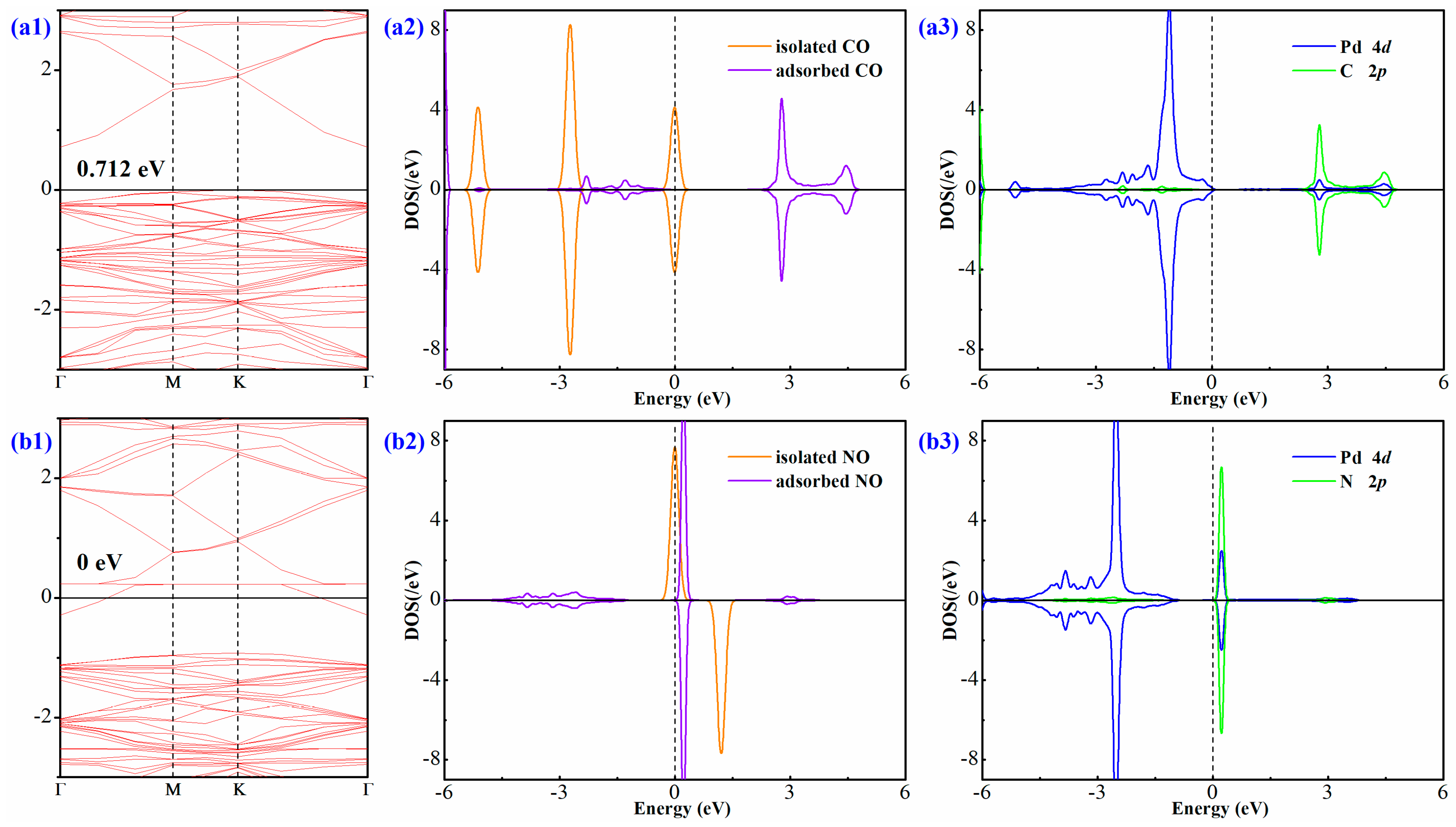
3.3. Electronic Behavior of Pd-InN upon Gas Adsorption
3.4. Application of the Pd-InN Monolayer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pierucci, D.; Zribi, J.; Henck, H.; Chaste, J.; Silly, M.G.; Bertran, F.; Le Fèvre, P.; Gil, B.; Summerfield, A.; Beton, P.H.; et al. Van der Waals epitaxy of two-dimensional single-layer h-BN on graphite by molecular beam epitaxy: Electronic properties and band structure. Appl. Phys. Lett. 2018, 112, 253102. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Yan, C.; Zhang, Y.; Zhang, X. Repairing the N-vacancy in an InN monolayer using NO molecules: A first-principles study. Nanoscale Adv. 2019, 1, 2003–2008. [Google Scholar] [CrossRef]
- Hashimoto, A.; Suenaga, K.; Gloter, A.; Urita, K.; Iijima, S. Direct evidence for atomic defects in graphene layers. Nature 2004, 430, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Su, W.S.; Chen, H.T. A new carbon allotrope: Penta-graphene as a metal-free catalyst for CO oxidation. Carbon 2017, 114, 465–472. [Google Scholar] [CrossRef]
- Sun, Z.; Chang, H. Graphene and Graphene-like Two-Dimensional Materials in Photodetection: Mechanisms and Methodology. ACS Nano 2014, 8, 4133–4156. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Li, Y.; Chen, D.; Zhang, Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. [Google Scholar] [CrossRef]
- Sarmazdeh, M.M.; Mendi, R.T.; Zelati, A.; Boochani, A.; Nofeli, F. First-principles study of optical properties of InN nanosheet. Int. J. Mod. Phys. B 2016, 30, 1650117. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Mota, F.B.; Rivelino, R.A.; Kakanakova-Georgieva, G.; Gueorguiev, K. Van der Waals stacks of few-layer h-AlN with graphene: An ab initio study of structural, interaction and electronic properties. Nanotechnology 2016, 27, 145601. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, S.; Hazar, F. First principles study on the spin unrestricted electronic structure properties of transition metal doped InN nanoribbons. Superlattices Microstruct. 2015, 84, 170–180. [Google Scholar] [CrossRef]
- Maleyre, B.; Briot, O.; Ruffenach, S.; Gil, B. Optical investigations on Si-doped InN films. Phys. Status Solidi 2010, 2, 1379–1383. [Google Scholar] [CrossRef]
- Yu, K.M.; Liliental-Weber, Z.; Walukiewicz, W.; Shan, W.; Ager, J.W.; Li, S.X.; Jones, R.E.; Haller, E.E.; Lu, H.; Schaff, W.J. On the crystalline structure, stoichiometry and band gap of InN thin films. Appl. Phys. Lett. 2005, 86, 071910. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Meng, R.; Tan, C.; Liang, Q.; Jiang, J.; Ye, H.; Chen, X. Adsorption of gas molecules on graphene-like InN monolayer: A first-principle study. Appl. Surf. Sci. 2017, 404, 291–299. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Zhang, Y.M.; Wu, W.X.; Liu, Y.X.; Zhou, Z.P. Transition metal (Pd, Pt, Ag, Au) decorated InN monolayer and their adsorption properties towards NO2: Density functional theory study. Appl. Surf. Sci. 2018, 455, 106–114. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Chen, D.C.; Tang, J. Pt & Pd decorated CNT as a workable media for SOF2 sensing: A DFT study. Appl. Surf. Sci. 2019, 471, 335–341. [Google Scholar]
- Cui, H.; Zhang, X.; Zhang, J.; Mehmood, M.A. Interaction of CO and CH4 Adsorption with Noble Metal (Rh, Pd, and Pt)-Decorated N3-CNTs: A First-Principles Study. ACS Omega 2018, 3, 16892–16898. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as a toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 1, 772–780. [Google Scholar] [CrossRef]
- Li, K.J.; Wang, W.C.; Cao, D.P. Metal (Pd, Pt)-decorated carbon nanotubes for CO and NO sensing. Sens. Actuators B Chem. 2011, 159, 171–177. [Google Scholar] [CrossRef]
- Ma, D.W.; Ju, W.W.; Li, T.X.; Zhang, X.W.; He, C.Z.; Ma, B.Y.; Lu, Z.S.; Yang, Z.X. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Cui, H.; Qiu, G.; Wang, J.; Li, Q. Carbon-chain inserting effect on electronic behavior of single-walled carbon nanotubes: A density functional theory study. MRS Commun. 2018, 8, 189–193. [Google Scholar] [CrossRef]
- Tamijani, A.A.; Salam, A.; De Lara-Castells, M.P. Adsorption of Noble-Gas Atoms on the TiO2 (110) Surface: An Ab Initio-Assisted Study with van der Waals-Corrected DFT. J. Phys. Chem. C 2016, 120, 18126–18139. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Chen, D.; Tang, J. Adsorption mechanism of SF6 decomposed species on pyridine-like PtN3 embedded CNT: A DFT study. Appl. Surf. Sci. 2018, 447, 594–598. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Saoud, F.S.; Plenet, J.C.; Henini, M. Structural, electronic and vibrational properties of InN under high pressure. Phys. B Condens. Matter 2012, 407, 1008–1013. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.S.; Zhang, J.; Tang, J. Adsorption behaviour of SF6 decomposed species onto Pd4-decorated single-walled CNT: A DFT study. Mol. Phys. 2018, 116, 1749–1755. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS 2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Chen, D.C. Borophene: A promising adsorbent material with strong ability and capacity for SO2 adsorption. Appl. Phys. A 2018, 124, 636. [Google Scholar] [CrossRef]
- Pyykko, P.; Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1-118. Chem. A Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef]
- Wu, P.; Yin, N.; Li, P.; Cheng, W.; Huang, M. The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS 2 monolayer: A first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 20713–20722. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, M.; Poh, H.L.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Šaněk, F.; Khezri, B.; Webster, R.D.; Pumera, M. Noble metal (Pd, Ru, Rh, Pt, Au, Ag) doped graphene hybrids for electrocatalysis. Nanoscale 2012, 4, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lv, P.; Ma, D.; Yang, X.; Li, S.; Yang, Z. Detection of gas molecules on single Mn adatom adsorbed graphyne: A DFT-D study. J. Phys. D Appl. Phys. 2018, 51, 065109. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Wu, J.; Junfeng, W. Layer-by-layer assembled In2O3 nanocubes/flower-like MoS2 nanofilm for room temperature formaldehyde sensing. Sens. Actuators B Chem. 2018, 273, 176–184. [Google Scholar] [CrossRef]
- Baek, D.H.; Kim, J. MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Ju, W.; Li, T.; Su, X.; Li, H.; Li, X.; Ma, D. Au cluster adsorption on perfect and defective MoS2 monolayers: Structural and electronic properties. Phys. Chem. Chem. Phys. 2017, 19, 20735–20748. [Google Scholar] [CrossRef]
- Zhao, B.; Li, C.; Liu, L.; Zhou, B.; Zhang, Q.; Chen, Z.; Tang, Z. Adsorption of gas molecules on Cu impurities embedded monolayer MoS2: A first- principles study. Appl. Surf. Sci. 2016, 382, 280–287. [Google Scholar] [CrossRef]
- Ma, D.W.; Wang, Q.G.; Li, T.X.; He, C.Z.; Ma, B.Y.; Tang, Y.N.; Lu, Z.S.; Yang, Z.X. Repairing sulfur vacancies in the MoS2 monolayer by using CO, NO and NO2 molecules. J. Mater. Chem. C 2016, 4, 7093–7101. [Google Scholar] [CrossRef]
- Xia, C.; Peng, Y.; Zhang, H.; Wang, T.; Wei, S.; Jia, Y. The characteristics of n- and p-type dopants in SnS2 monolayer nanosheets. Phys. Chem. Chem. Phys. 2014, 16, 19674. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, X.; Yao, Q.; Miao, Y.; Tang, J. Rh-doped carbon nanotubes as a superior media for the adsorption of O2 and O3 molecules: A density functional theory study. Carbon Lett. 2018, 28, 55–59. [Google Scholar]
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Semiconductor Physical Electronics; Springer: New York, NY, USA, 2006; Volume 28, pp. 363–364.
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.T.; Ao, Z.M.; Ji, Y.M.; Li, G.Y.; An, T.C. Adsorption mechanisms of different volatile organic compounds onto pristine C2N and Al-doped C2N monolayer: A DFT investigation. Appl. Surf. Sci. 2018, 450, 484–491. [Google Scholar] [CrossRef]

| System | Pd-InN | CO Molecule | NO Molecule | Pd-InN/CO | Pd-InN/NO |
|---|---|---|---|---|---|
| ZPVE (eV) | 1.91 | 0.13 | 0.12 | 2.11 | 2.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Ma, S. Adsorption and Sensing Behaviors of Pd-Doped InN Monolayer upon CO and NO Molecules: A First-Principles Study. Appl. Sci. 2019, 9, 3390. https://doi.org/10.3390/app9163390
Zhu S, Ma S. Adsorption and Sensing Behaviors of Pd-Doped InN Monolayer upon CO and NO Molecules: A First-Principles Study. Applied Sciences. 2019; 9(16):3390. https://doi.org/10.3390/app9163390
Chicago/Turabian StyleZhu, Shiyuan, and Shouxiao Ma. 2019. "Adsorption and Sensing Behaviors of Pd-Doped InN Monolayer upon CO and NO Molecules: A First-Principles Study" Applied Sciences 9, no. 16: 3390. https://doi.org/10.3390/app9163390
APA StyleZhu, S., & Ma, S. (2019). Adsorption and Sensing Behaviors of Pd-Doped InN Monolayer upon CO and NO Molecules: A First-Principles Study. Applied Sciences, 9(16), 3390. https://doi.org/10.3390/app9163390




