Preparation of an Asymmetric Membrane from Sugarcane Bagasse Using DMSO as Green Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Cellulose from Sugarcane Bagasse
2.3. Synthesis of Cellulose Acetate
2.4. Determination of Degree of Substitution and the Viscosity-Average Molecular Weight
2.5. Preparation of Asymmetric Cellulose Acetate Membranes
2.6. Characterizations and Analysis Methods
2.7. Membrane Performance of the Permeate Flux and the Antifouling Experiment
3. Results and Discussion
3.1. Extraction of Cellulose from Sugarcane Bagasse
3.1.1. Chemical Composition
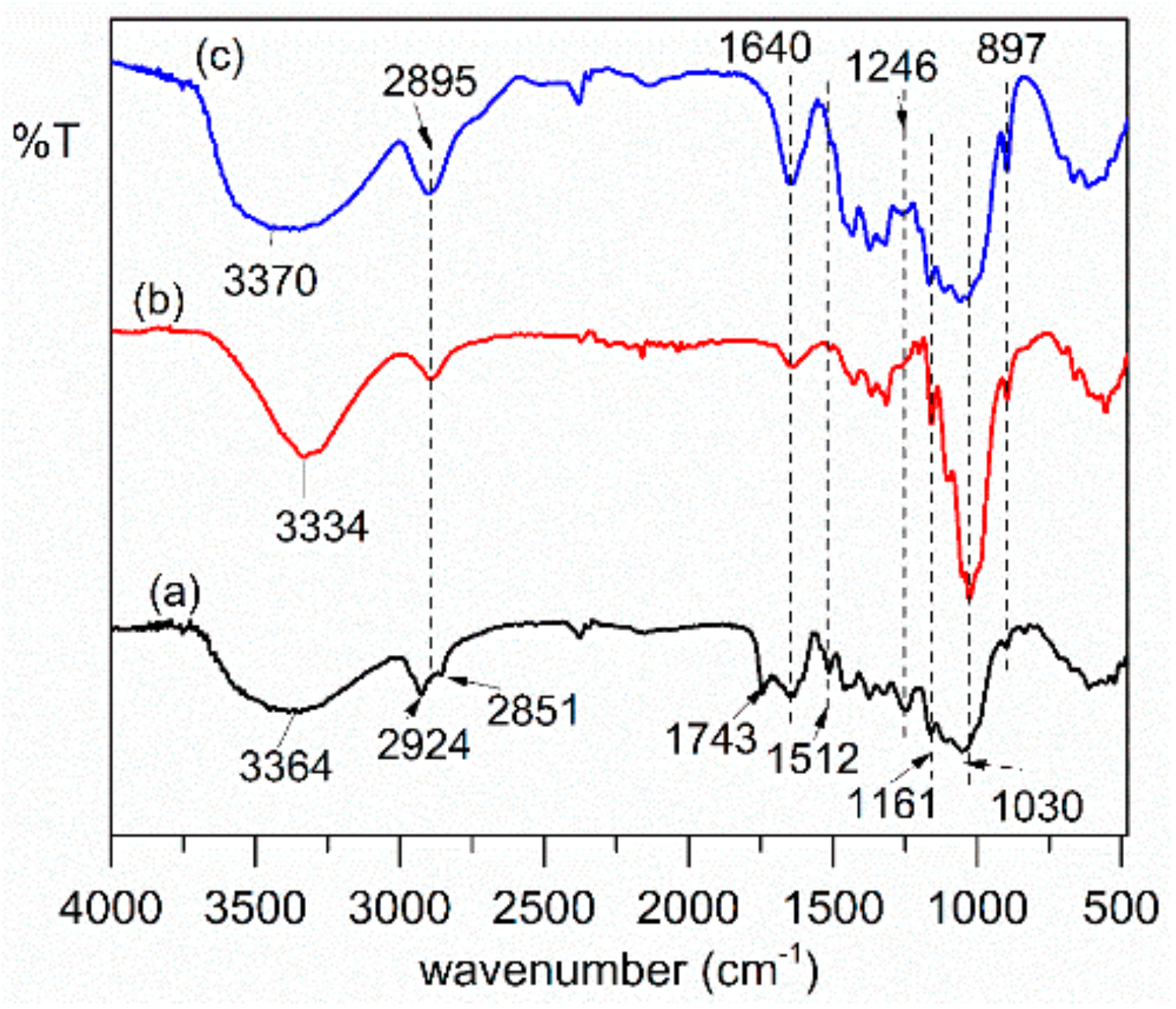
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2. Characterization of Cellulose Acetate
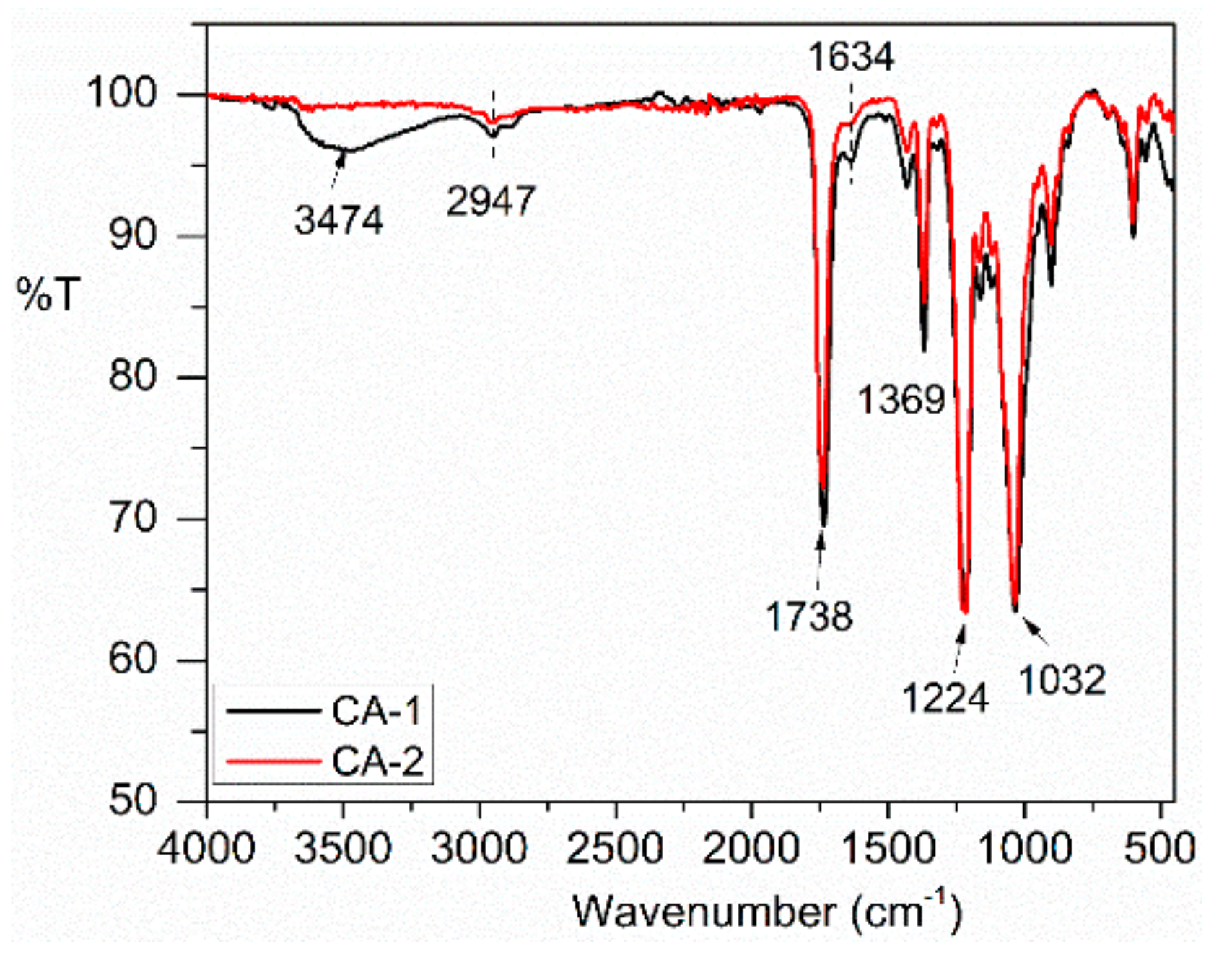
3.2.1. FT-IR
3.2.2. Degree of Substitution and Viscosity Average Molecular Weight
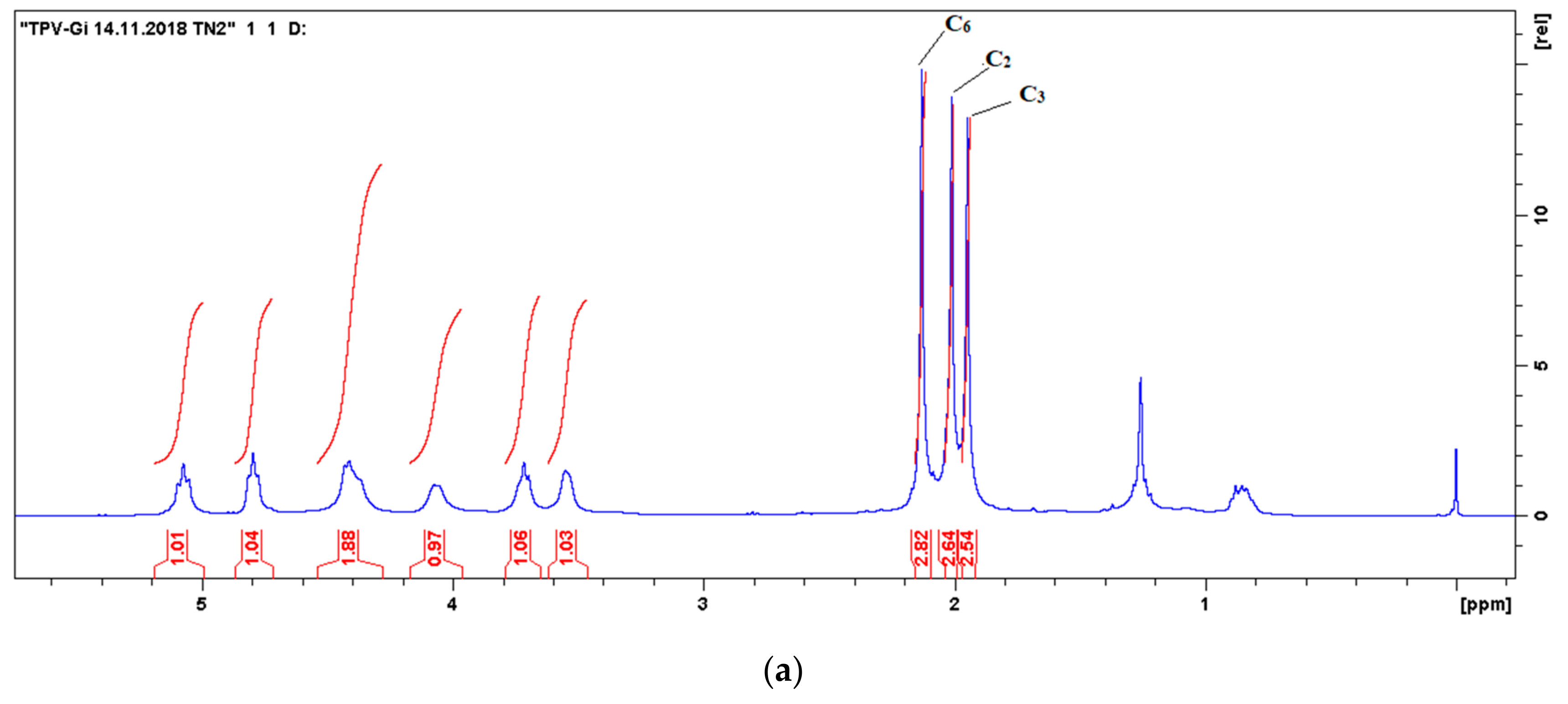
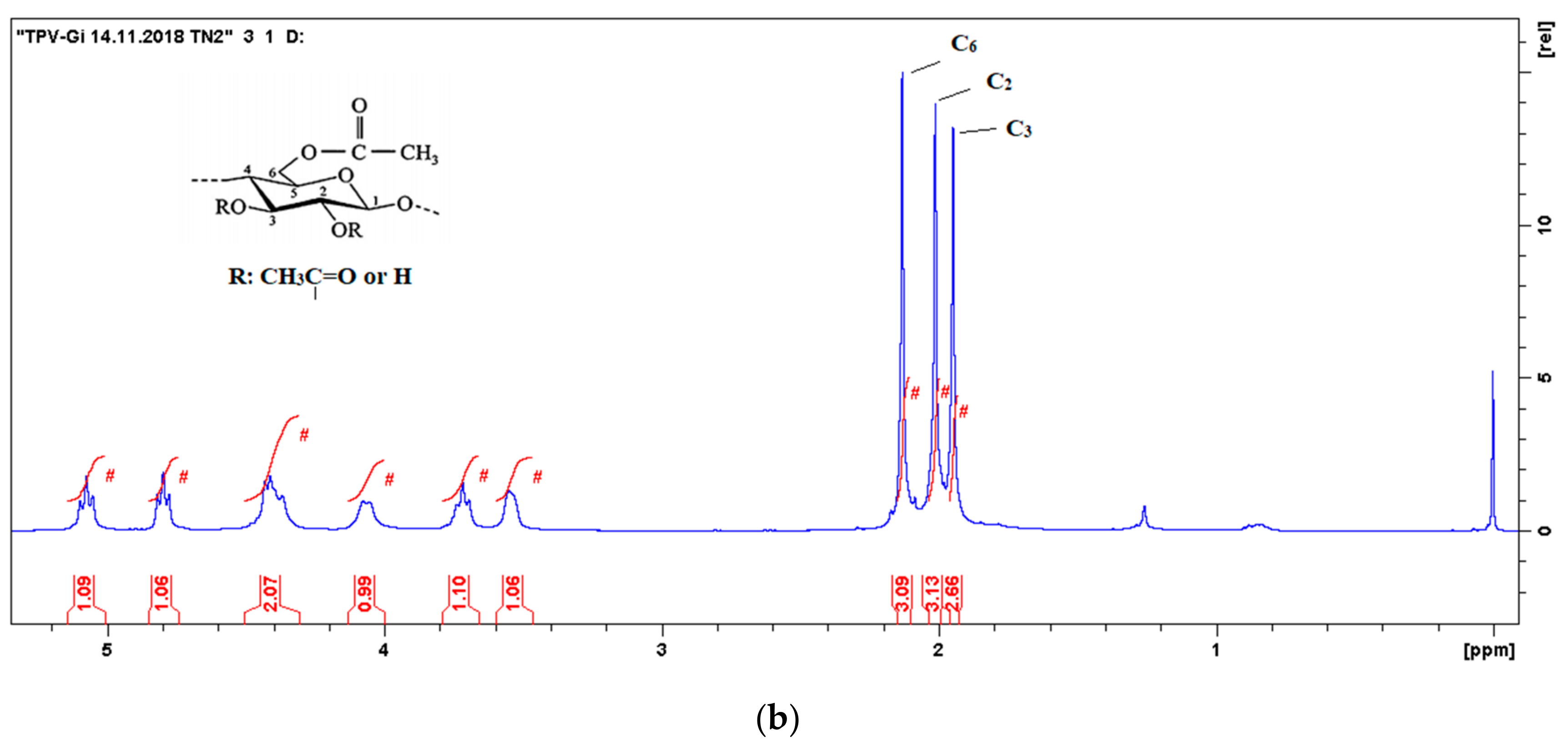
3.2.3. 1H-NMR Spectroscopy
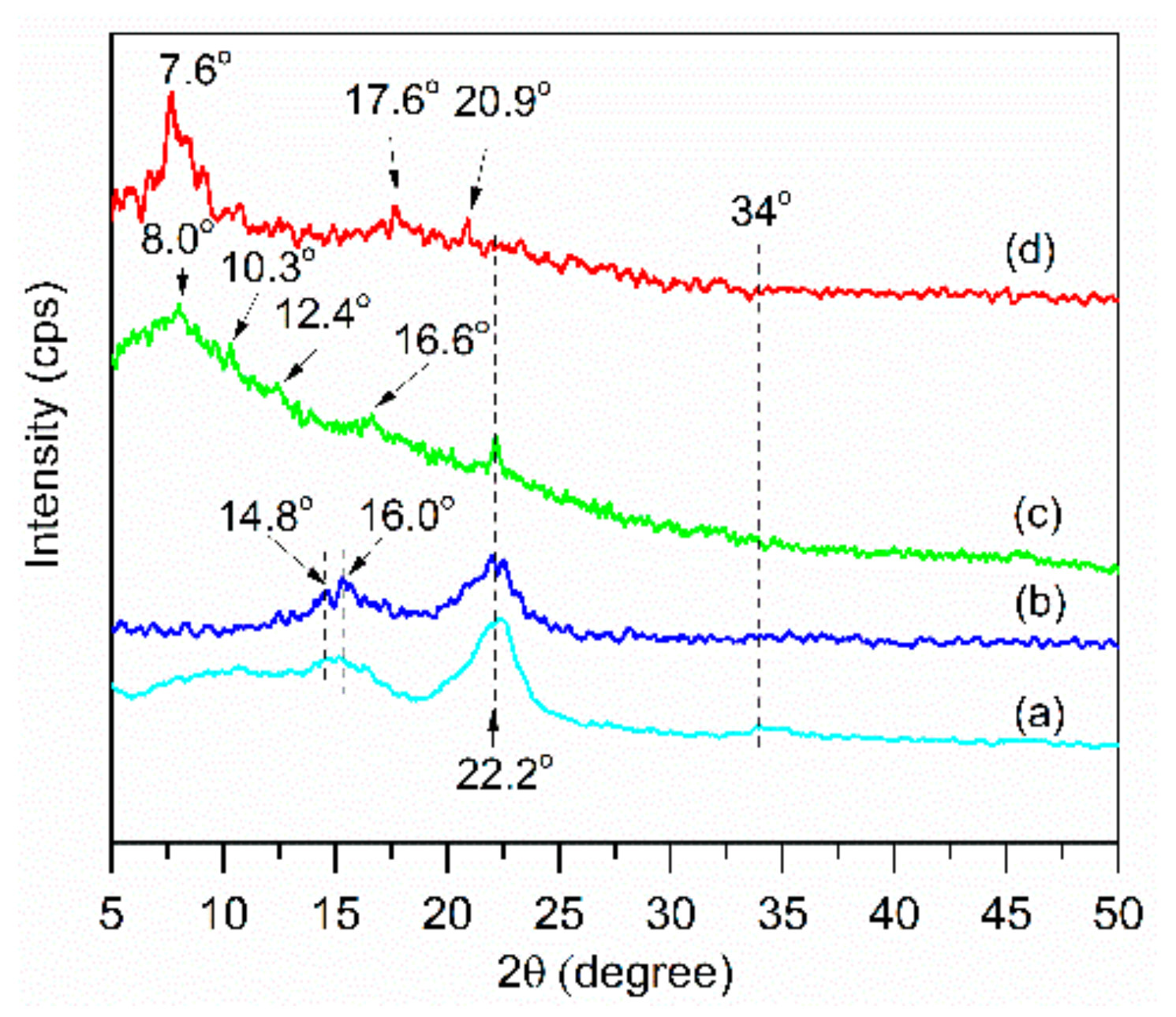
3.2.4. XRD of Extracted Cellulose and Cellulose Acetate
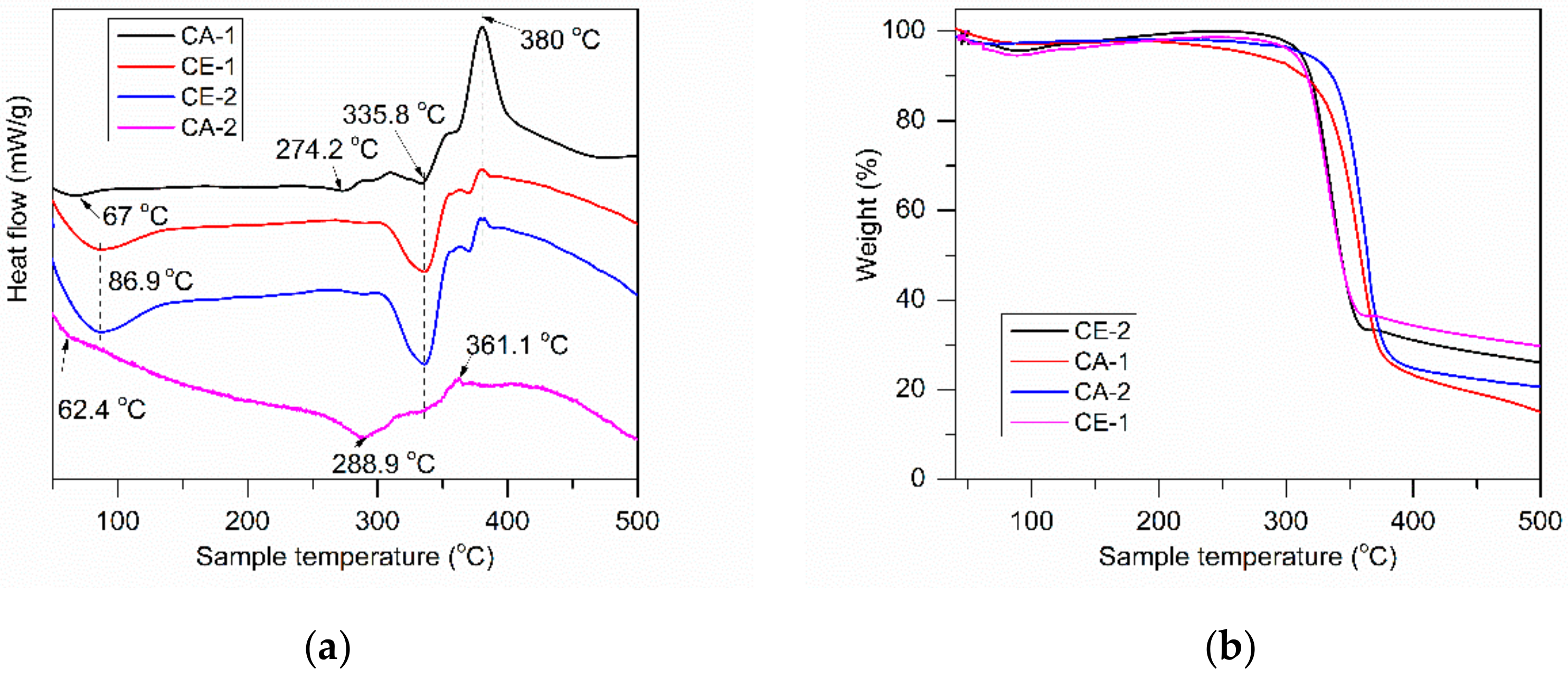
3.2.5. Differential Scanning Calorimetry-Thermogravimetric Analysis
3.3. Characterization of Asymmetric Membranes
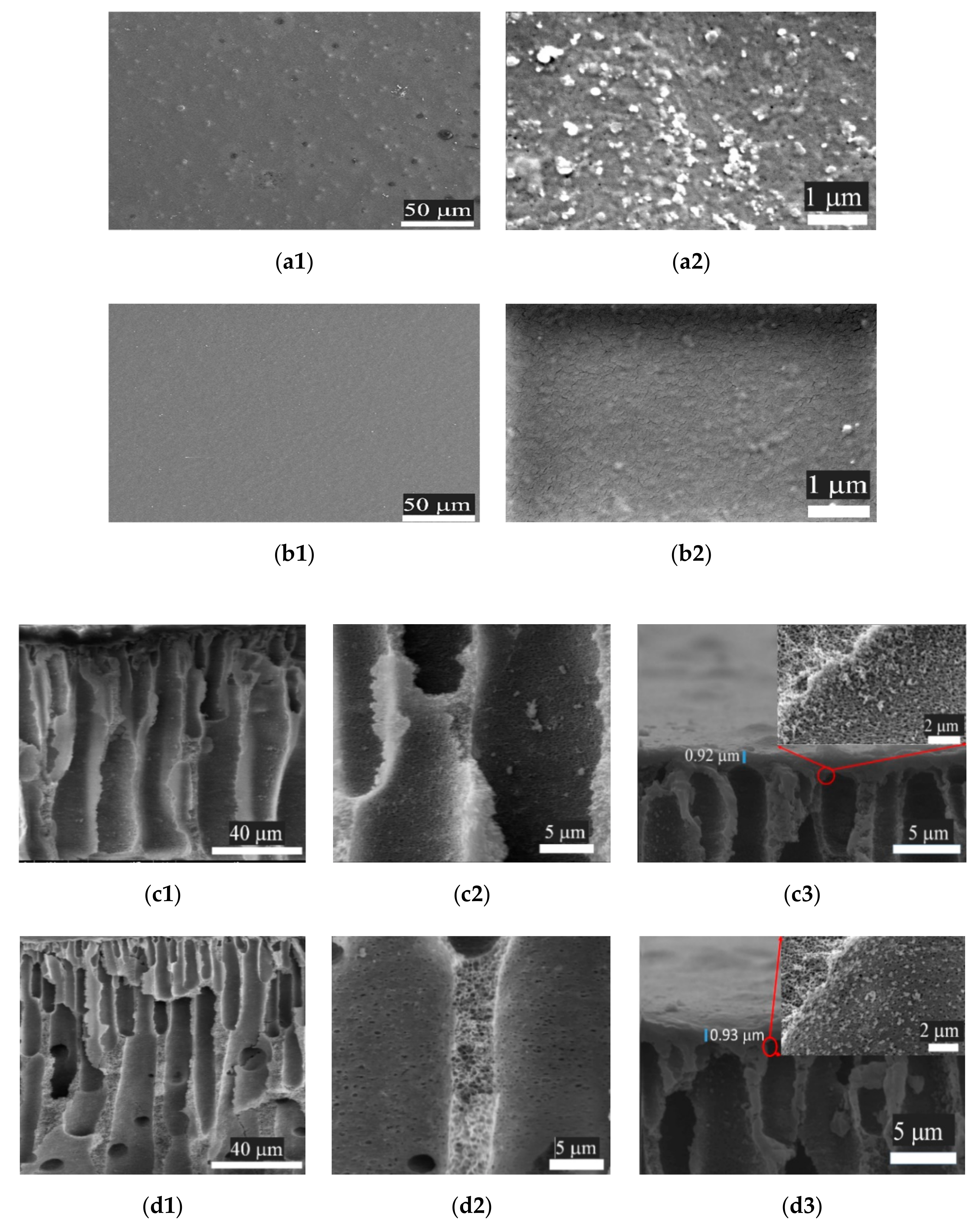
3.3.1. Scanning Electron Microscopy (SEM)
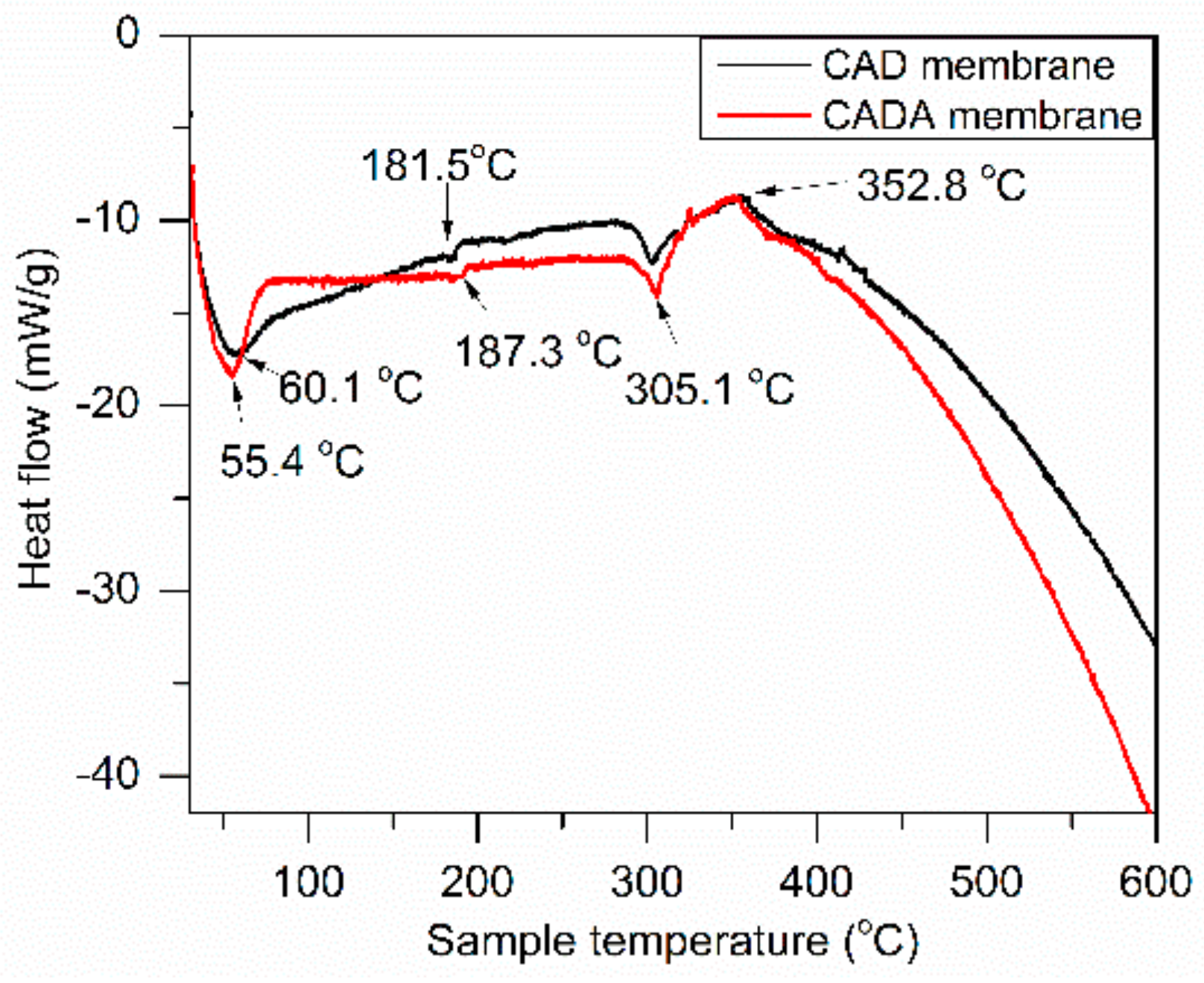
3.3.2. DSC Analysis
3.3.3. Atomic Force Microscopy (AFM) Analysis
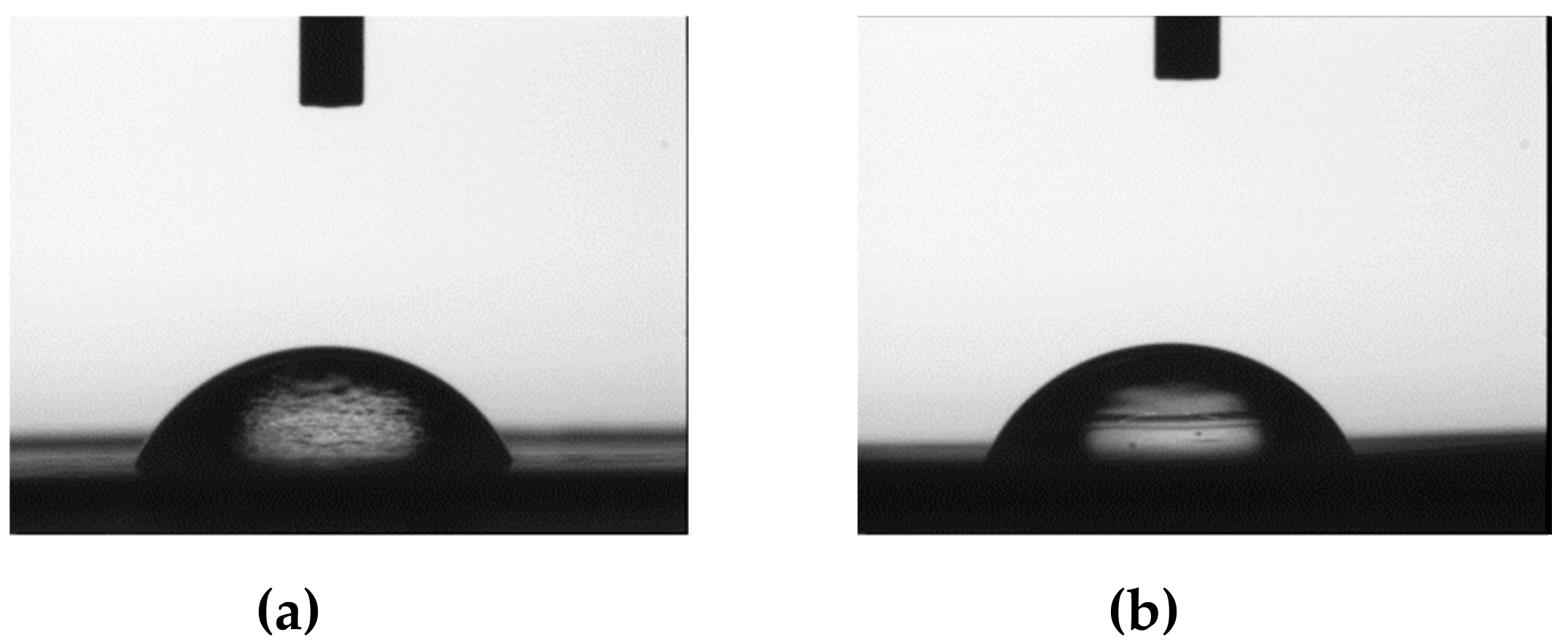
3.3.4. Contact Angle and Content Water Measurements
3.3.5. Performance and Fouling Evaluation of the Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Xu, Y. Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour. Technol. 2019, 282, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, D.A.; Filho, G.R.; Meireles, S. Optimization of sugarcane bagasse cellulose acetylation. Carbohydr. Polym. 2007, 69, 579–582. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.G.; Godoy, G.G.; Gonçalves, A. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr. Polym. 2017, 167, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and characterization of cellulose acetate from rice husk: Eco-friendly condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, D.A.; Valente, A.J.M.; Filho, G.R.; Burrows, H.D. Synthesis and properties of polyaniline-cellulose acetate blends: The use of sugarcane bagasse waste and the effect of the substitution degree. Carbohydr. Polym. 2009, 78, 402–408. [Google Scholar] [CrossRef]
- Candido, R.G.; Gonçalves, A.R. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Freitas, R.R.M.; Senna, A.M.; Botaro, V.R. Influence of degree of substitution on thermal dynamic mechanical and physicochemical properties of cellulose acetate. Ind. Crops Prod. 2017, 109, 452–458. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Barud, H.S.; Júnior, A.M.A.; Santos, D.B.; Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Filho, G.R.; Ribeiroa, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Jogunola, O.; Eta, V.; Hedenström, M.; Sundman, O.T.; Salmi, T.; Mikkola, J.P. Ionic liquid mediated technology for synthesis of cellulose acetates using different co-solvents. Carbohydr. Polym. 2016, 135, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.B.; Morais Júnior, W.G.; Silva, C.V.; Vieira, A.T.; Batista, A.C.F.; Faria, A.M.; Assunção, R.M.N. Preparation and Characterization of Cellulose Triacetate as Support for Lecitase Ultra Immobilization. Molecules 2017, 22, 1930. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, T.; Saljoughi, E. Effect of production conditions on morphology and permeability of asymmetric cellulose acetate membranes. Desalination 2009, 243, 1–7. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A. review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Zhang, Y.; Sun, S.; Liu, W. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling. J. Membr. Sci. 2019, 575, 50–59. [Google Scholar] [CrossRef]
- Filho, G.R.; Ribeiro, S.D.; Meireles, C.S.; Silva, L.G.; Ruggiero, R.; Junior, M.F.F.; Cerqueira, D.A.; Assunção, R.M.N.; Zeni, M.; Polleto, P. Release of doxycycline through cellulose acetate symmetric and asymmetric membranes produced from recycled agroindustrial residue: Sugarcane bagasse. Ind. Crops Prod. 2011, 33, 566–571. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Pandare, K.V.; Nair, G.; Varma, A.J. Utilization of sugarcane bagasse cellulose for producing cellulose acetates: Novel use of residual hemicellulose as plasticizer. Carbohydr. Polym. 2009, 76, 23–29. [Google Scholar] [CrossRef]
- Filho, R.G.; Toledo, L.C.; Silva, L.G.; Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Ruggiero, R. Membranes of Cellulose Triacetate Produced from Sugarcane Bagasse Cellulose as Alternative Matrices for Doxycycline Incorporation. Appl. Polym. Sci. 2009, 113, 3544–3549. [Google Scholar] [CrossRef]
- Nevárez, L.M.; Casarrubias, L.B.; Canto, O.S.; Celzard, A.; Fiero, V.; Gómez, R.I.; Sanschez, G.G. Biopolymers-based nanocomposites: Membranes from propionated lignin and cellulose for water purification. Carbohydr. Polym. 2011, 86, 732–741. [Google Scholar] [CrossRef]
- Evenepoel, N.; Wen, S.; Tilahun Tsehaye, M.; Bart, V.B. Potential of DMSO as greener solvent for PES ultra- and nanofiltration membrane preparation. J. Appl. Polym. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Meringolo, C.; Poerio, T.; Fontananova, E.; Mastropietro, T.F.; Nicoletta, F.P.; Filpo, G.D.; Curcio, E.; Profi, G.D. Exploiting Fluoropolymers Immiscibility to Tune Surface Properties and Mass Transfer in Blend Membranes for Membrane Contactor Applications. ACS Appl. Polym. Mater. 2019, 1, 326–334. [Google Scholar] [CrossRef]
- Xie, W.; Li, T.; Chen, C.; Wu, H.; Liang, H.; Chang, H.; Liu, B.; Drioli, E.; Wang, Q.; Crittenden, J.C. Using the Green Solvent Dimethyl Sulfoxide to Replace Traditional Solvents Partly and Fabricating PVC/PVC- g-PEGMA Blended Ultrafiltration Membranes with High Permeability and Rejection. Ind. Eng. Chem. Res. 2019, 58, 6413–6423. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.-M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation: A review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Cai, J.; Fei, P.; Xiong, Z.; Shi, Y.; Yan, K.; Xiong, H. Surface acetylation of bamboo cellulose: Preparation and rheological properties. Carbohydr. Polym. 2013, 92, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Viera, R.G.P.; Filho, G.R.; Assunção, R.M.N.; Carla, C.; Vieira, J.G.; Oliveira, G.S. Synthesis and characterization of methylcellulose from sugar cane bagasse cellulose. Carbohydr. Polym. 2007, 67, 182–189. [Google Scholar] [CrossRef]
- Meireles, C.S.; Filho, R.G.; Ferreira, M.F., Jr.; Cerqueira, A.D.; Assuncão, R.M.N.; Ribeiro, E.A.M.; Poletto, P.; Zeni, M. Characterization of asymmetric membranes of cellulose acetate from biomass: Newspaper and mango seed. Carbohydr. Polym. 2010, 80, 954–961. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Supplement 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ma, X.J.; Yang, X.F.; Zheng, X.; Lin, L.; Chen, L.H.; Huang, L.L.; Cao, S.L. Degradation and dissolution of hemicelluloses during bamboo hydrothermal pretreatment. Bioresour. Technol. 2014, 161, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Han, Y.; Lu, C.; Zhang, X.; Yuan, G. Acidic ionic liquid as ‘quasi-homogeneous’ catalyst for controllable synthesis of cellulose acetate. Carbohydr. Polym. 2014, 113, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, X.; Lu, C.; Zhou, Z.; Zhang, X.; Yuan, G. Water-soluble cellulose acetate from waste cotton fabrics and the aqueous processing of all-cellulose composites. Carbohydr. Polym. 2016, 149, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Matsumura, H.; Sugiyama, J.; Glasser, W.G. Cellulosic nanocomposites. I. Thermally deformable cellulose hexanoates from heterogeneous reaction. J. Appl. Polym. Sci. 2000, 78, 2242–2253. [Google Scholar] [CrossRef]
- Fan, G.; Wang, M.; Liao, C.; Fang, T.; Li, J.; Zhou, R. Isolation of cellulose from rice straw and its conversion into cellulose acetate catalyzed by phosphotungstic acid. Carbohydr. Polym. 2013, 94, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Numata, Y.; Nagai, N.; Erata, T.; Takai, M. CPMAS13C NMR and X-ray studies of cellooligosaccharide acetates as a model for cellulose triacetate. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4100–4107. [Google Scholar] [CrossRef]
- Wan Daud, W.R.; Djuned, F.M. Cellulose acetate from oil palm empty fruit bunch via a one step heterogeneous acetylation. Carbohydr. Polym. 2015, 132, 252–260. [Google Scholar] [CrossRef]
- Sun, X.F.; Sun, R.C.; Sun, J.X. Acetylation of sugarcane bagasse using NBS as a catalyst under mild reaction conditions for the production of oil sorption-active materials. Bioresour. Technol. 2004, 95, 343–350. [Google Scholar] [CrossRef]
- Xu, P.; Mujumdar, A.S.; Yu, B. Drying-Induced Cracks in Thin Film Fabricated from Colloidal Dispersions. Dry. Technol. 2009, 27, 636–652. [Google Scholar] [CrossRef]
- Singh, K.; Tirumkudulu, M.S. Cracking in Drying Colloidal Films. Phys. Rev. Lett. 2007, 98, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Huyen, T.D.; Roberto, M.N.; Takeshi, M.; Kailash, C.K. A Comparison of Commercial and Experimental Ultrafiltration Membranes via Surface Property Analysis and Fouling. Water Qual. Res. J. Can. 2006, 41, 84–93. [Google Scholar]
- Ferrand, E.L.; Li, D.; Lee, D.; Durning, C.J. All-Nanoparticle Layer-by-Layer Surface Modification of Micro- and Ultrafiltration Membranes. Langmuir 2014, 30, 5545–5556. [Google Scholar] [CrossRef] [PubMed]
- Majda, Z.; Ema, Z. Progress in Polymer Science Aliphatic hyperbranched polyesters based on 2,2-bis (methylol) propionic acid—Determination of structure, solution and bulk properties. Prog. Polym. Sci. 2011, 36, 53–88. [Google Scholar]
- Ghaemi, N.; Madaeni, S.S.; Alizadeh, A.; Daraei, P.; Vatanpour, V.; Falsa, M. Fabrication of cellulose acetate/sodium dodecyl sulfate nanofiltration membrane: Characterization and performance in rejection of pesticides. Desalination 2012, 290, 99–106. [Google Scholar] [CrossRef]
- Mahdavi, H.; Shahalizade, T. Preparation, characterization and performance study of cellulose acetate membranes modified by aliphatic hyperbranched polyester. J. Membr. Sci. 2015, 473, 256–266. [Google Scholar] [CrossRef]
- Kim, J.; Bart, V.D.B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Saraswathi, S.A.; Rana, M.S.; Alwarappan, D.; Gowrishankar, S.; Kanimozhi, S.; Nagendran, P.; Nagendran, A. Cellulose acetate ultrafiltration membranes customized with bio-inspired polydopamine coating and in situ immobilization of silver nanoparticles. New J. Chem. 2019, 43, 4216–4225. [Google Scholar] [CrossRef]
- Shen, S.; Chen, H.; Wang, R.; Ji, W.; Zhang, R.; Bai, R. Preparation of antifouling cellulose acetate membranes with good hydrophilic and oleophobic surface properties. Mater. Lett. 2019, 252, 1–4. [Google Scholar] [CrossRef]
- Jayalakshmi, A.; Rajesh, S.; Mohan, D. Applied Surface Science Fouling propensity and separation efficiency of epoxidated polyethersulfone incorporated cellulose acetate ultrafiltration membrane in the retention of proteins. Appl. Surf. Sci. 2012, 258, 9770–9781. [Google Scholar] [CrossRef]

| Samples a | H2O2 Treatment | Result | |||||
|---|---|---|---|---|---|---|---|
| Concentration of H2O2 (%, v/v) | Time (h) | Weight b (g) | Cellulose wt (%) | Hemicellulose wt (%) | Lignin wt (%) | Yield (%) | |
| CE-1 | 5 | 4 | 6.0 | 83.05 | 8.25 | 7.94 | 60% |
| CE-2 | 2 | 4 for once | 6.6 | 89.09 | 7.11 | 3.07 | 66% |
| Sample | % Acetyl Group (AG) | Degree of Substitution (DS) b | Viscosity η (mL/g) | Average Molecular Weight Mv (g/mol) | Yield (% Dry Weight) a |
|---|---|---|---|---|---|
| CA-1 | 41.68 | 2.65 | 103.2 | 43,760.8 | 45 |
| CA-2 | 43.05 | 2.80 | 112.8 | 48,685.9 | 51 |
| Membrane | Root Mean Square Height (Sq) | Arithmetic Mean Height (Sa) |
|---|---|---|
| CAD | 4.45 nm | 3.51 nm |
| CADA | 3.34 nm | 2.53 nm |
| Membranes | WC (%) | Contact Angle (°) |
|---|---|---|
| CAD | 78.34 | 55.6 ± 1.6 |
| CADA | 75.09 | 58.4 ± 1.8 |
| Membrane | Pure Water Flux, Jw1 (L m−2 h−1) | Permeation BSA Flux, Jp1 (L m−2 h−1) | Pure Water Flux after Cleaning, Jw2 (L m−2 h−1) | Permeation BSA Flux, Jp2 (L m−2 h−1) | Pure Water Flux after Cleaning, Jw3 (L m−2 h−1) |
|---|---|---|---|---|---|
| CAD | 314.41 | 136.24 | 288.21 | 128.38 | 248.91 |
| CADA | 301.31 | 131.01 | 248.91 | 99.56 | 196.51 |
| First Cycle | Second Cycle | |||||||
|---|---|---|---|---|---|---|---|---|
| FRR (%) | Rt (%) | Rr (%) | Rir (%) | FRR (%) | Rt (%) | Rr (%) | Rir (%) | |
| CAD | 91.67 | 56.67 | 48.33 | 8.33 | 86.36 | 55.45 | 41.82 | 13.64 |
| CADA | 82.61 | 56.52 | 39.13 | 17.39 | 78.95 | 60.00 | 38.95 | 21.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi To Nu, D.; Phi Hung, N.; Van Hoang, C.; Van der Bruggen, B. Preparation of an Asymmetric Membrane from Sugarcane Bagasse Using DMSO as Green Solvent. Appl. Sci. 2019, 9, 3347. https://doi.org/10.3390/app9163347
Thi To Nu D, Phi Hung N, Van Hoang C, Van der Bruggen B. Preparation of an Asymmetric Membrane from Sugarcane Bagasse Using DMSO as Green Solvent. Applied Sciences. 2019; 9(16):3347. https://doi.org/10.3390/app9163347
Chicago/Turabian StyleThi To Nu, Dang, Nguyen Phi Hung, Cao Van Hoang, and Bart Van der Bruggen. 2019. "Preparation of an Asymmetric Membrane from Sugarcane Bagasse Using DMSO as Green Solvent" Applied Sciences 9, no. 16: 3347. https://doi.org/10.3390/app9163347
APA StyleThi To Nu, D., Phi Hung, N., Van Hoang, C., & Van der Bruggen, B. (2019). Preparation of an Asymmetric Membrane from Sugarcane Bagasse Using DMSO as Green Solvent. Applied Sciences, 9(16), 3347. https://doi.org/10.3390/app9163347






