In Vitro Targeting and Imaging of Neurogenic Differentiation in Mouse Bone-Marrow Derived Mesenchymal Stem Cells with Superparamagnetic Iron Oxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Osteogenic, Chondrogenic, and Neurogenic Induction of D1 MSCs
2.2. Synthesis and Characterization of SPIONs
2.3. Surface Modification with Dopamine and 675-nm N-hydroxysuccinimide (NHS)
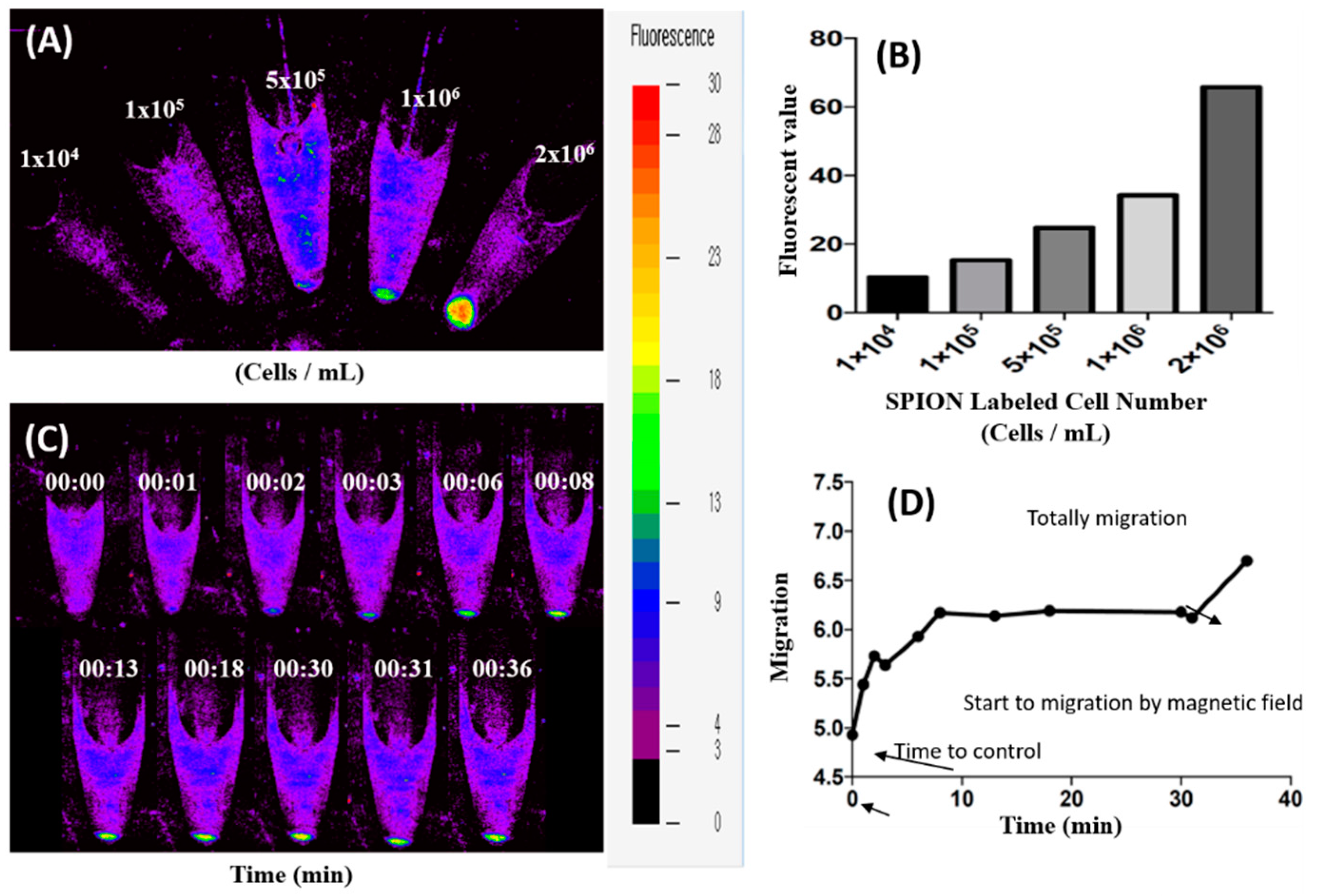
2.4. Cell Viability and Magnetic Migration of SPION-Labeled D1 MSCs
3. Results
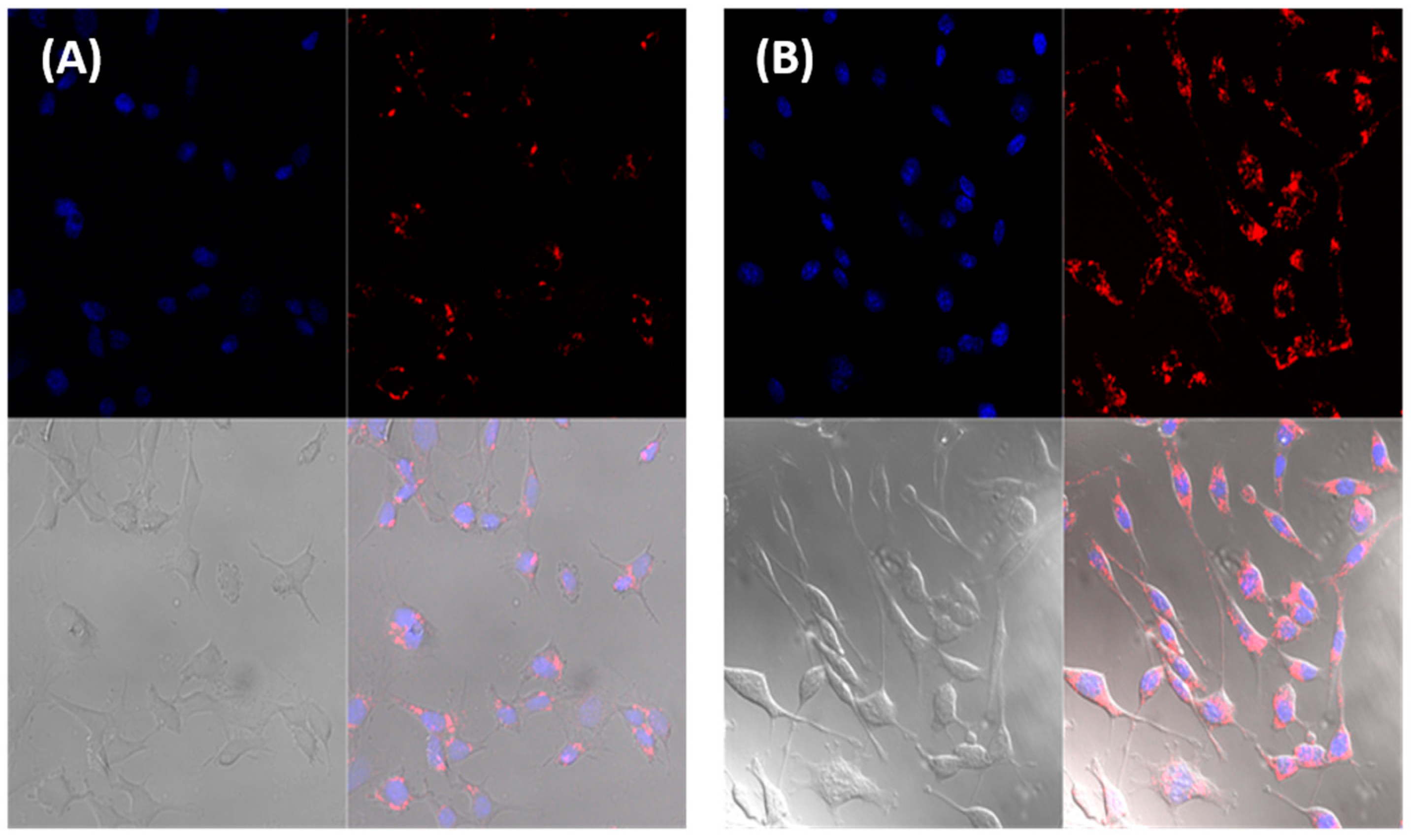
3.1. Differentiation Ability Potential of D1 MSCs
3.2. Cytotoxicity of SPIONs
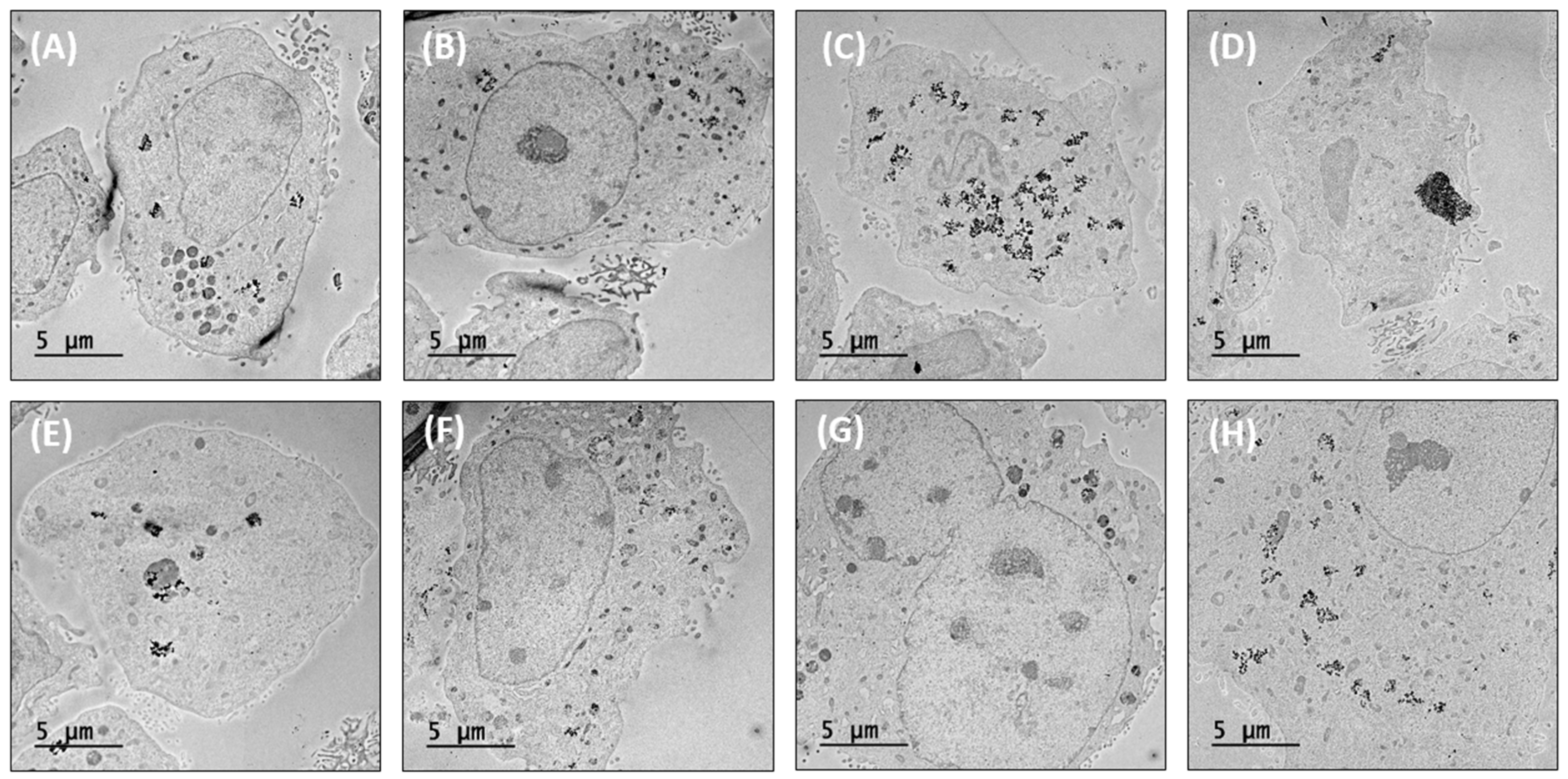
3.3. SPIONs Synthesis and Labeling with D1 MSCs
3.4. Magnetic Targeting and Migration of SPION-Labeled D1 MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vawda, R.; G Fehlings, M. Mesenchymal cells in the treatment of spinal cord injury: Current & future perspectives. Curr. Stem Cell Res. Ther. 2013, 8, 25–38. [Google Scholar] [PubMed]
- Shende, P.; Subedi, M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed. Pharmacother. 2017, 91, 693–706. [Google Scholar] [CrossRef]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Jones, L.; Snyder, E.; Tuszynski, M. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Ronaghi, M.; Erceg, S.; Moreno-Manzano, V.; Stojkovic, M. Challenges of stem cell therapy for spinal cord injury: Human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells 2010, 28, 93–99. [Google Scholar] [CrossRef]
- Pearse, D.D.; Bunge, M.B. Designing cell-and gene-based regeneration strategies to repair the injured spinal cord. J. Neurotrauma 2006, 23, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Chen, X.; Evseenko, D.; Vangsness, C.T., Jr. Nomenclature inconsistency and selective outcome reporting hinder understanding of stem cell therapy for the knee. JBJS 2019, 101, 186–195. [Google Scholar] [CrossRef]
- Li, J.; Lepski, G. Cell transplantation for spinal cord injury: A systematic review. BioMed Res. Int. 2013, 2013, 786475. [Google Scholar] [CrossRef]
- Grade, S.; Götz, M. Neuronal replacement therapy: Previous achievements and challenges ahead. NPJ Regen. Med. 2017, 2, 29. [Google Scholar] [CrossRef]
- Caplan, A. Why are MSCs therapeutic? New data: New insight. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2009, 217, 318–324. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, C.; Schwarz, E.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Amtenbrink, M.; Hofmann, H.; Montet, X. Superparamagnetic nanoparticles—A tool for early diagnostics. Swiss Med. Wkly. 2010, 140. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Takahashi, S.; Saito, S.; Endo, Y.; Nittami, T.; Nozaki, T.; Sobti, R.; Watanabe, M. Combined Effects of Fe3O4 Nanoparticles and Chemotherapeutic Agents on Prostate Cancer Cells In Vitro. Appl. Sci. 2018, 8, 134. [Google Scholar] [CrossRef]
- McDermott, S.; Guimaraes, A.R. Magnetic nanoparticles in the imaging of tumor angiogenesis. Appl. Sci. 2012, 2, 525–534. [Google Scholar] [CrossRef]
- Lee, S.J.; Muthiah, M.; Lee, H.J.; Lee, H.-J.; Moon, M.-J.; Che, H.-L.; Heo, S.U.; Lee, H.-C.; Jeong, Y.Y.; Park, I.-K. Synthesis and characterization of magnetic nanoparticle-embedded multi-functional polymeric micelles for MRI-guided gene delivery. Macromol. Res. 2012, 20, 188–196. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef]
- Kozissnik, B.; Dobson, J. Biomedical applications of mesoscale magnetic particles. MRS Bull. 2013, 38, 927–932. [Google Scholar] [CrossRef]
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- An, T.-Y.; Surendran, S.; Kim, H.; Choe, W.-S.; Kim, J.K.; Sim, U. A polydopamine-mediated biomimetic facile synthesis of molybdenum carbide-phosphide nanodots encapsulated in carbon shell for electrochemical hydrogen evolution reaction with long-term durability. Compos. Part B Eng. 2019, 175, 107071. [Google Scholar] [CrossRef]
- Yun, W.; Choi, J.; Ju, H.; Kim, M.; Choi, S.; Oh, E.; Seo, Y.; Key, J. Enhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse models. Int. J. Mol. Sci. 2018, 19, 1376. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Springer: Berlin, Germany, 2011; pp. 237–245. [Google Scholar]
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J. Stem Cells 2014, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995, 5, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef]
- Bulte, J.W. In vivo MRI cell tracking: Clinical studies. Am. J. Roentgenol. 2009, 193, 314–325. [Google Scholar] [CrossRef]
- Van Buul, G.; Farrell, E.; Kops, N.; Van Tiel, S.; Bos, P.; Weinans, H.; Krestin, G.; Van Osch, G.; Bernsen, M. Ferumoxides–protamine sulfate is more effective than ferucarbotran for cell labeling: Implications for clinically applicable cell tracking using MRI. Contrast Media Mol. Imaging 2009, 4, 230–236. [Google Scholar] [CrossRef]
- Prasad, A.; Teh, D.B.L.; Blasiak, A.; Chai, C.; Wu, Y.; Gharibani, P.M.; Yang, I.H.; Phan, T.T.; Lim, K.L.; Yang, H. Static magnetic field stimulation enhances oligodendrocyte differentiation and secretion of neurotrophic factors. Sci. Rep. 2017, 7, 6743. [Google Scholar] [CrossRef]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Syková, E.; Kubinová, Š. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef] [PubMed]
- Garate, A.; Ciriza, J.S.; Casado, J.G.; Blazquez, R.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. Assessment of the behavior of mesenchymal stem cells immobilized in biomimetic alginate microcapsules. Mol. Pharm. 2015, 12, 3953–3962. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Park, I.-K.; Jeong, Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-K.; Lee, D.-K.; Lim, H.-J.; Sim, U. In Vitro Targeting and Imaging of Neurogenic Differentiation in Mouse Bone-Marrow Derived Mesenchymal Stem Cells with Superparamagnetic Iron Oxide Nanoparticles. Appl. Sci. 2019, 9, 3259. https://doi.org/10.3390/app9163259
Kim S-K, Lee D-K, Lim H-J, Sim U. In Vitro Targeting and Imaging of Neurogenic Differentiation in Mouse Bone-Marrow Derived Mesenchymal Stem Cells with Superparamagnetic Iron Oxide Nanoparticles. Applied Sciences. 2019; 9(16):3259. https://doi.org/10.3390/app9163259
Chicago/Turabian StyleKim, Sung-Kyu, Dong-Kyu Lee, Hyung-Ju Lim, and Uk Sim. 2019. "In Vitro Targeting and Imaging of Neurogenic Differentiation in Mouse Bone-Marrow Derived Mesenchymal Stem Cells with Superparamagnetic Iron Oxide Nanoparticles" Applied Sciences 9, no. 16: 3259. https://doi.org/10.3390/app9163259
APA StyleKim, S.-K., Lee, D.-K., Lim, H.-J., & Sim, U. (2019). In Vitro Targeting and Imaging of Neurogenic Differentiation in Mouse Bone-Marrow Derived Mesenchymal Stem Cells with Superparamagnetic Iron Oxide Nanoparticles. Applied Sciences, 9(16), 3259. https://doi.org/10.3390/app9163259





