Differential Efficacy of Two Dental Implant Decontamination Techniques in Reducing Microbial Biofilm and Re-Growth onto Titanium Disks In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Disks
2.2. Pseudomonas Aeruginosa
2.3. Microbial Growth and Biofilm Formation onto Titanium Disks
2.4. Titanium Disk Decontamination Systems
2.5. Residual Biofilm and Microbial Re-Growth after Decontamination
2.6. Statistical Analysis
3. Results
3.1. Total Microbial Growth and Biofilm Formation onto Titanium Disks
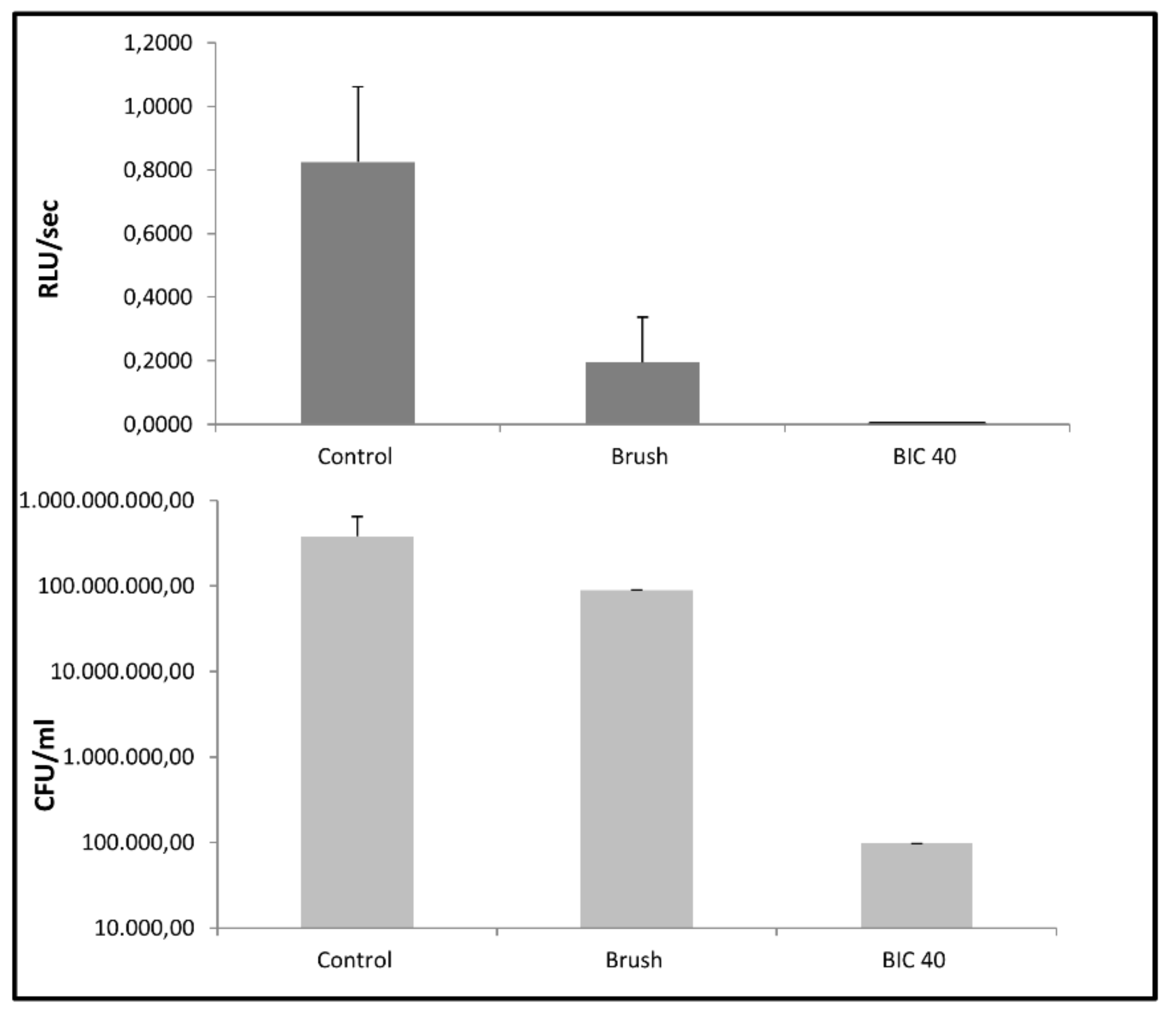
3.2. Microbial Biofilm Removal from Titanium Disks by the Two Decontamination Systems
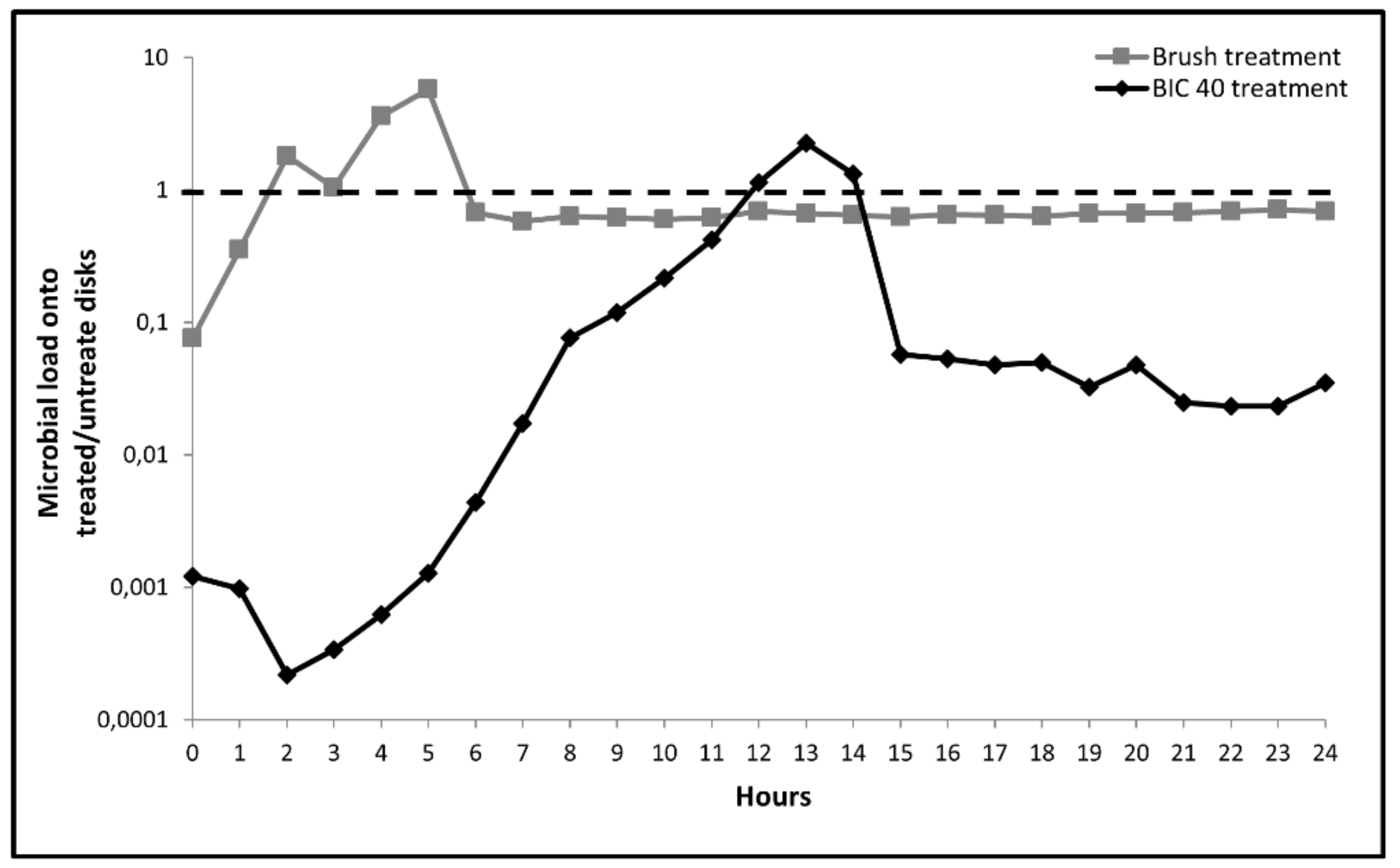
3.3. Microbial Re-Growth onto Treated Titanium Disks
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Singh, P. Understanding peri-implantitis: a strategic review. J. Oral Implants 2011, 37, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and Periodontal Disease: The Panacea to Problem Solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Botero, J.E.; González, A.M.; Mercado, R.A.; Olave, G.; Contreras, A. Subgingival microbiota in peri-implant mucosa lesions and adjacent teeth in partially edentulous patients. J. Periodontol. 2005, 76, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89, S267–CS290. [Google Scholar] [CrossRef]
- Klinge, B.; Flemming, T.; Cosyn, J.; De Bruyn, H.; Eisner, B.M.; Hultin, M.; Isidor, F.; Lang, N.P.; Lund, B.; Meyle, J.; et al. The patient undergoing implant therapy. Summary and consensus statements. Clin. Oral Implants Res. 2015, 26, 64–67. [Google Scholar] [CrossRef]
- Mellonig, J.T.; Griffiths, G.; Mayths, E.; Spitznagel, J. Treatment of the failing implant: case reports. Int. J. Periodont. Rest. Dent. 1995, 15, 384–395. [Google Scholar]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Penninger, J.M. RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv. Exp. Med. Biol. 2009, 649, 100–113. [Google Scholar]
- Mizoguchi, T.; Muto, A.; Udagawa, N.; Arai, A.; Yamashita, T.; Hosoya, A.; Ninomiya, T.; Nakamura, H.; Yamamoto, Y.; Kinugawa, S.; et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. Cell Biol. 2009, 184, 541–554. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Penninger, J.M. RANK/RANKL: Regulators of immune responses and bone physiology. Ann. NY Acad. Sci. 2008, 1143, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Takada, Y.; Ray, N.; Kishimoto, Y.; Penninger, J.M.; Yasuda, H.; Matsuo, K. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J. Immunol. 2006, 177, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in Osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, A.; Penninger, J.M. RANK(L) as a key target for controlling bone loss. Adv. Exp. Med. Biol. 2009, 647, 130–145. [Google Scholar] [PubMed]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef]
- Paulone, S.; Malavasi, G.; Ardizzoni, A.; Orsi, C.F.; Peppoloni, S.; Neglia, R.G.; Blasi, E. Candida albicans survival, growth and biofilm formation are differently affected by mouthwashes: An in vitro study. New Microbiol. 2017, 40, 45–52. [Google Scholar] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: a review of the literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar]
- De Freitas, A.R.; Silva, T.S.O.; Ribeiro, R.F.; de Albuquerque Junior, R.F.; Pedrazzi, V.; do Nascimento, C. Oral bacterial colonization on dental implants restored with titanium or zirconia abutments: 6-month follow-up. Clin. Oral Investig. 2018, 22, 2335–2343. [Google Scholar] [CrossRef]
- Albouy, J.P.; Abrahamsson, I.; Berglundh, T. Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2012, 39, 182–187. [Google Scholar] [CrossRef]
- Dennison, D.K.; Huerzeler, M.B.; Quinones, C.; Caffesse, R.G. Contaminated Implant Surfaces: An In Vitro Comparison of Implant Surface Coating and Treatment Modalities for Decontamination. J. Periodontol. 1994, 65, 942–948. [Google Scholar] [CrossRef]
- Sahrmann, P.; Ronay, V.; Hofer, D.; Attin, T.; Jung, R.E.; Schmidlin, P.R. In vitro cleaning potential of three different implant debridement methods. Clin. Oral Implant. Res. 2015, 26, 314–319. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. Influence of mechanical instruments on the biocompatibility of titanium dental implants surfaces: A systematic review. Clin. Oral Implant. Res. 2015, 26, 841–850. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Conserva, E.; Pisciotta, A.; Bertoni, L.; Bertani, G.; Meto, A.; Colombari, B.; Blasi, E.; Bellini, P.; de Pol, A.; Consolo, U.; et al. Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems. Int. J. Mol. Sci. 2019, 20, 1868. [Google Scholar] [CrossRef]
- Nemer Vieira, L.F.; Lopes de Chaves e Mello Dias, E.C.; Cardoso, E.S.; Machado, S.J.; Pereira da Silva, C.; Vidigal, G.M. Effectiveness of implant surface decontamination using a high-pressure sodium bicarbonate protocol: An in vitro study. Implant Dent. 2012, 21, 390–393. [Google Scholar] [CrossRef]
- Pericolini, E.; Colombari, B.; Ferretti, G.; Iseppi, R.; Ardizzoni, A.; Girardis, M.; Sala, A.; Peppoloni, S.; Blasi, E. Real-time monitoring of Pseudomonas aeruginosa biofilm formation on endotracheal tubes in vitro. BMC Microbiol. 2018, 18, 84. [Google Scholar] [CrossRef]
- Choi, K.H.; Schweizer, H.P. mini-Tn7 insertion in bacteria with single attTn7 sites: Example Pseudomonas aeruginosa. Nat. Protoc. 2006, 1, 153–161. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- Conserva, E.; Generali, L.; Bandieri, A.; Cavani, F.; Borghi, F.; Consolo, U. Plaque accumulation on titanium disks with different surface treatments: An in vivo investigation. Odontology 2018, 106, 145–153. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, F.; Rodríguez Andrés, C.; Arteagoitia, I. Which antibiotic regimen prevents implant failure or infection after dental implant surgery? A systematic review and meta-analysis. J. Cranio-Maxillofac. Surg. 2018, 46, 722–736. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin. Oral Implants Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef]
- Ramaglia, L.; di Lauro, A.E.; Morgese, F.; Squillace, A. Profilometric and standard error of the mean analysis of rough implant surfaces treated with different instrumentations. Implant Dent. 2006, 15, 77–82. [Google Scholar] [CrossRef]
- Speelman, J.A.; Collaert, B.; Klinge, B. Evaluation of different methods to clean titanium abutments. A scanning electron microscopic study. Clin. Oral Implants Res. 1992, 3, 120–127. [Google Scholar] [CrossRef]
- Kiska, D.L.; Gilligan, P.H. Pseudomonas. In Manual of clinical microbiology, 8th ed.; Murray, P.R., Baron, E.J., Jorgensen, J.H., Pfaller, M.A., Yolken, R.H., Eds.; ASM Press: Washington, DC, USA, 2003; pp. 719–728. [Google Scholar]
- Albertini, M.; López-Cerero, L.; O’Sullivan, M.G.; Chereguini, C.F.; Ballesta, S.; Ríos, V.; Herrero-Climent, M.; Bullón, P. Assessment of periodontal and opportunistic flora in patients with periimplantitis. Clin. Oral Impl. Res. 2015, 26, 937–941. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meto, A.; Conserva, E.; Liccardi, F.; Colombari, B.; Consolo, U.; Blasi, E. Differential Efficacy of Two Dental Implant Decontamination Techniques in Reducing Microbial Biofilm and Re-Growth onto Titanium Disks In Vitro. Appl. Sci. 2019, 9, 3191. https://doi.org/10.3390/app9153191
Meto A, Conserva E, Liccardi F, Colombari B, Consolo U, Blasi E. Differential Efficacy of Two Dental Implant Decontamination Techniques in Reducing Microbial Biofilm and Re-Growth onto Titanium Disks In Vitro. Applied Sciences. 2019; 9(15):3191. https://doi.org/10.3390/app9153191
Chicago/Turabian StyleMeto, Aida, Enrico Conserva, Francesco Liccardi, Bruna Colombari, Ugo Consolo, and Elisabetta Blasi. 2019. "Differential Efficacy of Two Dental Implant Decontamination Techniques in Reducing Microbial Biofilm and Re-Growth onto Titanium Disks In Vitro" Applied Sciences 9, no. 15: 3191. https://doi.org/10.3390/app9153191
APA StyleMeto, A., Conserva, E., Liccardi, F., Colombari, B., Consolo, U., & Blasi, E. (2019). Differential Efficacy of Two Dental Implant Decontamination Techniques in Reducing Microbial Biofilm and Re-Growth onto Titanium Disks In Vitro. Applied Sciences, 9(15), 3191. https://doi.org/10.3390/app9153191








